摘要/Abstract
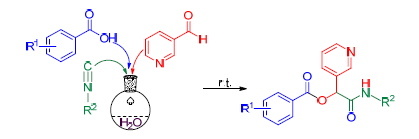
以3-吡啶甲醛、取代苯甲酸、叔丁基异腈(或1,1,3,3-四甲基丁基异腈)等为原料,利用Passerini三组分反应,在水相中简便合成了22种取代-2-(吡啶-3-基)-2-苯甲酰氧基乙酰胺,并用IR、1H NMR、13C NMR和HRMS确证了化合物的结构.该法具有反应条件温和、反应时间短、操作简便、收率高等优点.采用菌丝生长速率法测试了目标化合物对两种病原菌的离体抑制活性,结果表明在100 μg/mL时,8种化合物对灰霉菌显示出中等的抑制活性,抑制率为83%~89%;17种化合物对菌核菌有较强的抑制活性,抑制率为90%~100%.
关键词: 吡啶, 羧酸, 异腈, Passerini反应, 合成设计, 生物活性
Twenty-two novel substituted 2-(pyridin-3-yl)-2-benzoyloxy acetamides were synthesized via Passerini three-component reaction involving 3-pyridinecarboxaldehyde, substituted benzoic acid, and tert-butyl isocyanide (or 1,1,3,3-tetramethylbutyl isocyanide) in water, and characterized by IR, 1H NMR, 13C NMR and HRMS. This method provides some advantages such as mild conditions, short reaction time, facile operation and high yield. Moreover, the target compounds were evaluated for their in vitro antifungal activities against two plant pathogens by the mycelium growth rate method, and the results indicated that at the dosage of 100 μg/mL, eight compounds showed moderate activities against B. cinerea with inhibition rates of 83%~89%, and seventeen compounds exhibited strong activities against S. sclerotiorum with inhibition rates of 90%~100%.
Key words: pyridine, carboxylic acid, isocyanide, synthesis design, biological activity
PDF全文下载地址:
点我下载PDF
