

1. 温州医科大学 检验医学院 生命科学学院,浙江 温州 325035;
2. 温州医科大学 浙江启新生物技术有限公司,浙江 温州 325035
收稿日期:2020-04-09;接收日期:2020-05-21
基金项目:国家卫生健康委员会科学研究基金-浙江省医药卫生重大科技计划(No. WKJ-ZJ-1928), 温州市重大科技专项(Nos. ZS2017014, ZS2018ZY001)资助
摘要:本研究将microRNA插入EF1α启动子的内含子中,构建携带沉默PD-1基因的miRNA的新型慢病毒载体,并将其应用于CAR-T细胞。通过流式细胞术检测慢病毒载体转导效率和PD-1沉默效率;Western blotting检测PD-1蛋白表达差异;荧光定量PCR检测microRNA相对表达情况;荧光素酶生物发光法和流式细胞术检测CAR-T细胞的能力。结果显示与U6转录microRNA的载体相比较,将microRNA插入到EF1-α内含子中的病毒载体转导效率更显著,对PD-1的敲低效率均达90%以上,且Western blotting结果验证了PD-1的敲低效果。另外通过荧光定量PCR,可显示出转导该新型慢病毒载体的Jurkat细胞内microRNA的相对表达量。荧光素酶生物发光法证实了CAR-T细胞针对靶细胞的特异杀伤性,流式细胞术结果表明沉默PD-1的CAR-T细胞相较于正常CAR-T细胞显示出更强的特异性杀伤能力。本研究成功构建了经microRNA敲低PD-1的新型慢病毒载体并验证了其转导效率的优越性,以及基于此载体表达的microRNA可高效地沉默PD-1;且应用此载体的CAR-T细胞能发挥更强的杀伤活性,从而为后续该CAR-T细胞治疗表达PD-L1的肿瘤奠定基础。
关键词:程序性死亡受体1microRNA新型慢病毒载体嵌合抗原受体T细胞
Construction of a novel lentiviral vector knocking down PD-1 via microRNA and its application in CAR-T cells
Hui Chen1, Xi Jin1, Xiaoman Zhang1, Jimin Gao1,2


1. School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou 325035, Zhejiang, China;
2. Zhejiang Qixin Biotech, Wenzhou Medical University, Wenzhou 325035, Zhejiang, China
Received: April 9, 2020; Accepted: May 21, 2020
Supported by: National Health Commission Science Foundation-Major Medical and Health Science and Technology Program of Zhejiang Province (No. WKJ-ZJ-1928), Wenzhou Municipal Research Program (Nos. ZS2017014, 2018ZY001)
Corresponding author: Jimin Gao. Tel: +86-577-86699341; E-mail: jimingao64@163.com.
Abstract: By inserting microRNAs into the intron of EF1α promoter, we constructed a novel lentiviral vector knocking down PD-1 gene via microRNA and applied it to CAR-T cells. Lentiviral transduction efficiency and PD-1-silencing efficiency were detected by flow cytometry. PD-1 expression was detected by Western blotting. Relative expression of microRNA was measured by Q-PCR. Cytotoxicity of CAR-T cells based on this vector was tested by luciferase bioluminescence and flow cytometry. Compared with lentiviral vector with microRNA transcribed by U6 promotor, the transduction efficiency of lentiviral vector with microRNA which was inserted into the intron of EF1α promoter was more significant, and the knockdown rate of PD-1 was more than 90%, which was validated by flow cytometry and Western blotting. And the relative expression level of microRNA in Jurkat cells transduced with this novel lentiviral vector was shown by Q-PCR. Compared with normal CAR-T cells, CAR-T cells based on this vector showed stronger cytotoxicity against PD-L1 positive Raji cells. We successfully constructed a novel lentiviral vector that knocked down PD-1 via microRNA and verified the superiority of its transduction efficiency and knockdown efficiency of PD-1. CAR-T cells based on this vector can exert a more powerful cytotoxicity, thus providing theoretical support for the subsequent treatment of PD-L1 positive tumors.
Keywords: programmed cell death protein 1microRNAnovel lentiviral vectorCAR-T cells
CAR (Chimeric antigen receptor,嵌合抗原受体) T细胞疗法是利用病人自身的免疫细胞来清除癌细胞,CAR是改造后的受体,赋予T细胞非HLA (Human leukocyte antigen,人白细胞抗原)依赖的方式识别肿瘤抗原的能力,这使得经过CAR改造的T细胞相较于天然T细胞受体(TCR)能够识别更广泛的目标[1]。CAR由结合靶抗原的免疫球蛋白单链可变片段(Single-chain variable fragment,scFv)、跨膜结构域和细胞内T细胞信号传导结构域组成。目前,CAR-T细胞疗法在癌症免疫治疗中显示出显著的功效,特别是在血液系统疾病的治疗中[2-3]。
程序性死亡受体-1 (PD-1)是一种免疫抑制性受体,属于CD28家族成员的Ⅰ型跨膜蛋白[4],在激活的T细胞、B细胞、单核细胞和树突状细胞表面广泛表达[5]。PD-1与其配体PD-L1 (细胞程序性死亡-配体1,Programmed cell death 1 ligand 1)结合可抑制T细胞的活化、增殖和细胞因子的分泌,诱导T细胞的凋亡,从而负调控免疫应答[6]。肿瘤细胞通过高表达PD-L1分子,使表达PD-1的肿瘤抗原特异性T细胞凋亡,导致肿瘤细胞逃避免疫系统的监视和杀伤[7]。
慢病毒载体已广泛用于RNA分子递送或蛋白表达[8-11]。目前研究发现,基于慢病毒载体的小分子RNA转移至宿主细胞会通过各种机制导致慢病毒滴度降低[12-13],可能是由于病毒颗粒生产过程中mRNA(信使RNA)被降解导致包装失败,或者是因病毒转导宿主细胞后的基因表达降低。因此,在许多研究中都对慢病毒载体进行了修饰和优化,以增强基因表达或小分子RNA的传递[14-19]。
1 材料与方法1.1 细胞株与培养基293T细胞株(人肾上皮细胞系)购自ATCC,用含有10%胎牛血清的DMEM培养基培养;过表达人PD-L1和荧光素酶的Raji细胞株由本实验室自行构建并保种,Jurkat细胞株(人急性T细胞白血病细胞株)购自ATCC,以上两种细胞株用含有10%胎牛血清的RPMI 1640培养基培养。T细胞取自健康人外周血,用含5%人AB血清、1 ng/mL IL-2、10 μg/mL IL-7和10 μg/mL IL-15的X-Vivo培养基培养。DMEM、RPMI 1640和胎牛血清购自Gibco公司;X-Vivo购自Lonza公司;人AB血清购自Sigma公司;重组人IL2、IL7和IL15购自PRPROTECH公司。
1.2 试剂与仪器plenti-EF1α-GFP (Green fluorescent protein)、plenti-EF1α-anti-CD19 CAR表达质粒由本实验室保存;限制性内切酶购自New England Bio Labs;无缝克隆试剂盒购自南京诺唯赞生物科技有限公司;质粒抽提试剂盒购自QIAGEN公司;聚乙酰亚胺(Polyetherimide,PEI)购自Polysciences公司;生物素标记的人CD19蛋白购自ACRO Biosystems公司;PE标记的链霉亲和素、PE标记的抗人CD3抗体、Brilliant Violet 421标记的抗人PD-L1抗体、PB标记的抗人CD62L抗体、Alexa Fluor? 488标记的抗人CD45RO抗体、APC标记的抗人CD45RA抗体和APC标记的抗人PD-1抗体均购自BioLegend公司。
PCR仪、电泳装置、凝胶成像系统购自Bio-Rad;FACS Aria Ⅱ流式细胞仪购自BD公司;DynaMagTM-5磁力架、CO2恒温细胞培养箱、酶标仪等购自Thermo Fisher公司;超速离心机购自Beckman Coulter公司;荧光显微镜、台式低速离心机、超净工作台等购自Eppendorf公司。
1.3 实验方法1.3.1 慢病毒表达载体构建三条miRNA分别命名为miRNA-30#backbone (miRNA-30原始序列)、miRNA-30#PD-1#1 (靶向PD-1 3′UTR)、miRNA-30#PD-1#61 (靶向PD-1蛋白编码区),均由苏州金唯智生物科技有限公司合成,3条miRNA均使用同一对引物进行扩增,F:5′-TGCGGGCCAAGATCTTCTTCAGGTTAACCCA AC-3′和R:5′-CCAGTGTGCAGATCTTCCTAAAG TAGCCCCTTG-3′,获得目的片段Q1、Q2、Q3,以Bgl Ⅱ为酶切位点,将Q1、Q2、Q3以无缝连接插入plenti-EF1α-GFP质粒EF1α启动子的内含子中;以Bgl Ⅱ为酶切位点,将Q2、Q3以无缝连接插入plenti-EF1α-anti-CD19 CAR质粒EF1α启动子的内含子中。
1.3.2 慢病毒包装及其转导效率测定将上述目的质粒分别与包装质粒pLP1、pLP2、pMD2G共转染293T细胞得到慢病毒颗粒,设置一定比例转导Jurkat细胞,并于48 h后通过流式细胞仪检测转导效率。
1.3.3 CAR-T细胞的制备及扩增采集健康人静脉血,经Ficoll分离液密度梯度离心提取外周血单个核细胞,利用抗CD3/CD28抗体包被的磁珠筛选T细胞,并经磁珠活化12–24 h。将上述慢病毒转导至活化的T细胞,待T细胞扩增至一定数量时取部分细胞,经生物素标记的CD19蛋白和PE-SA先后染色,通过流式细胞术检测CAR表达率。
1.3.4 蛋白质印迹分析细胞裂解液通过SDS-PAGE,然后转移至PVDF膜(Bio-Rad)。抗PD-1抗体(CST:86163S),抗GAPDH (Beyotime)抗体分别被用于蛋白印迹一抗和二抗。
1.3.5 实时定量PCR使用TRIzol试剂从Jurkat细胞中提取总RNA。以U6为内参基因,通过特异性引物将miRNA和U6逆转录为cDNA。逆转录引物分别是Q-PCR miRNA#1 REV、Q-PCR miRNA#61 REV,实时荧光定量PCR引物如表 1所示。相对定量法通过2–ΔΔCt法计算。
表 1 荧光定量PCR引物Table 1 Primers for quantitative PCR
| Primer | Sequence (5′–3′) |
| Q-PCR U6 FOR | CTCGCTTCGGCAGCACA |
| Q-PCR U6 REV | GGAACGCTTCACGAATTT |
| Q-PCR miRNA#1 FOR | ACACTCCAGCTGGGTAATATAATAGAACCA |
| Q-PCR miRNA#1 REV | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCCTGTG |
| Q-PCR miRNA#61 FOR | ACACTCCAGCTGGGTTTAGCACGAAGCTCT |
| Q-PCR miRNA#61 REV | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCATCGGAG |
表选项
1.3.6 流式细胞仪以下抗体用于流式细胞仪实验:生物素化人CD19 (ACRO Biosystems),APC偶联抗PD-1 (BioLegend),Brilliant Violet 421偶联抗PD-L1 (BioLegend),PE偶联抗人CD3 (BioLegend),APC-SA (BioLegend),PE-SA (BioLegend),APC偶联抗CD45RA (BioLegend),PB偶联抗CD62L (BioLegend),FITC偶联抗CD45RO (BioLegend)。使用BD AriⅡ流式细胞仪收集细胞数据,并使用FlowJo软件进行分析。
1.3.7 荧光素酶生物发光法检测CAR-T细胞的杀伤效能96孔板中每孔铺10 000个PD-L1-Luc-GFP Raji细胞,然后以不同的效︰靶比添加CAR-T细胞,使每孔的终体积为200 μL。另外设置两组PD-L1-Luc-GFP Raji细胞孔,一个用RMPI-1640培养基重悬,另一个用ddH2O重悬以裂解细胞,将其用作最大背景值(MAX)和最小背景值(MIN) (每组重复3孔)。加入荧光素酶底物后,用酶标仪检测自身荧光值V。靶细胞的裂解率可通过以下公式计算:裂解率(%)=(MAX-V)/ (MAX-MIN)×100%。
1.3.8 统计学分析所有实验均独立重复3次,实验数据以均数±标准差(x±s)表示,数据统计分析和图表制作均采用GraphPad prim 6.0,差异在P时被认为具有统计学意义(*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.000 1)。
2 结果与分析2.1 携带miRNA的新型慢病毒载体的构建及验证我们设计了慢病毒质粒plenti-EF1α (miRNA- 30-backbone)-GFP、plenti-EF1α (miRNA#1)-GFP、plenti-EF1α (miRNA#61)-GFP,并以由U6启动子转录miRNA的慢病毒质粒plenti-EF1α-GFP- U6-miRNA#61作为对照(图 1A)。我们基于miRNA-30骨架(miRNA-30-backbone)设计了两种miRNA (分别针对PD-1基因3′UTR的miRNA-30#PD-1#1和CDS区的miRNA-30#PD- 1#61),其中miRNA序列经BglⅡ酶切位点插入到启动子EF1α的内含子中(表 2)。将上述目的质粒经第3代慢病毒包装系统得到相应慢病毒颗粒并转导至Jurkat细胞,通过流式细胞术检测Jurkat细胞中的报告基因GFP表达情况,以比较在相同条件下不同慢病毒载体上目的基因的表达效率。如图 1B所示,经慢病毒LV-EF1α-GFP (阳性对照)、LV-EF1α (miRNA-backbone)-GFP、LV-EF1α (miRNA#1)和LV-EF1α (miRNA#61)-GFP转导的Jurkat细胞的GFP表达率显著高于经慢病毒LV-EF1α-GFP-U6-miRNA#61转导的Jurkat细胞。该结果表明,相对于传统携带miRNA的慢病毒载体,将miRNA插至EF1α内含子中的慢病毒载体的转基因表达效率更高。
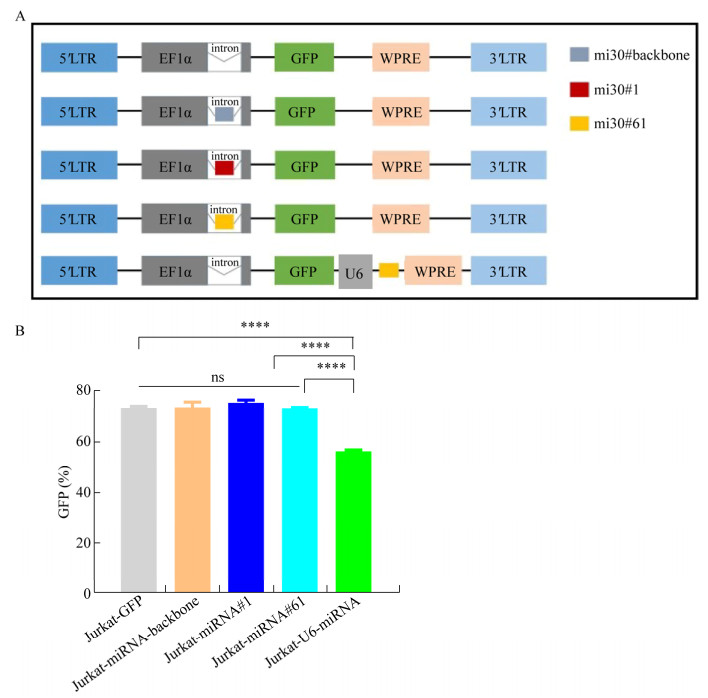 |
| 图 1 携带miRNA的新型慢病毒载体的构建及其目的基因表达效率的验证 Fig. 1 Construction of a novel lentiviral vector carrying miRNA and the verification of the target gene expression rate. (A) Schematic of the lentiviral vectors, miRNA-30#backbone, miRNA-30#1 and miRNA-30#61 were inserted into the intron respectively. GFP: reporter gene. U6: promotor for miRNA. EF1α: promotor for transgene. (B) Quantification of GFP expressing through different lentiviral vectors was detected by flow cytometry. Data shown were x±s from triplicates. Bars: s. ****P < 0.000 1 by One-Way ANOVA. |
| 图选项 |
表 2 miRNA序列Table 2 Sequences of miRNAs
| miRNA | Sequence (5′–3′) |
| miRNA-30-backbone | TCTTCAGGTTAACCCAACAGAAGGCTAAAGAAGGTATATTGCTGTTGACAGTGAGCGGGA |
| GACGTGATTACCGTCTCTTGCCTACTGCCTCGGACTTCAAGGGGCTACTTTAGGA | |
| miRNA-30#PD-1#1 | TCTTCAGGTTAACCCAACAGAAGGCTAAAGAAGGTATATTGCTGTTGACAGTGAGCGCCC |
| CTGTGGTTCTATTATATTATAGTGAAGCCACAGATGTATAATATAATAGAACCACAGGGA | |
| TGCCTACTGCCTCGGACTTCAAGGGGCTACTTTAGGA | |
| miRNA-30#PD-1#61 | TCTTCAGGTTAACCCAACAGAAGGCTAAAGAAGGTATATTGCTGTTGACAGTGAGCGAAT |
| CGGAGAGCTTCGTGCTAAATAGTGAAGCCACAGATGTATTTAGCACGAAGCTCTCCGATG | |
| TGCCTACTGCCTCGGACTTCAAGGGGCTACTTTAGGA |
表选项
2.2 miRNA介导的PD-1基因敲低效率我们通过植物血凝素(PHA)活化刺激Jurkat细胞,使其高表达PD-1,再通过转导上述不同慢病毒以评估不同新型慢病毒载体中miRNA介导的PD-1敲除效率。图 2A表明,成功转导病毒的GFP+细胞群中,LV-EF1α (miRNA#1)-GFP和LV-EF1α (miRNA#61)-GFP转导的Jurkat细胞表面几乎未检测到PD-1。如图 2B所示,与不靶向其他基因的miRNA骨架相比,miRNA#1和miRNA#61介导的PD-1沉默效率均超过了90%,具有显著的统计学差异。
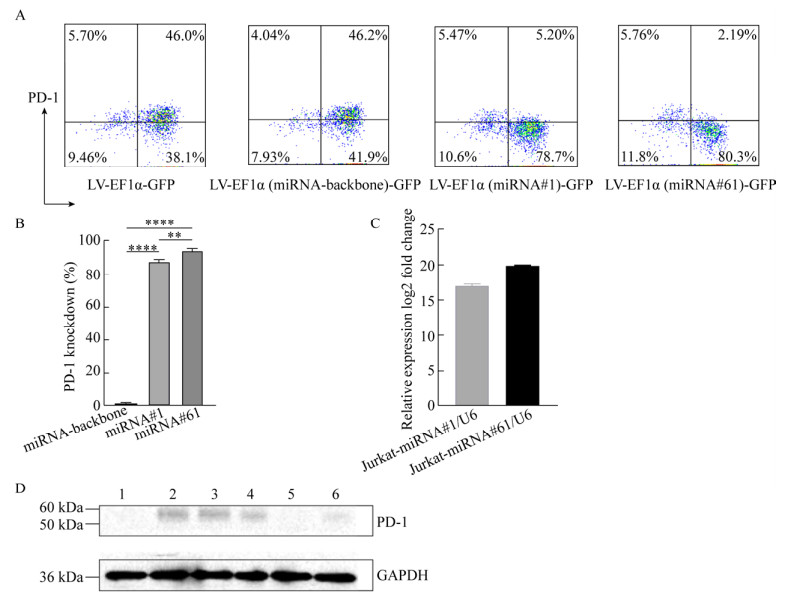 |
| 图 2 miRNAs介导的PD-1基因沉默的验证 Fig. 2 Efficiency of PD-1 knockdown mediated by miRNAs. (A) Expression of PD-1 on transduced Jurkat cells was detected by flow cytometry after stimulation with PHA for 48 h. Data shown were representative of three independent experiments. (B) Knockdown rates of PD-1 via miRNA#1 or miRNA#61. Data were x±s from triplicates. Bars, SD. (C) The relative expression levels of miRNA#1 or miRNA#61 in Jurkat cells transduced with LV-EF1α (miRNA#1)-GFP or LV-EF1α (miRNA#61)-GFP were evaluated by Q-PCR, respectively. Jurkat cells transduced with LV-EF1α-GFP as negative control. SnRNA U6 was used for internal control. Data were x±s from triplicates. Bars: s. (D). Expression of PD-1 in Jurkat cells transduced with different lentivirus particles was detected by Western blotting. 1: Jurkat cells (negative control); 2: Jurkat cells stimulated by PHA (positive control); 3: Jurkat cells transduced with LV-GFP (stimulated by PHA); 4: Jurkat cells transduced with LV-EF1α (miRNA-30-backbone)-GFP (stimulated by PHA); 5: Jurkat cells transduced with LV-EF1α (miRNA-30#PD-1#1)-GFP (stimulated by PHA); 6: Jurkat cells transduced with LV-EF1α (miRNA-30#PD-1#61)-GFP (stimulated by PHA). GAPDH: internal control. Data shown were representative of three independent experiments. |
| 图选项 |
接下来,我们通过荧光定量PCR检测了转导新型慢病毒载体后的Jurkat细胞中miRNA的相对表达量以验证miRNA是否由慢病毒载体递送。以LV-EF1α-GFP转导的Jurkat细胞作为对照,LV-EF1α (miRNA#1)-GFP和LV-EF1α (miRNA#61)-GFP转导的Jurkat细胞中,miRNA#1和miRNA#61分别显示出较高的表达水平(图 2C)。另外,通过Western blotting验证了LV-EF1α (miRNA#1)-GFP和LV-EF1α (miRNA#61)-GFP转导的Jurkat细胞中PD-1的表达明显减少(图 2D)。
2.3 经miRNA敲低PD-1的新型CAR慢病毒载体构建我们构建了第2代CAR慢病毒载体,其中包含识别CD19的嵌合抗原受体、CD8跨膜区、人CD28的细胞内信号传导域和CD3ζ T细胞信号传导域,其中miRNA#1和miRNA#61分别插入EF1α的内含子中(图 3A)。将基于此载体的慢病毒颗粒转导至人原代T细胞以构建CAR-T细胞。如图 3B所示,3种T细胞表面的CAR阳性率分别为88.6%、80.0%和73.8%,显示成功构建anti-CD19 CAR-T细胞。此外,我们将3种不同的CAR-T细胞分别与高表达CD19的Raji细胞以效靶比为1︰1、5︰1和10︰1共培养4 h,结果显示Raji细胞被3种CAR-T特异杀伤(图 3C)。
 |
| 图 3 经miRNA敲低PD-1的新型CAR慢病毒载体构建 Fig. 3 Construction of a novel CAR lentiviral vector knocking down PD-1 via miRNA. (A) Schematic of second-generation CAR vectors, anti-CD19 scFv; leader: signal peptide; CD8 TM: CD8 transmembrane region; CD28: intracellular signaling domain of human CD28; CD3ζ: intracellular signaling domain of CD3 zeta, miRNA30#1 and miRNA30#61 were inserted into the intron respectively. (B) CAR expression rates were detected by flow cytometry. (C) The specific cytotoxicity of CAR-T cells against tumor cells were assayed by luciferase bioluminescence. Data were x±s from triplicates. Bars: s. |
| 图选项 |
2.4 CAR-T细胞表面PD-1的敲除增强了其抗肿瘤效应使用CD3/CD28抗体包被的磁珠刺激CAR-T细胞48 h后,如图 4A所示,流式细胞术检测结果显示,在携带miRNA#1或miRNA#61的CAR-T细胞中PD-1表达率明显下降,表明miRNA成功地在CAR-T细胞中介导了PD-1的沉默。
 |
| 图 4 PD-1沉默的anti-CD19 CAR-T细胞显示出更强的抗肿瘤活性 Fig. 4 PD-1-silencing anti-CD19 CAR T cells enhanced anti-tumor efficiency. (A) PD-1 and CAR expression on the surface of T cells were detected by flow cytometry on day 2 after re-stimulation with CD3/CD28 antibody coated beads. (B) The cytotoxicity of CAR-T cells against PD-L1+ Raji cells was detected by flow cytometry after co-incubation for 72 h with re-stimulation by CD3/CD28 antibody coated beads at effector-to-target ratio of 1:1. Data shown above were representative of three independent experiments. Data below were x±s from triplicates. Bars: s. |
| 图选项 |
为检测上述经miRNA敲低PD-1的CAR-T细胞的杀伤活性差异,将CAR-T细胞与PD-L1+并转有荧光素酶基因和GFP报告基因的Raji细胞以效靶比为1︰1共培养72 h。如图 4B所示,与anti-CD19 CAR-T细胞杀伤效率相比,经miRNA敲低PD-1的两种CAR-T细胞均显示出杀伤的优越性,其靶细胞裂解率明显提高。
3 讨论在本研究中,我们以pri-miRNA-30骨架为基础设计了靶向PD-1的miRNA,从而介导CAR-T细胞PD-1蛋白的敲低。基于慢病毒载体,我们将miRNA插入人延伸因子1α (EF1α)启动子的内含子中[14, 20],miRNA会随着目的基因的转录而同时转录表达,而不是加入额外的U6等启动子单独转录miRNA[21]。Cooper等的研究表明慢病毒包装过程中EF1α启动子中的内含子不会被剪接丢失[14]。基于以上发现,将miRNA插入内含子的方式是可行的。将应用此方法的慢病毒载体转导宿主细胞,而目的基因由启动子EF1α驱动。后续的结果显示,与携带U6启动子转录的miRNA的慢病毒转导的宿主细胞(Jurkat)相比,将miRNA插入EF1α内含子的慢病毒载体传递的基因表达明显更高。这种现象可能是由于此载体可以避免目的基因的mRNA被RNase Ⅲ[22]识别和切割,因为EF1α启动转录后mRNA会进行剪接从而其内含子和插入其中的miRNA会在剪接过程中被去除。因此,目的基因的mRNA和miRNA将分别由一个启动子EF1α驱动,这为同时表达miRNA和CAR结构的CAR-T细胞疗法提供了更高效的方法。
本研究证实了miRNA介导的PD-1敲低效率保持在较高水平,且不亚于Cas9的基因敲除[23-24]。更重要的是,经miRNA敲低PD-1的CAR慢病毒载体可直接转导T细胞而用于CAR-T免疫疗法,而Cas9基因编辑方法相对繁琐[25],且其在加工过程中可能影响到T细胞的免疫活性或者产生脱靶效应,从而造成不良影响。另外,Rafiq等构建了一种分泌抗PD-1 scFv的CAR-T细胞,它可以通过旁分泌和自分泌两种方式阻断免疫检查点的抑制以增强抗肿瘤功效[26]。尽管miRNA沉默PD-1仅限于CAR-T细胞本身,但其PD-1的敲低效率超过90%,而抗PD-1 scFv阻断T细胞的效率在某种程度上较低,导致在很大程度上引起CAR-T细胞衰竭。
本研究结果显示,随着T细胞被CD3/CD28抗体活化从而其表面高表PD-1蛋白,与PD-L1阳性的靶细胞共孵育后,Raji细胞表面PD-L1与CAR-T细胞表面PD-1的结合所发挥的负调控作用,可抑制CAR-T细胞的活化、增殖,并诱导其凋亡,而anti-CD19 CAR-T (miRNA-30#1或miRNA-30#61)细胞中PD-1的高效敲低可避免该负调控所引起的抑制作用,从而介导了CAR-T细胞发挥更强的杀伤能力。另外,我们可以设计靶向其他免疫检查点的miRNA,将其插入该种新型慢病毒载体,如CTLA-4 (cytotoxic T-lymphocyte-associated protein 4)[27]、LAG-3 (Lymphocyte-activation gene 3)[28]、TIM-3 (T cell immunoglobulin and mucin domain-containing protein 3)[29]等,从而达到沉默CAR-T细胞表面多种免疫检查点以进一步增强其抗肿瘤活性,进而为CAR-T细胞疗法提供了一种可靠策略,使CAR-T细胞免受免疫检查点的抑制并改善其生物学效应。
参考文献
| [1] | Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol, 2016, 13(6): 370-383. DOI:10.1038/nrclinonc.2016.36 |
| [2] | Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med, 2013, 5(177): 177ra138. |
| [3] | Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med, 2014, 371(16): 1507-1517. |
| [4] | Han YY, Liu DD, Li LH. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res, 2020, 10(3): 727-742. |
| [5] | Payandeh Z, Khalili S, Somi MH, et al. PD-1/PD-L1-dependent immune response in colorectal cancer. J Cell Physiol, 2020, 235(7/8): 5461-5475. |
| [6] | Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol, 2004, 173(2): 945-954. |
| [7] | Dong HD, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med, 2002, 8(8): 793-800. |
| [8] | Merlin S, Follenzi A. Transcriptional targeting and MicroRNA regulation of lentiviral vectors. Mol Ther Methods Clin Dev, 2019, 12: 223-232. |
| [9] | ?kerblom M, Sachdeva R, Quintino L, 等. Visualization and genetic modification of resident brain microglia using lentiviral vectors regulated by microRNA-9. Nat Commun, 2013, 4: 1770. DOI:10.1038/ncomms2801 |
| [10] | Sachdeva R, J?nsson ME, Nelander J, et al. Tracking differentiating neural progenitors in pluripotent cultures using microRNA-regulated lentiviral vectors. Proc Natl Acad Sci USA, 2010, 107(25): 11602-11607. |
| [11] | Gentner B, Schira G, Giustacchini A, et al. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Methods, 2009, 6(1): 63-66. |
| [12] | Liu YP, Vink MA, Westerink JT, et al. Titers of lentiviral vectors encoding shRNAs and miRNAs are reduced by different mechanisms that require distinct repair strategies. RNA, 2010, 16(7): 1328-1339. |
| [13] | Poluri A, Sutton RE. Titers of HIV-based vectors encoding shRNAs are reduced by a dicer-dependent mechanism. Mol Ther, 2008, 16(2): 378-386. |
| [14] | Cooper AR, Lill GR, Gschweng EH, et al. Rescue of splicing-mediated intron loss maximizes expression in lentiviral vectors containing the human ubiquitin C promoter. Nucl Acids Res, 2015, 43(1): 682-690. |
| [15] | Fowler DK, Williams C, Gerritsen AT, et al. Improved knockdown from artificial microRNAs in an enhanced miR-155 backbone: a designer's guide to potent multi-target RNAi. Nucleic Acids Res, 2016, 44(5): e48. |
| [16] | Herrera-Carrillo E, Liu YP, Berkhout B. Improving miRNA delivery by optimizing miRNA expression cassettes in diverse virus vectors. Hum Gene Ther Methods, 2017, 28(4): 177-190. |
| [17] | Hu T, Fu Q, Chen P, et al. Construction of an artificial MicroRNA expression vector for simultaneous inhibition of multiple genes in mammalian cells. Int J Mol Sci, 2009, 10(5): 2158-2168. |
| [18] | Poling BC, Tsai K, Kang D, et al. A lentiviral vector bearing a reverse intron demonstrates superior expression of both proteins and microRNAs. RNA Biol, 2017, 14(11): 1570-1579. |
| [19] | Sun DQ, Melegari M, Sridhar S, et al. Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. Biotechniques, 2006, 41(1): 59-63. DOI:10.1016/j.vaccine.2004.04.033 |
| [20] | Amendola M, Passerini L, Pucci F, et al. Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol Ther, 2009, 17(6): 1039-1052. |
| [21] | Nie LH, Thakur MD, Wang YM, et al. Regulation of U6 promoter activity by transcriptional interference in viral vector-based RNAi. Genom Proteom Bioinformat, 2010, 8(3): 170-179. |
| [22] | Filippov V, Solovyev V, Filippova M, et al. A novel type of RNase Ⅲ family proteins in eukaryotes. Gene, 2000, 245(1): 213-221. DOI:10.1016/S0378-1119(99)00571-5 |
| [23] | Guo XL, Jiang H, Shi BZ, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol, 2018, 9: 1118. DOI:10.3389/fphar.2018.01118 |
| [24] | Hu WH, Zi ZG, Jin YL, et al. CRISPR/Cas9- mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother, 2019, 68(3): 365-377. |
| [25] | Barrangou R, Doudna JA. Applications of CRISPR technologies in research and beyond. Nat Biotechnol, 2016, 34(9): 933-941. DOI:10.1038/nbt.3659 |
| [26] | Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol, 2018, 36(9): 847-856. |
| [27] | Syn NL, Teng MWL, Mok TSK, et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol, 2017, 18(12): e731-e741. |
| [28] | Que Y, Fang ZX, Guan YX, et al. LAG-3 expression on tumor-infiltrating T cells in soft tissue sarcoma correlates with poor survival. Cancer Biol Med, 2019, 16(2): 331-340. |
| [29] | Tang RH, Rangachari M, Kuchroo VK. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin Immunol, 2019, 42: 101302. |
