摘要/Abstract
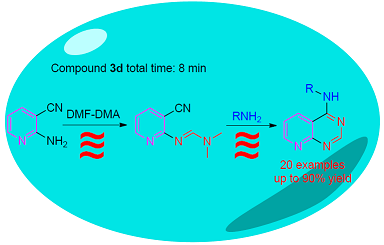
研究了N-(3,5-二氯苯基)吡啶并[2,3-d]嘧啶-4-胺的合成新方法.在微波辐射条件下,以2-氨基-3-氰基吡啶为原料,依次通过缩合、环化和Dimroth重排两步反应,得到目标产物N-(3,5-二氯苯基)吡啶并[2,3-d]嘧啶-4-胺,总收率90%.并应用该方法,合成了20个吡啶并[2,3-d]嘧啶-4-胺类化合物.同时,比较了微波辐射和传统油浴加热条件下的反应结果.结果表明,微波辐射条件下,反应时间短,产率高.此方法有望成为一种高效、温和、对环境友好的合成吡啶并[2,3-d]嘧啶-4-胺的方法.
关键词: 吡啶并[2,3-d]嘧啶-4-胺, 达摩斯重排, 有机合成, 微波辐射
In this paper, a novel synthetic method for N-(3, 5-dichlorophenyl)pyrido [2, 3-d]pyrimidin-4-amine was reported, which began from 2-aminonicotinonitrile, following condensation, cyclization and then Dimroth rearrangement reaction under microwave irradiation conditions. The over yield was 90%. Employing the same synthetic method, 20 pyrido [2, 3-d]pyrimidin- 4-amine derivatives were synthesized. We also compared microwave irradiation and oil bath heating for synthetizing the target products. The results showed that the method under microwave irradiation for the preparation of pyrido [2, 3-d]pyrimidin- 4-amine was time-saving and high yield. It is expected to become an efficient, gentle and environmentally friendly synthetic method of pyrido [2, 3-d]pyrimidin-4-amine.
Key words: pyrido[2, 3-d]pyrimidin-4-amine, Dimroth rearrangement, organic synthesis, microwave irradiation
PDF全文下载地址:
点我下载PDF
