摘要/Abstract
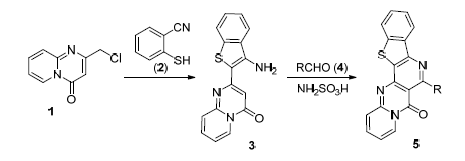
利用Thorpe-Ziegler反应,通过2-氯甲基-4H-吡啶并[1,2-a]嘧啶-4-酮与2-巯基苯甲腈的环化制得2-(3-氨基苯并噻吩-2-基)-4H-吡啶并[1,2-a]嘧啶酮,继而在氨基磺酸作用下,通过Pictet-Spengler反应,设计合成了新型苯并噻吩并[3',2':2,3]吡啶并[4,5-d]吡啶并[1,2-a]嘧啶衍生物.初步抑菌活性试验表明,当浓度为50 mg/L时,化合物5b对黄瓜灰霉病菌和小麦赤霉病菌的抑制率达96%以上,5f对油菜菌核病菌的抑制率为98%,5g和5i对烟草赤星病菌的抑制率达93%以上.
关键词: 吡啶并[1,2-a]嘧啶, 苯并噻吩, 2-巯基苯甲腈, Pictet-Spengler反应, 合成, 抑菌活性
A series of novel benzothieno[3',2':2,3]pyrido[4,5-d]pyrido[1,2-a]pyrimidines are prepared via Pictet-Spengler reaction of 2-(3-aminobenzothiophene-2-yl)-4H-pyrido[1,2-a]pyrimidin-4-one using sulfamic acid as a catalyst, which in turn were obtained from the Thorpe-Ziegler isomerization of 2-(chloromethyl)-4H-pyrido[1,2-a]pyrimidin-4-one with 2-mercapto-benzonitrile. The structures of the products were characterized by FT-IR, 1H NMR, 13C NMR spectra and elemental analysis. The fungicidal activities of the prepared compounds were also preliminarily evaluated. For example, 5b exhibited more than 96% inhibition rate to Botrytis cinerea and Gibberella zeae at 50 mg/L, 5f exhibited 98% inhibition rate to Sclerotonia sclerotiorum at 50 mg/L, and 5g, 5i exhibited more than 93% inhibition rate to Alternaria alternata at 50 mg/L.
Key words: pyrido[1,2-a]pyrimidine, benzothiophene, 2-mercaptobenzonitrile, Pictet-Spengler reaction, synthesis, fungicidal activity
PDF全文下载地址:
点我下载PDF
