马天天#, 杭奕#, 陶旗, 俞晓蓉, 祝艺然, 宋厚辉, 程昌勇


浙江农林大学动物科技学院, 动物医学院, 浙江 杭州 311300
收稿日期:2018-04-11;修回日期:2018-07-07;网络出版日期:2019-11-12
基金项目:国家自然科学基金(31872620,31770040);浙江省自然科学基金(LY17C180001,LZ19C180001,LQ19C180002);国家级大学生创新创业训练计划(201810341024);浙江省大学生科技创新活动计划基金(2018R412037)
*通信作者:程昌勇, E-mail:lamge@zafu.edu.cn.
#并列第一作者.
摘要:[目的] 本研究旨在构建单核细胞增多性李斯特菌(Listeria monocytogenes,简称单增李斯特菌)溶血素O(Listeriolysin O,LLO)的关键结构域PEST序列(包含S44、S48和T51关键磷酸化位点)突变体,并针对其生物学功能展开研究。[方法] 以李斯特菌参考菌株EGD-e为模板扩增编码LLO的hly基因,克隆至pET30a(+)原核表达载体,在此基础上利用氨基酸突变技术获得表达PEST突变体(LLOΔPEST、LLOS44A、LLOS48A和LLOT51A)的重组质粒,转入E.coli Rosetta感受态细胞中,诱导表达重组蛋白经镍离子亲和层析纯化后进行SDS-PAGE分析。利用红细胞裂解试验检测重组蛋白的溶血活性,并通过Western blotting检测重组突变蛋白刺激Caco-2细胞后对MAPK关键信号分子ERK1/2磷酸化水平变化的影响。[结果] 结果显示,本研究成功获得重组LLO及其突变体蛋白LLOΔPEST、LLOS44A、LLOS48A和LLOT51A。在pH5.5和7.4条件下,LLOΔPEST、LLOS44A、LLOS48A和LLOT51A均具有和LLO相当的溶血活性,说明PEST序列缺失或突变并不影响LLO的膜裂解活性。研究进一步发现,重组LLO及其突变蛋白刺激Caco-2细胞后均能激活ERK1/2的磷酸化。[结论] 研究表明LLO的关键结构域PEST序列对于维持该蛋白的膜裂解能力及穿孔活性并非必需,且该结构域的缺失不影响李斯特菌在感染宿主时依赖LLO介导ERK1/2磷酸化的生物学过程。本研究将为进一步探索细菌感染过程中PEST序列对于LLO发挥生物学功能的潜在作用及分子机制奠定基础。
关键词:单核细胞增多性李斯特菌溶血素OPEST序列膜裂解活性ERK1/2磷酸化
Roles of the PEST-like sequence of Listeriolysin O from Listeria monocytogenes in activating ERK1/2 phosphorylation
Tiantian Ma#, Yi Hang#, Qi Tao, Xiaorong Yu, Yiran Zhu, Houhui Song, Changyong Cheng


College of Animal Science and Technology, College of Veterinary Medicine of Zhejiang A & F University, Hangzhou 311300, Zhejiang Province, China
Received: 11 April 2018; Revised: 7 July 2018; Published online: 12 November 2019
*Corresponding author: Cheng Changyong, E-mail:lamge@zafu.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (31872620, 31770040), by the Natural Science Foundation of Zhejiang Province (LY17C180001, LZ19C180001, LQ19C180002), by the National College Students Innovation and Entrepreneurship Training Program (201810341024) and by the Zhejiang University Student Science and Technology Innovation Program Fund Project (2018R412037)
#These authors contributed equally to this work.
Abstract: [Objective] The determinant virulence factor Listeriolysin O (LLO) of Listeria monocytogenes, a foodborne pathogen, contains a unique N-terminal amino acid sequence that is absent in other cytolysins and was previously referred as the PEST-like sequence (containing three putative phosphorylation sites, S44, S48, and T51). We here, therefore, aimed to explore the biological roles of the PEST-like sequence in LLO-induced ERK1/2 kinases phosphorylation in human epithelial cells (Caco-2). [Methods] The plasmid for expressing the recombinant LLO was constructed and transformed into E. coli Rosetta, and the his-tagged soluble protein was purified using the nickel-chelated affinity column chromatography. The LLO variants (LLOΔPEST, LLOS44A, LLOS48A, and LLOT51A) were then obtained by using the site-directed mutagenesis strategy and expressed as above for LLO. The hemolytic activity for these recombinant proteins was assessed by lysis the erythrocytes, and moreover, effects of LLO or its variants on ERK1/2 kinases phosphorylation in Caco-2 cells was detected by using the Western blotting method. [Results] The results in the present study showed that the recombinant LLO, as well as the four LLO variants were able to lysis the erythrocytes at pH 5.5 and pH 7.4, suggesting that the PEST-like sequence was not required for the pore-forming activity of LLO. Besides, treatment of the LLO or its variants at the cytolytic concentration of 5 nmol/L could significantly induce ERK1/2 kinases phosphorylation in Caco-2 cells. [Conclusion] Our data collectively showed that the PEST-like sequence was not necessary for the LLO-mediated perforation ability on host membranes and not required for the LLO-triggered ERK1/2 signaling, which laid the foundation for further exploration of the potential roles of this motif during L. monocytogenes infection.
Keywords: Listeria monocytogenesListeriolysin Othe PEST-like sequencepore-forming activityERK1/2 kinases phosphorylation
病原体能够在受感染的宿主细胞内建立并维持生态壁龛以隐藏自己免受宿主免疫系统的攻击[1-2]。细胞内的生态壁龛能够为病原体提供营养和增殖环境[3-4]。革兰氏阳性病原菌单核细胞增生性李斯特菌(简称单增李斯特菌)利用宿主细胞质构建生态壁龛[5-7]。单增李斯特菌内化进入哺乳动物细胞后从吞噬体逃逸,进入宿主细胞质并开始复制增殖。在胞内,其利用表面蛋白ActA招募宿主肌动蛋白以提供自身动力,后扩散到邻近的新细胞继续新一轮增殖感染[2, 4-8]。细菌裂解吞噬泡并逃逸到宿主胞质的过程对于持续感染至关重要,而这一过程在很大程度上依赖于李斯特菌溶血素O (LLO)[4, 9-11]。
LLO作为成孔毒素,属于胆固醇依赖性胞质溶素(CDCs)家族,能够与宿主细胞含胆固醇的膜结合,形成直径达30 nm的孔,以协助李斯特菌进行逃逸[3, 12-15]。另外,LLO蛋白的3个酸性氨基酸残基E247、D208和D320构成的pH传感器使得其对于环境pH值变化相当敏感,LLO在pH值小于7时具备溶血活性,在pH为5.5时该活性最强[16-17]。LLO作为一种多功能毒力因子,能够激活宿主细胞内多种信号通路反应[18],其中包括细胞胞质蛋白ERK1/2的磷酸化[19-20],以及介导宿主细胞核内组氨酸的修饰[21]。但LLO对细胞也具有潜在的毒性,为了维持胞内生长环境,李斯特菌LLO的强大成孔活性需要受到一定限制以防止细胞质膜受损而影响细菌生存。Decatur等通过比对LLO与PFO (Perfringolysin O)氨基酸序列[22],发现LLO蛋白氨基末端特异性存在26个氨基酸,这26个氨基酸序列富含氨基酸脯氨酸(P)、谷氨酸(E)、丝氨酸(S)和苏氨酸(T),并因此命名为PEST序列。在真核细胞中PEST序列主要与蛋白酶体介导的蛋白降解有关[23],且已有研究表明该段序列调节LLO蛋白活性[24]。此外,LLO的PEST序列区域还存在多个潜在的磷酸化位点,包括S44、S48和T51。然而LLO是否通过PEST序列尤其是上述磷酸化位点直接或间接介导宿主细胞反应及其潜在的分子机制尚不得知。因此本研究将LLO蛋白PEST序列上3个潜在磷酸化位点定点突变为丙氨酸或对PEST序列进行缺失,分析LLO蛋白活性变化并研究该序列在李斯特菌感染触发的ERK1/2磷酸化过程中的生物学功能。本研究发现突变蛋白LLOΔPEST、LLOS44A、LLOS48A和LLOT51A均具有和LLO相当的溶血活性,说明PEST序列缺失或突变并不影响LLO的膜裂解活性。研究进一步发现,重组LLO及其突变蛋白刺激Caco-2细胞后均能激活ERK1/2的磷酸化。研究表明LLO的关键结构域PEST序列对于维持该蛋白的膜裂解能力及穿孔活性并非必需,且该结构域的缺失不影响李斯特菌在感染宿主时依赖LLO介导ERK1/2磷酸化的生物学过程,这对下一步深入研究LLO在李斯特菌从吞噬体逃逸机制中发挥的生物学功能及分子机理具有重要意义。
1 材料和方法 1.1 材料
1.1.1 菌株、质粒和培养条件: 试验用到的菌株为单增李斯特菌EGD-e和大肠杆菌DH5α Rosetta,来自本实验冻存库。EGD-e菌株需要接种于脑心浸出液肉汤(Brain Heart Infusion, BHI),DH5α菌株需要接种于肉汤(Luria-Bertani,LB),37 ℃振荡培养过夜。质粒pSL270和pET30a(+)均来自本实验室冻存库。
1.1.2 主要试剂: BHI培养基购自Oxoid公司;LB培养基购自上海生工生物工程有限公司;实验所用几种限制性内切酶购自NEB公司;高保真聚合酶2× phanta max master mix购自南京诺唯赞生物科技有限公司;T4 DNA ligase购自Thermo Fisher Scientific公司;PCR产物和胶回收纯化试剂盒购自上海莱枫生物科技有限公司;质粒提取试剂盒购自天根生化科技有限公司;BCA蛋白浓度测定试剂盒购自碧云天生物技术公司;ERK1/2、p-ERK1/2和β-actin单克隆抗体购自CST公司;HRP标记二抗购自Sigma-Aldrich公司。卡那霉素(50 mg/mL)储存于-20 ℃,在LB培养基中卡那霉素使用浓度为50 μg/mL。
1.1.3 引物: 对于重组质粒pSL1553的构建,所用的模板为EGD-e菌株全基因组,设计一对用于扩增缺失PEST序列基因hlyΔPEST的特异性引物。对于含hlyS44A、hlyS48A和hlyT51A突变质粒的构建,以实验室已有的hly重组表达质粒pSL270为模板,设计点突变引物将PEST序列区的3个关键氨基酸位点S44、S48和T51进行定点突变,分别得到突变载体。本研究中用到的引物均由苏州金唯智生物科技有限公司合成,引物序列详见表 1。
表 1. 本试验所用引物 Table 1. Primers used in this study
| Primers | Sequences (5′→3′) | Products |
| pSL1553-fwd (Nde Ⅰ)) | CGCCATATGGAAATCGATAAGTATATACAAGGATTGGATTAC | hlyΔPEST (1410 bp) |
| pSL1553-rev (Xho Ⅰ)) | CCGCTCGAGTTCGATTGGATTATCTACTTTATTACTATATTTCG | |
| EGD-e LLOS44A-fwd | GGCACCACCAGCAGCTCCGCCTGCAAG | hlyS44A (1515 bp) |
| EGD-e LLOS44A-rev | CTTGCAGGCGGAGCTGCTGGTGGTGCC | |
| EGD-e LLOS48A-fwd | CAGCATCTCCGCCTGCAGCTCCTAAGACGCC | hlyS48A (1515 bp) |
| EGD-e LLOS48A-rev | GGCGTCTTAGGAGCTGCAGGCGGAGATGCTG | |
| EGD-e LLOT51A-fwd | TCCGCCTGCAAGTCCTAAGGCGCCAATCGAAAA | hlyT51A (1515 bp) |
| EGD-e LLOT51A-rev | TTTTCGATTGGCGCCTTAGGACTTGCAGGCGGA | |
| The restriction enzyme sites are underlined. | ||
表选项
1.2 方法hlyΔPEST重组质粒构建和筛选 从NCBI上获取hly基因序列(登录号NC_003210.1)下载并导入到Vector NTI软件中,通过Vector NTI软件设计hlyΔPEST基因片段的引物pSL1553-fwd (Nde Ⅰ))和pSL1553-rev (Xho Ⅰ))。以EGD-e为模板,采用引物pSL1553-fwd (Nde Ⅰ))和pSL1553-rev (Xho Ⅰ))从单增李斯特菌EGD-e基因组中扩增得到hlyΔPEST基因。表达载体质粒pET30a(+)及PCR扩增的目的基因片段经Nde Ⅰ)和Xho Ⅰ)双酶切并纯化后,利用T4连接酶过夜连接后转化至大肠杆菌DH5α感受态细胞中,通过PCR筛选得到带有重组质粒(根据实验室规定命名为pSL1553)的重组菌,经测序验证正确后提取质粒用于下一步蛋白表达。
1.3 hlyS44A、hlyS48A和hlyT51A点突变质粒构建和筛选 以实验室已有表达hly重组质粒pSL270为模板,分别通过特定的引物EGD-e LLOS44A-fwd、EGD-e LLOS44A-rev、EGD-e LLOS48A-fwd、EGD-e LLOS48A-rev、EGD-e LLOT51A-fwd和EGD-e LLOT51A-rev对hly上3个位点S44、S48和T51分别进行单点突变。将得到的PCR产物用Dpn Ⅰ处理3 h后进行纯化并转化至大肠杆菌DH5α感受态细胞中,经测序验证正确后提取质粒用于下一步蛋白表达。
1.4 LLO重组蛋白及PEST序列突变蛋白原核表达与纯化 将测序结果正确的重组质粒及突变质粒转入大肠杆菌Rosetta感受态细胞中,于LB培养基中37 ℃培养至OD600为0.6,加入IPTG 16 ℃诱导12 h使其大量表达目的蛋白,离心收集菌体并重悬于50 mmol/L PBS缓冲液,对其进行超声破碎。破碎后溶液离心收集上清并与镍柱4 ℃结合6 h。使用30 mmol/L咪唑洗脱杂蛋白和300 mmol/L咪唑收集目的蛋白。BCA试剂盒测定纯化的蛋白浓度后,进行SDS-PAGE验证。用同样的方法表达纯化LLO重组蛋白。最后将纯化的蛋白用半透膜透析后适当浓缩,使用酶标仪检测其浓度,用0.22 μmol/L滤器过滤除菌并置于4 ℃待用。
1.5 LLO的PEST序列缺失及突变蛋白的红细胞裂解试验 分别制备pH 7.4和5.5的含5%的绵羊血红细胞和含2 ng/μL的蛋白溶液。将绵羊血1000 r/min、4 ℃离心10 min,弃上清和白细胞层。加入生理盐水轻轻重悬红细胞,1000 r/min、4 ℃离心10 min,弃上清并重复上述步骤2次。将红细胞和生理盐水以1:20混匀,配制成5%的绵羊血红细胞-生理盐水悬液。经上述步骤处理后所获悬液与蛋白等体积混匀,静置于37 ℃培养箱内反应30 min。将反应结束的混合液12000 r/min离心1 min。取上清200 μL加入96孔板中,测OD550。
1.6 Western blotting检测突变蛋白对细胞ERK1/2蛋白磷酸化影响 Caco-2细胞以1×106个/mL密度铺于12孔板并过夜培养,将透析并除菌保存的蛋白以5 nmol/L浓度与细胞混合,置于培养箱中培育30 min。去除细胞培养液,用PBS洗涤3次后裂解细胞并收集,12000 r/min、4 ℃离心10 min。去除沉淀,检测上清蛋白浓度并计算蛋白量,后与染色液混合并以5倍体系制备样品,沸水煮5 min后瞬离。将样品进行SDS-PAGE后利用半干转膜法转移至PVDF膜上,用5%脱脂奶粉将PVDF膜封闭1 h,TBST洗涤3遍后分别按比例加入ERK1/2、p-ERK1/2和β-actin单克隆抗体后4 ℃孵育过夜,洗涤3遍后加入HRP标记的二抗(羊抗兔或羊抗鼠)继续孵育1 h,最后利用成像系统拍摄并分析结果。
2 结果和分析 2.1 hlyΔPEST重组质粒的构建 以单增李斯特菌EGD-e基因组为模板,引物如表 1所示,通过PCR扩增得到hlyΔPEST基因片段。结果如图 1-A和B所示,PCR扩增的基因片段长度约为1400 bp,与已知的hlyΔPEST基因的长度(1410 bp)相吻合,PCR扩增产物和质粒pET30a(+)经酶切、酶连后,转入DH5α感受态细胞,涂布于含卡那霉素的LB平板上,挑取单克隆经PCR验证后提取质粒送公司测序,测序结果与已知hly序列比对,确认不含PEST序列,说明重组质粒构建成功,得到重组质粒pSL1553。
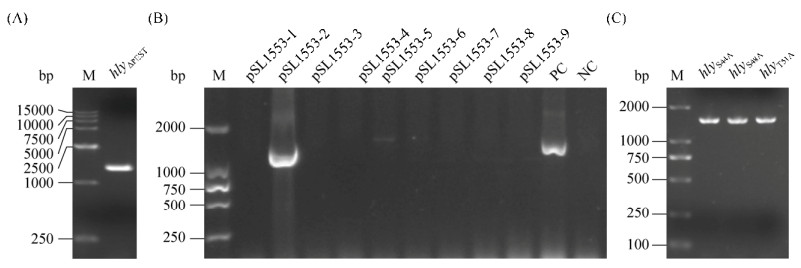 |
| 图 1 PEST序列重组质粒的构建 Figure 1 Construction of PEST sequence recombinant plasmids. A-B: hly PEST sequence deletion PCR amplification and verification; C: amino acid mutations PCR amplification; M: DNA marker. |
| 图选项 |
2.2 hlyS44A、hlyS48A和hlyT51A点突变质粒的构建 以pSL270为模板,引物如表 1所示,通过PCR扩增得到hlyS44A、hlyS48A和hlyT51A的点突变重组质粒, 结果如图 1-C所示,PCR扩增的基因片段约为1500 bp,与已知序列基因的长度(1515 bp)相吻合。将PCR产物用Dpn Ⅰ去甲基化后纯化,热激转化入DH5α感受态细胞,涂布于含卡那霉素的LB平板上,挑取单克隆培养12 h后提取质粒送公司测序,比对结果显示,成功得到3个突变质粒。
2.3 成功获得重组蛋白LLOΔPEST、LLOS44A、LLOS48A和LLOT51A 将重组质粒pSL1553及3个点突变质粒分别热激转化入大肠杆菌Rosetta感受态细胞中,使用IPTG诱导蛋白表达。收集菌体,超声破碎使表达的重组蛋白从菌体的胞质释放出来。获得含有重组蛋白的样品,经镍柱亲和层析后,纯化得到重组蛋白LLOΔPEST、LLOS44A、LLOS48A和LLOT51A。重组蛋白样品透析处理后,进行SDS-PAGE,结果如图 2-A所示,与已有重组蛋白LLO条带对比,均有明显的目的蛋白条带。
 |
| 图 2 重组蛋白LLOΔPEST、LLOS44A、LLOS48A、LLOT51A的SDS-PAGE分析及溶血活性分析 Figure 2 SDS-PAGE analysis of recombinant proteins LLO, LLOΔPEST, LLOS44A, LLOS48A, LLOT51A and hemolytic activity analysis. A: SDS-PAGE analysis of LLO and variants purification; M: Protein marker. B: Hemolysis of proteins at different pH environment. C: Hemolytic activity analysis. ns: no significant level; *: P < 0.05. |
| 图选项 |
2.4 PEST序列突变后蛋白溶血活性分析 pH 5.5和7.4环境下,以LLO重组蛋白为阳性对照,PBS组作为阴性对照。结果发现LLOΔPEST、LLOS44A、LLOS48A和LLOT51A均具有较明显的溶血现象。如图 2-B所示,pH 5.5环境下的红细胞细胞膜全部破碎,pH 7.4环境下的红细胞部分破碎,即pH 5.5时的溶血活性较pH 7.4时的更高。如图 2-C所示,在pH 5.5和7.4条件下,LLOΔPEST、LLOS44A、LLOS48A和LLOT51A均具有和LLO相当的溶血活性,说明PEST序列缺失或突变并不影响LLO的膜裂解活性。
2.5 LLO的PEST序列突变蛋白均能引起细胞ERK1/2磷酸化表达 已有研究表明LLO能够引起细胞中ERK1/2蛋白发生磷酸化,但对PEST序列的S44、S48和T51位点进行突变后是否影响LLO蛋白对宿主细胞的磷酸化尚未知,因此利用突变蛋白分别与Caco-2细胞进行孵育并利用Western blotting检测细胞ERK1/2磷酸化表达水平。结果发现,与空白组对比,LLO及其突变蛋白均能导致细胞ERK1/2蛋白磷酸化,表明LLO PEST序列及其3个潜在的磷酸化位点并不影响细胞ERK1/2蛋白的磷酸化,如图 3。
 |
| 图 3 重组LLO及其突变体蛋白激活细胞ERK1/2磷酸化水平分析 Figure 3 Analysis of cellular ERK1/2 phosphorylation level. A: Western blotting analysis of ERK1/2 and p-ERK1/2 protein expression in Caco-2 cells incubated with LLO and variants; B: The gray scale analysis results of proteins expression; ns: no significant level. |
| 图选项 |
3 讨论 单增李斯特菌通过类似吞噬作用的过程内化到哺乳动物细胞中后,最初存在于源自质膜的液泡中,随后通过成孔毒素LLO穿透液泡,在两种细菌磷脂酶(PlcA和PlcB)的共同参与下,介导单增李斯特菌逃逸到宿主细胞质,并大量复制[2, 25-26]。单增李斯特菌通过这种方式为自身提供有利的生长环境并保护自身免受宿主体液免疫应答清除[3]。在这一过程中,LLO作为胆固醇依赖性胞质溶素(CDC)家族的一员[9]发挥了关键作用。而在所有CDC中,包括来自产气荚膜梭菌的穿孔毒素O、炭疽杆菌中的炭疽素O、肺炎链球菌的肺炎球菌溶血素和来自化脓性链球菌的链球菌溶血素,只有LLO进化为仅在液泡中起作用,其他CDC均作用于质膜外部造成宿主细胞的裂解。因此,确保胞质中LLO不会穿透宿主质膜、维持细胞内生存环境对于李斯特菌发病机制至关重要。
首先,LLO具有的pH传感器保证其在液泡中的活性,并控制其对宿主细胞的裂解。另外,由于其他CDC中不存在PEST样序列,把LLO的PEST序列加入链球菌溶血素O(SLO)的氨基末端会降低SLO的细胞毒性[27-28],因此PEST序列对LLO活性的调控显得尤为重要。尽管PEST序列与蛋白酶体介导的降解有关,但已有研究表明LLO中的PEST样序列不影响蛋白质稳定性[22]。本研究通过体外表达PEST全部缺失以及PEST序列三个位点突变的重组蛋白,在不同pH下检测突变蛋白溶血活性,发现PEST序列在酸性条件下对LLO体外溶血能力略有影响。而PEST序列中的磷酸化残基特别是S44,作为MAPK共有位点的一部分,并没有影响LLO对于宿主肠上皮细胞ERK1/2蛋白磷酸化的激活。那么PEST对于LLO究竟有着怎样的生物学意义?最近Chen等的研究发现全长LLO一旦接触质膜后,随即迅速从膜上消失,而缺乏PEST样序列的LLO大部分保留在质膜上[29]。由于异四聚体衔接蛋白2(AP2)的组分Ap2a2唯一已知的作用是募集有助于胞吞作用的辅助蛋白[30],而LLO的PEST序列能够通过Ap2a2阻止质膜损伤,但LLO通过PEST序列调节细胞内吞机制的具体原因尚未被阐明。另外其这一研究中使用的是小鼠的cDNA文库,单增李斯特菌作为高致病性食源性病原菌[31],从人源基因进行进一步探索也十分必要。
LLO蛋白作为胆固醇依赖的穿孔素家族蛋白主要协助李斯特菌逃逸吞噬体,在感染过程中主要和细胞膜或吞噬体膜发生作用或联系[9],已有研究表明该蛋白穿透宿主细胞质膜并迅速诱导细胞内Ca2+内流、K+外流[32-33],从而激活包括ERK在内的多条信号传导途径。该蛋白单体也能在细胞胞质内短时间存在[34],这一过程中有可能直接与胞质蛋白发生互作。我们猜测LLO跟ERK及其通路上游关键激酶可能发生互作,目前该研究正在进一步开展中。
4 结论 本研究成功构建并表达了重组突变蛋白LLOΔPEST、LLOS44A、LLOS48A和LLOT51A,证实在PEST序列缺失或突变后,在pH 5.5和7.4条件下,LLO溶血活性不受影响。说明PEST序列缺失或突变并不影响LLO的膜裂解活性。且PEST序列上3个潜在的磷酸化位点并不影响LLO激活ERK1/2磷酸化,该结果为进一步探索李斯特菌感染过程中PEST序列对LLO发挥生物学功能的潜在分子机制奠定基础。
References
| [1] | Mitchell G, Chen C, Portnoy DA. Strategies used by bacteria to grow in macrophages. Microbiology Spectrum, 2016, 4(3). DOI:10.1128/microbiolspec.MCHD-0012-2015 |
| [2] | Radoshevich L, Cossart P. Listeria monocytogenes:towards a complete picture of its physiology and pathogenesis. Nature Reviews Microbiology, 2018, 16(1): 32-46. DOI:10.1038/nrmicro.2017.126 |
| [3] | Vdovikova S, Luhr M, Szalai P, Nyg?rd Skalman L, Francis MK, Lundmark R, Engedal N, Johansson J, Wai SN. A novel role of Listeria monocytogenes membrane vesicles in inhibition of autophagy and cell death. Frontiers in Cellular and Infection Microbiology, 2017, 7: 154. DOI:10.3389/fcimb.2017.00154 |
| [4] | Kortebi M, Milohanic E, Mitchell G, Péchoux C, Prevost MC, Cossart P, Bierne H. Listeria monocytogenes switches from dissemination to persistence by adopting a vacuolar lifestyle in epithelial cells. PLoS Pathogens, 2017, 13(11): e1006734. DOI:10.1371/journal.ppat.1006734 |
| [5] | Studer P, Staubli T, Wieser N, Wolf P, Schuppler M, Loessner MJ. Proliferation of Listeria monocytogenesL-form cells by formation of internal and external vesicles. Nature Communications, 2016, 7: 13631. DOI:10.1038/ncomms13631 |
| [6] | Osborne SE, Sit B, Shaker A, Currie E, Tan JMJ, van Rijn J, Higgins DE, Brumell JH. Type I interferon promotes cell-to-cell spread of Listeria monocytogenes. Cellular Microbiology, 2017, 19(3): e12660. DOI:10.1111/cmi.12660 |
| [7] | Chen GY, Mcdougal CE, D'Antonio MA, Portman JL, Sauer JD. A genetic screen reveals that synthesis of 1, 4-dihydroxy-2-naphthoate (DHNA), but not full-length menaquinone, is required for Listeria monocytogenes cytosolic survival. mBio, 2017, 8(2): e00119-17. DOI:10.1128/mBio.00119-17 |
| [8] | Quereda JJ, Dussurget O, Nahori MA, Ghozlane A, Volant S, Dillies MA, Regnault B, Kennedy S, Mondot S, Villoing B, Cossart P, Pizarro-Cerda J. Bacteriocin from epidemic Listeria strains alters the host intestinal microbiota to favor infection. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(20): 5706-5711. DOI:10.1073/pnas.1523899113 |
| [9] | Nguyen BN, Peterson BN, Portnoy DA. Listeriolysin O:a phagosome-specific cytolysin revisited. Cellular Microbiology, 2019, 21(3): e12988. DOI:10.1111/cmi.12988 |
| [10] | Cheng CY, Jiang L, Ma TT, Wang H, Han X, Sun J, Yang YC, Chen ZW, Yu HF, Hang Y, Liu FD, Wang BS, Fang WH, Huang HR, Fang C, Cai C, Freitag N, Song HH. Carboxyl-terminal residues N478 and V479 required for the cytolytic activity of listeriolysin O play a critical role in Listeria monocytogenes pathogenicity. Frontiers in Immunology, 2017, 8: 1439. DOI:10.3389/fimmu.2017.01439 |
| [11] | Vadia S, Arnett E, Haghighat AC, Wilson-Kubalek EM, Tweten RK, Seveau S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathogens, 2011, 7(11): e1002356. DOI:10.1371/journal.ppat.1002356 |
| [12] | Osborne SE, Brumell JH. Listeriolysin O:from bazooka to Swiss army knife. Philosophical Transactions of the Royal Society B:Biological Sciences, 2017, 372(1726): 20160222. DOI:10.1098/rstb.2016.0222 |
| [13] | Seveau S. Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes//Anderluh G, Gilbert R. MACPF/CDC Proteins-Agents of Defence, Attack and Invasion. Dordrecht: Springer, 2014: 161-195. |
| [14] | Cassidy SKB, O'Riordan MXD. More than a pore:the cellular response to cholesterol-dependent cytolysins. Toxins, 2013, 5(4): 618-636. DOI:10.3390/toxins5040618 |
| [15] | Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(9): 4341-4346. DOI:10.1073/pnas.0911581107 |
| [16] | Bavdek A, Kostanj?ek R, Antonini V, Lakey JH, Dalla Serra M, Gilbert RJC, Anderluh G. pH dependence of listeriolysin O aggregation and pore-forming ability. The FEBS Journal, 2012, 279(1): 126-141. DOI:10.1111/j.1742-4658.2011.08405.x |
| [17] | Schuerch DW, Wilson-Kubalek EM, Tweten RK. Molecular basis of listeriolysin O pH dependence. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(35): 12537-12542. DOI:10.1073/pnas.0500558102 |
| [18] | Lam JGT, Vadia S, Pathak-Sharma S, McLaughlin E, Zhang XL, Swanson J, Seveau S. Host cell perforation by listeriolysin O (LLO) activates a Ca2+-dependent cPKC/Rac1/Arp2/3 signaling pathway that promotes Listeria monocytogenes internalization independently of membrane resealing. Molecular Biology of the Cell, 2018, 29(3): 270-284. DOI:10.1091/mbc.E17-09-0561 |
| [19] | Weiglein I, Goebel W, Troppmair J, Rapp UR, Demuth A, Kuhn M. Listeria monocytogenes infection of HeLa cells results in listeriolysin O-mediated transient activation of the Raf-MEK-MAP kinase pathway. FEMS Microbiology Letters, 1997, 148(2): 189-195. DOI:10.1111/j.1574-6968.1997.tb10287.x |
| [20] | Hashino M, Tachibana M, Nishida T, Hara H, Tsuchiya K, Mitsuyama M, Watanabe K, Shimizu T, Watarai M. Inactivation of the MAPK signaling pathway by Listeria monocytogenes infection promotes trophoblast giant cell death. Frontiers in Microbiology, 2015, 6: 1145. |
| [21] | Hamon MA, Batsché E, Régnault B, Tham TN, Seveau S, Muchardt C, Cossart P. Histone modifications induced by a family of bacterial toxins. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(33): 13467-13472. DOI:10.1073/pnas.0702729104 |
| [22] | Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science, 2000, 290(5493): 992-995. DOI:10.1126/science.290.5493.992 |
| [23] | Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends in Biochemical Sciences, 1996, 21(7): 267-271. DOI:10.1016/S0968-0004(96)10031-1 |
| [24] | Lety MA, Frehel C, Dubail I, Beretti JL, Kayal S, Berche P, Charbit A. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence in Listeria monocytogenes. Molecular Microbiology, 2001, 39(5): 1124-1139. DOI:10.1111/j.1365-2958.2001.02281.x |
| [25] | Suryawanshi RD, Malik SVS, Jayarao B, Chaudhari SP, Savage E, Vergis J, Kurkure NV, Barbuddhe SB, Rawool DB. Comparative diagnostic efficacy of recombinant LLO and PI-PLC-based ELISAs for detection of listeriosis in animals. Journal of Microbiological Methods, 2017, 137: 40-45. DOI:10.1016/j.mimet.2017.04.005 |
| [26] | Pizarro-Cerdá J, Charbit A, Enninga J, Lafont F, Cossart P. Manipulation of host membranes by the bacterial pathogens Listeria, Francisella, Shigella and Yersinia. Seminars in Cell & Developmental Biology, 2016, 60: 155-167. |
| [27] | Schnupf P, Zhou JM, Varshavsky A, Portnoy DA. Listeriolysin O secreted by Listeria monocytogenes into the host cell cytosol is degraded by the N-end rule pathway. Infection and Immunity, 2007, 75(11): 5135-5147. DOI:10.1128/IAI.00164-07 |
| [28] | Lety MA, Frehel C, Berche P, Charbit A. Critical role of the N-terminal residues of listeriolysin O in phagosomal escape and virulence of Listeria monocytogenes. Molecular Microbiology, 2002, 46(2): 367-379. DOI:10.1046/j.1365-2958.2002.03176.x |
| [29] | Chen C, Nguyen BN, Mitchell G, Margolis SR, Ma D, Portnoy DA. The listeriolysin O PEST-like sequence co-opts AP-2-mediated endocytosis to prevent plasma membrane damage during Listeria infection. Cell Host & Microbe, 2018, 23(6): 786-795.e5. |
| [30] | Smith SM, Baker M, Halebian M, Smith CJ. Weak molecular interactions in clathrin-mediated endocytosis. Frontiers in Molecular Biosciences, 2017, 4: 72. DOI:10.3389/fmolb.2017.00072 |
| [31] | Witter AR, Okunnu BM, Berg RE. The essential role of neutrophils during infection with the intracellular bacterial pathogen Listeria monocytogenes. The Journal of Immunology, 2016, 197(5): 1557-1565. DOI:10.4049/jimmunol.1600599 |
| [32] | Chen RQ, Ji GQ, Wang L, Ren H, Xi LY. Activation of ERK1/2 and TNF-α production are regulated by calcium/calmodulin signaling pathway during Penicillium marneffei infection within human macrophages. Microbial Pathogenesis, 2016, 93: 95-99. DOI:10.1016/j.micpath.2016.01.026 |
| [33] | Vadia S, Seveau S. Fluxes of Ca2+ and K+ are required for the listeriolysin O-dependent internalization pathway of Listeria monocytogenes. Infection and Immunity, 2014, 82(3): 1084-1091. DOI:10.1128/IAI.01067-13 |
| [34] | Schnupf P, Portnoy DA, Decatur AL. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells:role of the PEST-like sequence. Cellular Microbiology, 2006, 8(2): 353-364. DOI:10.1111/j.1462-5822.2005.00631.x |
