摘要/Abstract
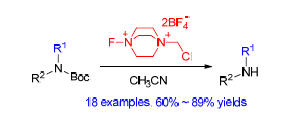
商品化的selectfluor[即1-氯甲基-4-氟-1,4-重氮化二环2.2.2辛烷双(四氟硼酸)盐]具有不吸潮的特性,是一种被应用得最为广泛的氟代试剂.因此,发现并理解其新的反应特性对于应用该试剂具有非常重要的意义.本工作报道了selectflour可以用于选择性脱除双保护氨基上的一个叔丁氧羰基保护基.该方法具有条件温和、操作简单、化学选择性好等特点.跟其他方法相比,该脱保护方法有一定的应用价值,被成功应用于一系列氨基酸衍生物的脱保护,在医药上合成有潜在用途的嘌呤衍生物.以氘代乙腈为溶剂的核磁反应实验解释了为什么该反应需要等物质的量的selectfluor试剂.
关键词: selectfluor, 叔丁氧羰基, 脱保护, 选择性, 合成
Selectfluor, 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2] octane bis(tetrafluoroborate), is among the most popular fluorinating reagents owning to its commercially availability and non-hygroscopic property. The discovery and understanding of new reactivities of selectfluor are thus important for reaction design and optimization when this popular reagent is employed. It has been found that selectfluor could selectively remove Boc group from doubly protected amines in acetonitrile. This deprotection could be of interest when compared to other reported methods, not only because selectfluor is a solid and easy-to-handle, but also because the reaction is mild, operationally simple and chemoselective. The potential usefulness of this method is demonstrated by the deprotection of a series of protected amino acids and a one-step synthesis of pharmaceutically important purine derivative. The NMR experiments conducted in CD3CN explain why stoichiometric amount of selectfluor is needed for a successful reaction.
Key words: selectfluor, t-butyloxycarbonyl, deprotection, selectivity, synthesis
PDF全文下载地址:
点我下载PDF
