摘要/Abstract
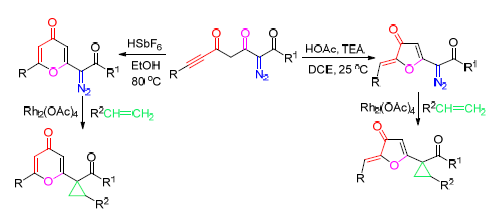
在六氟锑酸/乙醇的体系中,80℃条件下,2-重氮-3,5-二氧代-6-炔酯/炔酮/炔酰胺环化生成重氮γ-吡喃酮衍生物;然而,在乙酸/三乙胺/1,2-二氯乙烷体系中,25℃条件下,其环化反应主要生成重氮3(2H)-呋喃酮.在Rh(Ⅱ)催化下,这些重氮化合物与烯烃可以进行有效的分子间环丙烷化.
关键词: 重氮g-吡喃酮, 重氮3(2H)-呋喃酮, 合成, 环丙烷化, 区域选择性
For HSbF6/EtOH system, diazo γ-pyrones were cleanly obtained starting from 2-diazo-3,5-dioxo-6-ynoates/ynones/ynamide at 80℃, whereas diazo 3(2H)-furanones were predominantly generated in HOAc-Et3N-1,2-dichloroethane system at 25℃. These diazo compounds can undergo an efficient Rh(Ⅱ)-catalyzed intermolecular cyclopropanation with alkene.
Key words: diazo γ-pyrone, diazo 3(2H)-furanone, synthesis, cyclopropanation, regioselectivity
PDF全文下载地址:
点我下载PDF
