摘要/Abstract
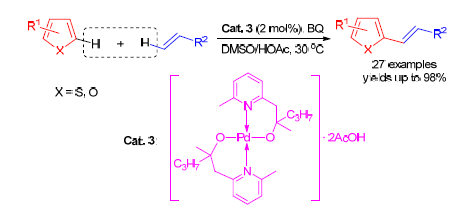
在前期工作的基础上,设计合成了一系列吡啶乙醇类双[N,O]环钯配合物,以该类环钯配合物为催化剂,通过Fujiwara-Moritani反应,在相对温和的反应条件下(2 mol%催化剂,30℃,空气环境中)即可高效实现噻吩、呋喃类芳杂环与各类烯烃的偶联,并以中等到高等的分离产率得到相应的目标化合物.进一步通过动力学实验和ESI(+)-MS同步检测,推测该反应是通过协同的金属化/去质子化(CMD)机理进行.
关键词: 双[N,O]环钯配合物, 吡啶乙醇类配体, Fujiwara-Moritani反应, 协同的金属化/去质子化
A series of bis(alkoxo)palladium complexes (2 mol%) based on pyridine-containing alcohol ligand were tested for Fujiwara-Moritani reaction of thiophenes/furans with various olefins. The desired products were isolated in moderate to excellent yields under mild conditions. A possible concerted metalation-deprotonation (CMD) pathway for this transformation was proved by control experiments and ESI(+)-MS analysis.
Key words: bis(alkoxo)palladium complex, Fujiwara-Moritani reaction, CMD pathway, pyridine-containing alcohol ligand
PDF全文下载地址:
点我下载PDF
