摘要/Abstract
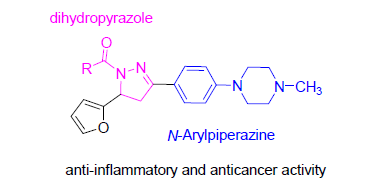
吡唑是一类具有优良生物活性的五元杂环化合物.以4-氟苯乙酮和2-呋喃甲醛为原料出发,经Aldol缩合、取代、环化和酰化等反应,合成得到16个未见文献报道的新型哌嗪取代的3-芳基-5-呋喃基-4,5-二氢吡唑酰胺衍生物.采用小鼠巨噬细胞Raw264.7模型和噻唑蓝(MTT)法分别测试了目标化合物的体外抗炎活性和抗肿瘤活性(A549、Hela和SGC7901).研究结果发现,二氢吡唑类化合物能有效抑制炎症因子NO的生成,并对肿瘤细胞株表现出选择性的抑制活性.其中3个化合物与地塞米松活性相当,能有效抑制NO的生成;3个化合物对肿瘤细胞株的体外选择性抑制活性与5-氟尿嘧啶(5-FU)相当,均可做进一步构效关系研究.
关键词: 二氢吡唑, 酰胺, 抗炎活性, 抗肿瘤活性
Pyrazole is a five-membered heterocyclic molecule with a broad range of biological activities. In this study, a series of new 3-aryl-5-furanyl-4,5-dihydropyrazole derivatives have been designed and synthesized by the general principle of molecular hybridization. The structures were characterized by 1H NMR, 13C NMR and HRMS. We screened in vitro anti-inflammatory in lipopolysaccharide (LPS)-stimulated RAW-264.7 macrophages and anticancer activity against 3 strains human tumor cell lines (A549, Hela and SGC7901) by the methyl thiazolyl tetrazolium (MTT) assay. The result indicated that dihydropyrazole compounds showed good inhibitory effect on the generation of NO and selective cytotoxic activity against tumor cell lines, and the acyl moieties of amides had an obvious influence on biological activities. Especially, 3 compounds were found to be similar anti-inflammatory to positive control dexamethasone, and 3 compounds displayed similar selective anti-tumor activity to positive control 5-fluorouracil (5-FU), which were to be lead compounds for further SAR research.
Key words: dihydropyrazole, amide, anti-inflammatory activity, anticancer activity
PDF全文下载地址:
点我下载PDF
