
 , 刘鹏, 赵文阁
, 刘鹏, 赵文阁 哈尔滨师范大学 生命科学与技术学院,黑龙江 哈尔滨 150025
收稿日期:2019-11-13;接收日期:2020-03-11;网络出版时间:2020-03-26
基金项目:哈尔滨市科技创新人才研究专项资金(No. 2014FQXJ169)资助
摘要:本研究旨在探讨嗜水气单胞菌(Aeromonas hydrophila,Ah)胁迫下MHCⅠ基因在东北林蛙不同组织的表达特征,为揭示两栖类抗感染免疫应答机制提供依据。文中首先构建嗜水气单胞菌感染下的试验动物模型,通过苏木精-伊红染色法(Hematoxylin-eosin staining,HE染色)观察病理学变化;利用RT-PCR克隆东北林蛙MHCⅠ基因α1+α2肽结合区并进行生物信息学分析,再运用荧光定量PCR技术检测Ah胁迫下MHCⅠ在不同组织中的转录水平。结果表明:在Ah感染后,皮肤、肝脏和肌肉等组织均出现细胞结构消失和纹理紊乱等现象;获得MHCⅠ基因α1+α2肽结合区片段494 bp,可编码164个氨基酸,与两栖类同源性在77%以上,与哺乳类同源性低至14.96%,表明MHCⅠ基因α1+α2区在不同物种间保守性较低;荧光定量PCR结果显示,在Ah胁迫下,实验组肝脏、脾脏、肾脏、皮肤和肌肉组织MHCⅠ基因的转录水平在72 h前均高于对照组,但是各组织到达峰值的时间具有差异性(P < 0.01),表明Ah胁迫下MHCⅠ基因在不同组织的表达时间存在差异。研究结果为进一步探究MHC分子在抗感染中的免疫功能提供了参考依据。
关键词:东北林蛙嗜水气单胞菌MHCⅠ基因表达
Expression of MHCⅠ genes in different tissues of Rana dybowskii under the stress of Aeromonas hydrophila
Ruofei Bian, Xiao Xu, Yufen Liu

 , Peng Liu, Wenge Zhao
, Peng Liu, Wenge Zhao College of Life Science and Technology, Harbin Normal University, Harbin 150025, Heilongjiang, China
Received: November 13, 2019; Accepted: March 11, 2020; Published: March 26, 2020
Supported by: Harbin Science and Technology Innovation Talent Research Project (No. 2014FQXJ169)
Corresponding author: Yufen Liu. Tel: +86-451-88060784; E-mail: liuyufen0825@126.com.
Abstract: The aim of this study was to investigate the expression of MHCⅠ gene in different tissues of Rana dybowskii under the stress of Aeromonas hydrophila (Ah), and to provide evidence for revealing the anti-infective immune response mechanism of amphibians. The experimental animal model of Aeromonas hydrophila infection was first constructed, and the pathological changes were observed by HE staining. The MHCⅠ gene α1+α2 peptide binding region of Rana dybowskii was cloned by RT-PCR and analyzed by bioinformatics. Real-time PCR was used to detect the transcription level of MHCⅠ in different tissues under Ah stress. After Ah infection, the skin, liver and muscle tissues showed signs of cell structure disappearance and texture disorder. The MHCⅠ gene α1+α2 peptide binding region fragment was 494 bp, encoding 164 amino acids, and homology with amphibians. Above 77%, the homology with mammals was as low as 14.96%, indicating that the α1+α2 region of MHC gene was less conserved among different species. The results of real-time PCR show that the liver, spleen and kidney of the experimental group were under Ah stress. The transcript levels of MHCⅠ gene in skin and muscle tissues were higher than those in the control group at 72 h, but the time to peak of each tissue was different (P < 0.01), indicating that the response time of MHCⅠ gene in different tissues was different under Ah stress. This study provides a reference for further exploring the immune function of MHC molecules in anti-infection.
Keywords: Rana dybowskiiAeromonas hydrophilaMHCⅠ geneexpression
主要组织相容性复合体(Major histocompatibility complex,MHC)是一组与免疫密切相关、紧密连锁的基因群,其产物广泛存在于脊椎动物中并在免疫应答中扮演极为重要的角色[1-2]。研究表明[3-5],MHC主要由3类分子组成,其中Ⅰ类和Ⅱ类分子广泛参与免疫应答的过程,已成为疾病抗性和易感性的候选标记基因。MHCⅠ类分子主要识别内源性抗原,并呈递给CD8+ T细胞,从而完成经典的内源性抗原呈递过程,但是在感染的过程中经常出现抗原的交叉呈递现象,即外源性抗原也可以通过MHCⅠ类分子呈递[3]。近年来,MHCⅠ类分子在疾病发生中的作用研究主要集中于人和其他哺乳动物以及禽类,在两栖类中的研究目前大部分局限于MHC分子的遗传多样性和进化机制[6-11]。
东北林蛙Rana dybowskii隶属蛙科(Ranidae)、林蛙属(Rana),是一种对严寒气候有较强适应能力的无尾两栖类,属于东北地区优势两栖物种[12-13]。其雌蛙的输卵管是我国传统中药材哈什蟆油的主要成分[14-15]。随着生态环境不断恶化,东北林蛙种群数量锐减,已被列为易危物种[16-18]。微生物侵袭被认为是其减少的元凶之一,其中嗜水气单胞菌是造成蛙群患败血病的主要病原体[19-21]。本研究对东北林蛙MHCⅠ基因的α1+α2肽结合区进行克隆和生物信息学分析,通过人工感染嗜水气单胞菌建立感染模型,采用荧光定量PCR技术检测MHCⅠ基因在不同组织的转录情况,为MHCⅠ基因的免疫功能研究提供参考,并为两栖类传染病的预防和治疗提供理论基础。
1 材料与方法1.1 材料1.1.1 试验动物和菌株东北林蛙,雄性,实验室人工养殖;嗜水气单胞菌株由本实验室分离鉴定。
1.1.2 主要试剂克隆载体pMD18-T、感受态细胞DH5α、DL2000 Marker、Ex Taq DNA聚合酶、T4 DNA连接酶均购自宝生物工程(大连)有限公司;dNTPs、琼脂糖胶回收试剂盒购自哈尔滨伊事达生物工程有限公司;Trizol试剂盒购自生工生物工程(上海)股份有限公司;HiScript Ⅱ Q RT Super Mix for qPCR (+gDNA wiper),ChamQ SYBR qPCR Master Mix,均购于南京诺唯赞(Vazyme)生物科技有限公司;10%福尔马林固定液、伊红染液和其他试剂均为国产分析纯。
1.1.3 仪器设备凝胶成像系统(Smart Gel Ⅲ;北京赛智创业科技有限公司);低温循环水浴箱(HX-1050;北京博医康技术公司);Olympus BH-2型显微镜;组织切片机(820型;日本Nikon公司);ABI7500实时荧光定量PCR仪;高速冷冻离心机(Allegra-64R;美国BECKMAN公司)。
1.2 方法1.2.1 东北林蛙的人工感染和样品采集将菌株活化后,依据参考文献[22],采用紫外分光光度计和平板菌落计数法测定菌液浓度,通过预实验,确定攻毒浓度。将东北林蛙随机分为两组:即对照组(LB组)和试验组(Ah组),每组15只,试验组每只东北林蛙采用腹腔注射的方式注射1 mL嗜水气单胞菌液(1.12×107 CFU/mL),对照组东北林蛙注射同样体积的LB液体培养基,分别取6、12、24、48、72 h时两组东北林蛙心脏、肝脏、脾脏、肺脏、肾脏、皮肤和肌肉组织样品,每个时间点取3只,用液氮速冻后于?80 ℃保存。
1.2.2 引物设计根据已发表的中国林蛙MHCⅠ类基因的序列(GenBank登录号:FJ385693.1),利用Primer Premier 5.0设计引物,MHCⅠ-α1+α2扩增片段跨度为494 bp左右,MHCⅠ-q扩增片段跨度为227 bp左右;内参引物β-actin依据参考文献[19]设计,长度为176 bp左右,引物由哈尔滨博仕生物工程有限公司,合成引物序列如表 1所示。
表 1 引物序列Table 1 Primers used in this study
| Primer name | Primer sequence (5′–3′) | Product size (bp) |
| MHCⅠ-α1+α2-F | CGGGGTACCCGGGTCTCGGATAAAGGAT | 494 |
| MHCⅠ-α1+α2-R | CGCTCGAGCGGTCCCGTACTCTATGTATTTCT | |
| MHCⅠ-q-F | GTCTCATCTGGCTCGTCC | 227 |
| MHCⅠ-q-R | ATCCGTACTGCTGATACCC | |
| β-actin-F | AAGAATGAGGGCTGGAACA | 176 |
| β-actin-R | GTGCGTGACATCAAGGAGAAGC |
表选项
1.2.3 东北林蛙MHCⅠ类α1+α2的克隆及生物信息学分析依据Trizol试剂盒说明书,提取对照组和试验组5个时间点7种组织样品的总RNA,采用1%的水平式琼脂糖凝胶电泳检测其完整性并用分光光度计检测确定浓度。再按照反转录试剂盒操作步骤,合成cDNA第一链,用于后续实验及qRT-PCR。以上述肌肉的cDNA第一链为模板,按照Taq plus DNA聚合酶说明书进行PCR反应体系的配置,反应条件为:94 ℃预变性5 min;94 ℃变性30 s,53 ℃退火30 s,72 ℃延伸30 s,30个循环;72 ℃终延伸10 min。获得的PCR产物进行胶回收纯化后,与pMD18-T载体进行连接,并转入到大肠杆菌DH5α中,经PCR检测,获得的疑似阳性菌株命名为pMD18-T-MHCⅠ-α1+α2,将该菌株送哈尔滨博仕生物工程有限公司进行序列测定。利用SWISS-MODEL (http://www.expasy.ch/swissmod/SWISS-MODEL.html)、DNAMAN6.0、MEGA7和SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl)等软件对MHCⅠ基因α1+α2肽结合区的核苷酸和氨基酸序列进行生物信息学分析。选择的参考物种信息如表 2所示。
表 2 参考物种信息Table 2 List of Reference species
| Species | Accession No of nucleotide | Accession No of amino acid |
| Rana chensinensis | FJ385693.1 | ACJ63978.1 |
| Xenopus laevis | FJ589643.1 | AGM17468.1 |
| Polypedates megacephalus | KC261643.1 | AGN74545.1 |
| Rhacophorus omeimontis | KC261663.1 | AGN74565.1 |
| Xenopus tropicalis | BC161748.1 | AAI61748.1 |
| Bufo gargarizans | KY302858.1 | APW83890.1 |
| Gallus gallus | KT321041 | ALS55373.1 |
| Homo sapiens | M24097.1 | AAA59656.1 |
| Mus muscμlus | NM153760.2 | NP715641.1 |
| Oryctolagus cuniculus | NM001190434 | NP001177363 |
| Bos taurus | NM001040532.1 | NP001035622.1 |
| Sus scrofa | NM001097427 | NP001090896.1 |
| Pan troglodytes | NM001045494 | NP001038959.1 |
表选项
1.2.4 东北林蛙病理组织切片的制备取感染后0、6、12、24、48、72 h东北林蛙的肝脏、皮肤和肌肉组织,用10%福尔马林固定液进行固定。采用石蜡包埋法进行处理,以约5 μm厚度进行切片。脱蜡复水即二甲苯Ⅰ 5 min,二甲苯Ⅱ 5 min,1/2二甲苯5 min,无水乙醇3 min,95%乙醇Ⅰ 3 min,95%乙醇Ⅱ 3 min,90%乙醇3 min,80%乙醇3 min,70%乙醇3 min,双蒸水Ⅰ 5 min后苏木精染色10 min,流水冲洗去余色,将切片放入1%的盐酸乙醇中分化10 s,再放入1%的氨水中蓝化10 s,清水清洗数分钟,0.5%伊红溶液染色3 min后进行梯度脱水透明并用树胶进行封片。
1.2.5 东北林蛙MHCⅠ类基因的荧光定量PCR分析以β-actin的表达作为内参,采用SYBR GreenⅠ嵌合荧光法检测MHCⅠ基因在东北林蛙心脏、肝脏、脾脏、肺脏、肾脏、皮肤和肌肉等7种组织中的转录情况。反应体系如下(20 μL):2×ChamQ SYBR qPCR Master Mix 10 μL,Primer1 (10 μmol/L) 0.4 μL,Primer 2 (10 μmol/L) 0.4 μL,50×ROX Reference Dye2 0.4 μL,ddH2O 7.8 μL,cDNA模板1 μL。反应程序:94 ℃ 10 min;94 ℃ 30 s,60 ℃ 30 s,72 ℃ 30 s,40个循环;72 ℃ 10 min。每个处理设置3次重复。相对表达量采用2–ΔΔCt法计算,利用SPSS25软件对所得数据进行单因素方差分析,并利用GraphPad Prism 8软件进行作图。
2 结果与分析2.1 东北林蛙MHCⅠ基因α1+α2区的克隆与鉴定采用Trizol法提取东北林蛙肌肉RNA后,进行琼脂糖凝胶电泳检测,有清晰的28S和18S的条带,再利用紫外分光光度计进行检测,A260/A280值在1.8–2.0之间,可以进行后续的反转录实验。以肌肉的cDNA为模板,扩增MHCⅠ-α1+α2区,电泳检测结果如图 1A所示,目的带大小约为500 bp左右,与预期相符,使用回收试剂盒进行回收,结果如图 1B所示。将目的片段α1+α2和pMD18-T连接后,转入大肠杆菌DH5α后,通过菌落PCR鉴定,电泳检验结果如图 1C所示,可见PCR结果条带单一,与预计大小相符。
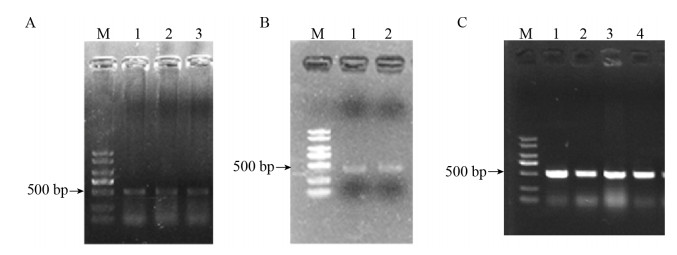 |
| 图 1 东北林蛙MHCⅠ基因α1+α2区的克隆 Fig. 1 Cloning of MHC classⅠ α1+α2 in Rana dybowskii. (A) PCR amplification results of MHC classⅠ α1+α2 in Rana dybowskii. M: DL2000 marker; lane 1–3: PCR product of MHC classⅠα1+α2 gene. (B) PCR amplification results of MHC classⅠ α1+α2 after gel extraction in Rana dybowskii. M: DL2000 marker; lane 1–2: The products of gel extraction. (C) Colony PCR amplification results of MHC classⅠα1+α2 in Rana dybowskii. M: DL2000 marker; lane 1–4: the products of colony PCR |
| 图选项 |
2.2 东北林蛙MHCⅠ基因α1+α2区序列的生物信息学分析测序结果表明,获得的东北林蛙MHCⅠ类α1+α2序列长为494 bp,与GenBank公布的中国林蛙MHCⅠ α1+α2核苷酸序列的同源性达94%;该核苷酸序列编码164个氨基酸残基(图 2)。SOPMA软件分析表明,MHCⅠ基因α1+α2蛋白二级结构包含α-螺旋占36.59% (60AA),无规卷曲占28.66% (47AA),延伸链占20.12% (33AA)和β折叠占14.63% (24AA)等几种结构形成。利用SWISS-MODEL软件对三级结构进行模拟预测,其结构(图 3A)与模式生物非洲爪蟾的三级结构类似(图 3B)。通过与表 2中13个物种进行核苷酸序列比较,发现该区域与两栖类同源性在77%以上,其中与中国林蛙同源性最高,与哺乳类相比低至14.96%,禽类为24.44%。核苷酸(图 4A)和氨基酸(图 4B)系统进化树显示其与两栖类亲缘关系最近,在一个分支内,而与人、牛、猪和禽类等亲缘关系较远。
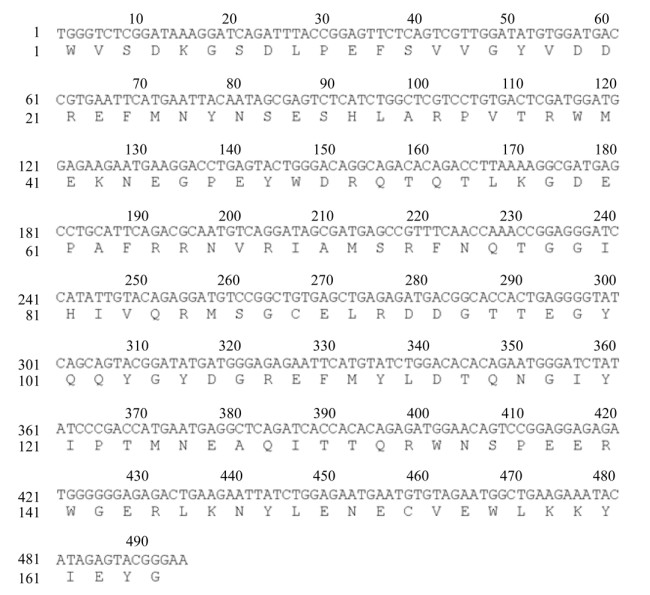 |
| 图 2 东北林蛙MHCⅠ基因α1+α2区核苷酸和氨基酸序列 Fig. 2 Nucleotide and amino acid sequences of the MHC classⅠ α1+α2 of Rana dybowskii |
| 图选项 |
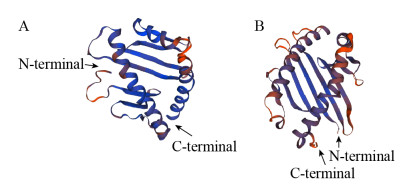 |
| 图 3 MHCⅠ α1+α2区的三级结构模型 Fig. 3 Tertiary structure model of MHC classⅠα1+α2 protein. 3-D structure of MHCⅠ α1+α2 designed using Swiss Model workspace. (A) 3-D structure of MHCⅠ α1+α2 of Rana dybowskii. (B) 3-D structure of MHCⅠ α1+α2 of Xenopus laevis. Their 3-D structure is similar |
| 图选项 |
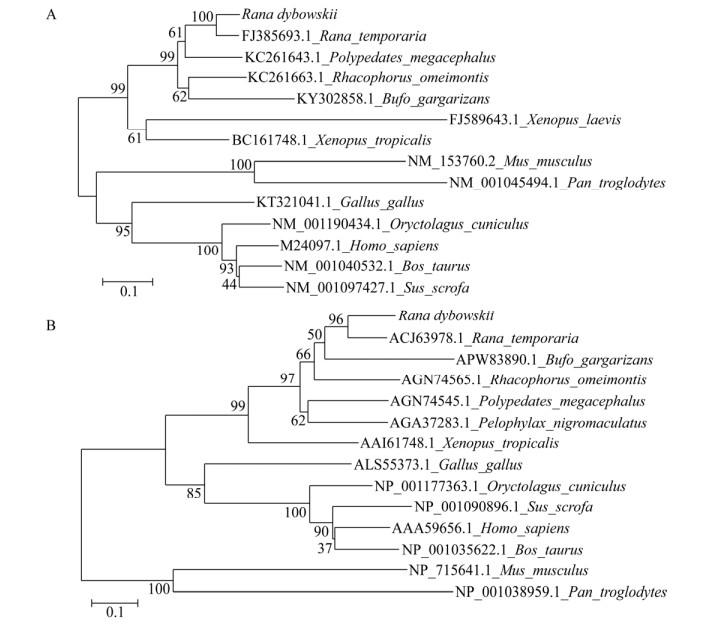 |
| 图 4 MHCⅠ α1+α2系统进化树分析 Fig. 4 Phylogenetic tree of MHC classⅠ α1+α2. Homology analysis of MHCⅠ α1+α2. Analysis was done using the MEGA software. The phylogenetic tree was constructed using the neighbor-joining (N-J) method within MEGA7.0. Numbers on the branches represent bootstrap values for 1000 replications. (A) Evolutionary analysis of MHCⅠ α1+α2 nucleotide acid sequences. (B) Evolutionary analysis of MHCⅠ α1+α2 amino acid sequences |
| 图选项 |
2.3 嗜水气单胞菌胁迫下东北林蛙组织的病理鉴定取感染后0、6、12、24、48、72 h的东北林蛙肝脏、皮肤和肌肉组织,进行HE染色。结果表明,感染后肝脏组织病理学观察所见(图 5A1–A6),随着时间延长(6–72 h)细胞呈空泡样变,细胞间隙增大,肝细胞核固缩,炎性细胞浸润逐渐明显。在皮肤组织中(图 5B1–B6),可见数量较多染色呈蓝紫色的碱性细胞,呈浅粉色的胶原纤维和呈紫色的纤维细胞,此外可见有大量的黑色素细胞在表皮下与真皮层之间,随着感染时间的延长,细胞间的界限不清,整体结构变得松散。在肌肉组织中(图 5C1–C6),随着感染时间的逐渐延长,肌纤维纹理变得紊乱。在解剖过程中也发现随着感染嗜水气单胞菌的时间延长,东北林蛙头部和背部出现溃烂,背部和腿部出现出血点,解剖过程也可见腹水、肝脏肿大出现充血、腿部肌肉有出血点等现象,表明人工感染成功。
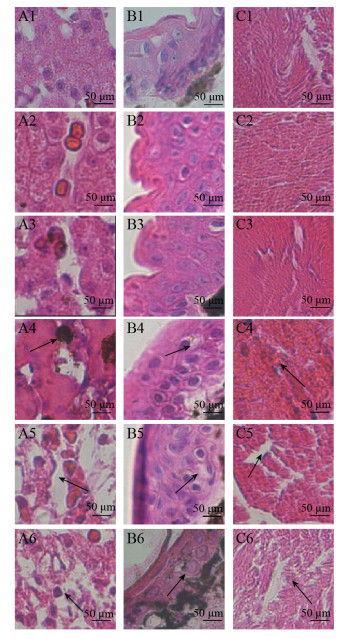 |
| 图 5 东北林蛙病理组织切片 Fig. 5 Histopathological section of Rana dybowskii challenged with Aeromonas hydrophila. Observation by hematoxylin-eosin staining in different tissues of Rana dybowskii challenged with Aeromonas hydrophila. A: liver; B: skin; C: muscular, 1: 0 h; 2: 6 h; 3: 12 h; 4: 24 h; 5: 48 h; 6: 72 h |
| 图选项 |
2.4 东北林蛙MHCⅠ基因在不同组织中的转录水平在嗜水气单胞菌胁迫下,东北林蛙心脏MHCⅠ mRNA的表达量在6 h达到峰值,为对照组的4.7倍(P < 0.01),在12 h下调至略高于本底水平,在24–72 h表达量始终略低于本底水平(图 6A)。东北林蛙肝脏MHCⅠ mRNA的表达量在各时间点均高于对照,在6 h到达峰值后表达有所下调,在24 h时为对照的1.8倍(P < 0.05),48–72 h又出现上调表达(图 6B)。东北林蛙脾脏MHCⅠ mRNA的表达量除6 h为对照组的4.9倍(P < 0.01),其余时间表达量均为对照组的2倍左右(图 6C)。东北林蛙肺脏MHCⅠ mRNA的表达量在12 h达到峰值为对照组的13倍后逐渐下降,在72 h时低于本底水平(图 6D)。东北林蛙肾脏MHCⅠ mRNA的表达量呈上调表达,在24 h时达到峰值(P < 0.01),48–72 h表达量虽有下调,但仍高于本底水平(图 6E)。东北林蛙皮肤MHCⅠ mRNA的表达量始终维持高表达水平,在6 h达到峰值为对照组的11倍(P < 0.01) (图 6F)。东北林蛙肌肉MHCⅠ mRNA的表达量在6 h时略低于本底水平,在12 h达到峰值(P < 0.01),为对照的3.3倍后逐渐下降,但始终高于本底水平(图 6G)。东北林蛙在Ah胁迫下,试验组肝脏、脾脏、肾脏、皮肤和肌肉组织MHCⅠ基因的转录水平在72 h前均高于对照组,但是这些组织到达峰值的时间具有差异性(P < 0.01),心脏MHCⅠ基因的转录水平与其他组织相比差异较大(P < 0.01),6 h即达到高峰,随后迅速下降。表明Ah胁迫下MHCⅠ基因在不同组织的应答时间和水平存在差异。
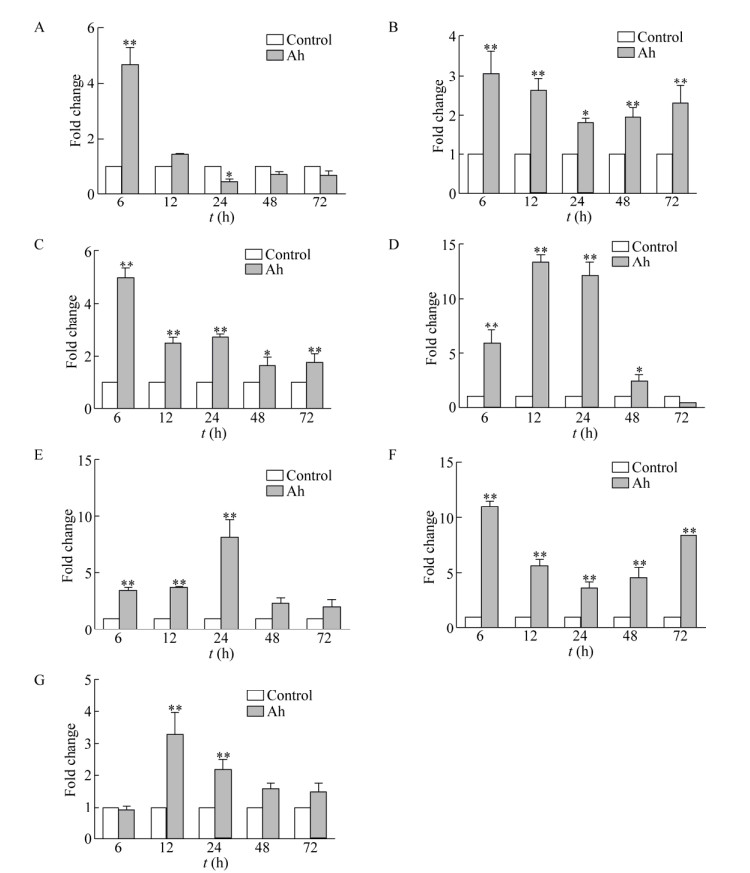 |
| 图 6 嗜水气单胞菌胁迫下东北林蛙各组织MHCⅠ转录水平变化检测 Fig. 6 Graphical presentation of qPCR analysis shows changes in MHCⅠ gene expression in healthy Rana dybowskii or in Rana dybowskii challenged with Aeromonas hydrophila. (A–G) MHCⅠ transcripts in different tissues of Rana dybowskii challenged with Aeromonas hydrophila. Each infected Rana dybowskii was injected intraperitoneally with 1 mL bacterial suspension (1.12×107 CFU/mL), whereas the control group was injected with the same volume of sterile tryptone liquid medium. (A) The heart. (B) The liver. (C) The spleen. (D) The lung. (E) The kidneys. (F) The skin. (G) The muscular. The samples were collected at different time points after Aeromonas hydrophila challenge (x-axis).β-Actin was used as an internal control. The results were expressed as the mean ± standard error (bars) of three independent exposures. *: P < 0.05; **:P < 0.01 |
| 图选项 |
3 讨论作为最早开始陆生的脊椎动物,两栖动物的生活环境使它们接触病原体的机会更多[23]。在感染过程中,免疫系统如何发挥调控作用,对物种生存至关重要。作为脊椎动物免疫系统重要成分的主要组织相容性复合体,可以首先识别和呈递抗原启动免疫应答,从而发挥抗感染作用。MHC基因目前已经成为研究两栖类适应性进化和物种保护的最佳候选基因之一[24-26]。MHCⅠ基因的α1+α2区为肽结合区,该区域可以识别不同的抗原肽,呈递给T细胞完成细胞免疫应答过程。以往研究显示[27],该区域具有一定的多态性。陶久臣等[28]曾对东北林蛙的MHC基因多态性进行分析,其研究表明东北林蛙MHCⅠ类基因有丰富的多态性变化,在进化过程中经历了基因突变、正选择等作用。本研究证明东北林蛙与中国林蛙亲缘关系较相近,与哺乳动物和禽类亲缘关系较远,可以划分成两个大的分支,这也表明MHCⅠ类基因作为一种古老的基因,在物种进化中经历了一系列分化,形成各自物种的特点[29]。
东北林蛙在感染嗜水气单胞菌后,MHCⅠ的转录水平发生了明显的变化,且在不同组织中有显著的差异,其中MHCⅠ分子在皮肤响应更积极,推测这与东北林蛙的水中生活习性呈现相关性。在斜带石斑鱼研究[30]中,也发现MHCⅠ类基因在皮肤中表达量最高,这表明皮肤或许是两栖动物及水生动物受到胁迫后抗原呈递的主要位点。李伟等[31]在研究嗜水气单胞菌感染黄鳝时,对肝脏、脾脏和肾脏的MHCⅠ分子的表达量进行了分析,变化趋势与本试验一致。当用嗜水气单胞菌感染团头鲂[32]和无乳链球菌感染尼罗罗非鱼[33]时,肾脏和脾脏中MHCⅠ分子也具有较高水平的表达。在牙鲆[34]和淡水鲤鱼[35]MHCⅠ类基因抵御病原体的研究中,微生物感染后肾的MHCⅠ分子的表达量变化趋势均与本文一致,从而说明MHCⅠ分子在抵抗病原微生物的过程中发挥了重要作用。
综上,在东北林蛙感染嗜水气单胞菌的过程中,不同组织中MHCⅠ分子的转录水平均出现上升趋势,从而推测MHCⅠ基因在免疫反应中发挥重要作用,是东北林蛙重要的免疫应答基因,这为进一步研究东北林蛙MHCⅠ基因免疫功能及其作用机制提供了参考。
参考文献
| [1] | Bjorkman PJ, Saper MA, Samraoui B, et al. Structure of the human class Ⅰ histocompatibility antigen, HLA-A2. Nature, 1987, 329(6139): 506-512. |
| [2] | Cao XT. Medical Immunology. 6th ed. Beijing: People's Health Press, 2013: 68-74 (in Chinese). 曹雪涛. 医学免疫学. 6版. 北京: 人民卫生出版社, 2013: 68-74. |
| [3] | Klein J. Natural History of the Major Histocompatibility Complex. New York: Wiley, 1986. |
| [4] | Apanius V, Penn D, Slev PR, et al. The nature of selection on the major histocompatibility complex. Crit Rev Immunol, 1997, 17(2): 179-224. |
| [5] | Edwards SV, Hedrick PW. Evolution and ecology of MHC molecules: from genomics to sexual selection. Trends Ecol Evol, 1998, 13(8): 305-311. |
| [6] | Reche PA, Reinherz EL. Sequence variability analysis of human Class Ⅰ and Class Ⅱ MHC molecules: functional and structural correlates of amino acid polymorphisms. J Mol Biol, 2003, 331(3): 630-641. |
| [7] | Li HY, Ma Y, Xu YC. Cloning and sequence analysis of MHC Class Ⅰ gene of tiger (Panthera tigris). Chin J Wildl, 2010, 31(3): 115-120 (in Chinese). 李慧一, 马跃, 徐艳春. 虎MHC ClassⅠ基因的克隆及测序. 野生动物, 2010, 31(3): 115-120. |
| [8] | Yu S, Wu J, Bai J, et al. Polymorphic analysis of peptide binding domain of major histocompatibility complex class Ⅰ in domestic ducks. Pol J Vet Sci, 2019, 22(2): 415-422. |
| [9] | Cloutier A, Mills JA, Baker AJ. Characterization and locus-specific typing of MHC class Ⅰ genes in the red-billed gull (Larus scopulinus) provides evidence for major, minor, and nonclassical loci. Immunogenetics, 2011, 63(6): 377-394. |
| [10] | Kuhner M, Watts S, Klitz W, et al. Gene conversion in the evolution of both the H-2 and Qa class Ⅰ genes of the murine major histocompatibility complex. Genetics, 1990, 126(4): 1115-1126. |
| [11] | Wang YZ. Polymorphism and evolution of MHC class Ⅰ genes of Rhscophorus omeimontis and Rhscophorus megacephalus[D]. Wuhan: Central China Normal University, 2012 (in Chinese). 王永珍.峨眉树蛙和斑腿树蛙MHCⅠ类基因的多态性及其进化[D].武汉: 华中师范大学, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10511-1013179929.htm |
| [12] | Zhao WG, Liu P, Chen H. Amphibians and Reptiles in Heilongjiang Province. Beijing: Science Press, 2008 (in Chinese). 赵文阁, 刘鹏, 陈辉. 黑龙江省两栖爬行动物志. 北京: 科学出版社, 2008. |
| [13] | Li PP, Lu YY, Li A. Taxonomy and distribution of Chinese brown frog Rana chensinensis. J Snake, 2014, 26(2): 156-158 (in Chinese). 李丕鹏, 陆宇燕, 李昂. 中国林蛙的分类及分布. 蛇志, 2014, 26(2): 156-158. |
| [14] | Liu P, Liu H, Zhang DC, et al. Sexual dimorphism and morphological correlates of mating individuals in the frog Rana dybowskii. Chin J Zool, 2013, 48(2): 188-192 (in Chinese). 刘鹏, 刘恒, 张德成, 等. 东北林蛙的两性异形和抱对个体的形态相关性. 动物学杂志, 2013, 48(2): 188-192. |
| [15] | Tao JC, Yu M, Xing BJ, et al. Comparison of different extraction methods for genomic DNA of Rana dybowskii. Heilongjiang Anim Sci Vet Med, 2018(17): 179-181 (in Chinese). 陶久臣, 于淼, 邢波建, 等. 东北林蛙基因组DNA不同提取方法的比较. 黑龙江畜牧兽医, 2018(17): 179-181. |
| [16] | Gong J, Sun QP, Xue F, et al. Molecular Characterization of the major histocompatibility complex class ia gene in the black-spotted frog, Pelophylax nigromaculata. Biochem Genet, 2013, 51(11/12): 876-888. |
| [17] | Zhao EM. China Red Data Book of Endangered Animals (Amphibians & Reptiles). Beijing: Science Press, 1998 (in Chinese). 赵尔宓. 中国濒危动物红皮书(两栖类和爬行类). 北京: 科学出版社, 1998. |
| [18] | Xu XX, Zhao WG, Liu P. Effect of environmental temperature on body temperature during reproductive period and embryonic development in different geographic populations of Rana dybowskii. Acta Ecol Sin, 2018, 38(8): 2965-2973 (in Chinese). 徐骁骁, 赵文阁, 刘鹏. 环境温度对东北林蛙不同地理种群繁殖期体温和胚胎发育的影响. 生态学报, 2018, 38(8): 2965-2973. |
| [19] | Shi XC, Chai LH, Niu SD, et al. Expression dynamics of MyD88 and TRAF6 genes in Rana dybowskii infected with Aeromonas hydrophila. Progress Vet Med, 2015, 36(6): 59-63 (in Chinese). 史雪灿, 柴龙会, 牛曙东, 等. 嗜水气单胞菌胁迫下东北林蛙皮肤MyD88和TRAF6基因表达的动态变化. 动物医学进展, 2015, 36(6): 59-63. |
| [20] | Du JN, Wu CW, Ye YX, et al. Advances in the introduction and pathogenic identification of common infectious diseases in frogs. Today Anim Husband Vet Med, 2019, 35(8): 63-64 (in Chinese). 杜嘉楠, 吴晨薇, 叶颖萱, 等. 蛙类常见传染病介绍及病原学鉴定研究进展. 今日畜牧兽医, 2019, 35(8): 63-64. |
| [21] | Qu LL, Chai LH, Wang BJ, et al. Expression dynamics of TBK1 transcripts in different tissues of Rana dybowskii under LPS and Aeromonas hydrophila stress. Chin J Wildlife, 2019, 40(1): 142-146 (in Chinese). 曲俐俐, 柴龙会, 王伯驹, 等. LPS和嗜水气单胞菌胁迫下东北林蛙不同组织内TBK1转录产物表达动态的研究. 野生动物学报, 2019, 40(1): 142-146. |
| [22] | Yang GZ, Ye H, Rong T. Determination of growth curve of Aeromonas hydrophila and drug sensitivity test. Chin J Anim Husband Vet Med, 2011(9): 27-30 (in Chinese). 杨国钊, 叶红, 容庭. 嗜水气单胞菌生长曲线的测定及药物敏感性试验. 畜牧兽医科技信息, 2011(9): 27-30. |
| [23] | Xu YG, Chai LH, Shi W, et al. Transcriptome profiling and digital gene expression analysis of the skin of Dybowski's frog (Rana dybowskii) exposed to Aeromonas hydrophila. Appl Microbiol Biotechnol, 2017, 101(14): 5799-5808. |
| [24] | Li ZB, Zhang N, Ma LZ, et al. Distribution of ancient α1 and α2 domain lineages between two classical MHC class Ⅰ genes and their alleles in grass carp. Immunogenetics, 2019, 71(5/6): 395-405. |
| [25] | Kiemnec-Tyburczy KM, Tracy KE, Lips KR, et al. Genetic variation and selection of MHC class Ⅰ loci differ in two congeneric frogs. Genetica, 2018, 146(2): 125-136. |
| [26] | Savage AE, Mulder KP, Torres T, et al. Lost but not forgotten: MHC genotypes predict overwinter survival despite depauperate MHC diversity in a declining frog. Conservat Genet, 2017, 19(2): 309-322. |
| [27] | Kosch TA, Eimes JA, Didinger C, et al. Characterization of MHC class ⅠA in the endangered southern corroboree frog. Immunogenetics, 2016, 69(3): 165-174. |
| [28] | Tao JC. Polmorphism and genetic evolution of MHC class genes in Rana Dybowskii[D]. Harbin: Harbin Normal University, 2019 (in Chinese). 陶久臣.东北林蛙MHC基因的多态性及其遗传进化[D].哈尔滨: 哈尔滨师范大学, 2019. http://cdmd.cnki.com.cn/Article/CDMD-10231-1019141800.htm |
| [29] | Piontkivska H, Nei M. Birth-and-death evolution in primate MHC Class Ⅰ genes: divergence time estimates. Mol Biol Evol, 2003, 20(4): 601-609. |
| [30] | Xu DD, Xu S, Wu FY, et al. Expression pattern analysis on MHC Iα and β2m genes in Epinephelus coioides infected by Cryptocaryon irritans. J Southern Agric, 2016, 47(12): 2145-2150 (in Chinese). 徐冬冬, 徐舜, 吴凤依, 等. 刺激隐核虫感染诱导斜带石斑鱼MHC Iα和β2m基因的表达谱分析. 南方农业学报, 2016, 47(12): 2145-2150. |
| [31] | Li W, Jiang A, Wu H. Molecular clone and polymorphism analysis on exon 3 of MHC Class Iα gene in swamp eel, Monopterus albus. J Hunan Agric Univ (Nat Sci), 2015, 41(4): 396-400 (in Chinese). 李伟, 江翱, 吴辉. 黄鳝主要组织相容性复合体Iα基因的克隆及多态性分析. 湖南农业大学学报:自然科学版, 2015, 41(4): 396-400. |
| [32] | Luo W, Zhang J, Wen JF, et al. Molecular cloning and expression analysis of major histocompatibility complex class Ⅰ, ⅡA and ⅡB genes of blunt snout bream (Megalobrama amblycephala). Dev Comparat Immunol, 2014, 42(2): 169-173. |
| [33] | Li JP, Gao FY, Lu MX, et al. cDNA isolation, polymorphism analysis, and tissue expression of MHC Iα of Nile tilapia (Oreochromis niloticus). J Fishery Sci China, 2014, 21(6): 1134-1145 (in Chinese). 黎建平, 高风英, 卢迈新, 等. 尼罗罗非鱼MHC Ⅰα全长cDNA的克隆、多态性及组织表达特征. 中国水产科学, 2014, 21(6): 1134-1145. |
| [34] | Wang B, Du HH, Huang HQ, et al. Major histocompatibility complex class Ⅰ (MHC Iα) of Japanese flounder (Paralichthys olivaceus) plays a critical role in defense against intracellular pathogen infection. Fish Shellfish Immunol, 2019, 94: 122-131. |
| [35] | Banerjee R, Roy S, Samanta M, et al. Molecular cloning, characterization and expression analysis of MHCⅠ and chemokines CXCR3 and CXCR4 gene from freshwater carp, Catla catla. Microbiol Immunol, 2019, 63(9): 379-391. |
