程颍州, 慕春龙

 , 朱伟云
, 朱伟云 南京农业大学消化道微生物研究室, 国家动物消化道营养国际联合研究中心, 江苏省消化道营养与动物健康重点实验室, 江苏 南京 210095
收稿日期:2019-02-24;修回日期:2019-05-11;网络出版日期:2019-06-14
基金项目:国家自然科学基金(31430082)
作者简介:慕春龙,博士,2016年毕业于南京农业大学动物科技学院动物营养与饲料科学专业,现任南京农业大学讲师,从事动物营养和肠道微生物研究。主要研究领域为猪肠道微生物功能及宿主-微生物互作、微生物-肠-脑轴。已在The Journal of Nutrition、Applied Environmental and Microbiology、Journal of Proteome Research、Frontiers in Microbiology等期刊发表SCI论文12篇,参与英文专著1部。先后参与国家973项目、国家自然科学基金和江苏省自然科学基金项目的研究工作。荣获2017年江苏省优秀博士学位论文,并获得2016年度“帝斯曼缤纷科技奖-中国青年****动物营养科学奖”优胜奖.
*通信作者:慕春龙, E-mail: muchunlong@njau.edu.cn.
摘要:[目的] 从体外和体内研究Lactobacillus animalis LGM对Th细胞分化转录因子T-bet、GATA-3、ROR-γt和Foxp3的调节作用,以及探究L. animalis LGM对小鼠结肠炎的影响。[方法] 本试验采用改良型的Hungate滚管技术从猪结肠内容物中分离一株L. animalis LGM,根据其16S rRNA序列进行鉴定。收集L. animalis LGM培养液上清,与细菌脂多糖(LPS,2μg/mL)同时孵育Caco-2细胞24 h,体外研究L. animalis LGM对Caco-2细胞内Th细胞分化转录因子(T-bet,GATA3,ROR-γt和Foxp3)mRNA表达的影响;配制L. animalis LGM细菌悬液,研究L. animalis LGM灌胃对DSS诱导结肠炎小鼠症状及结肠Th细胞分化转录因子和细胞因子(IFN-γ,IL-4,IL-17和IL-10)mRNA表达的影响,表达结果采用荧光定量PCR法检测。[结果] 与对照组相比,L. animalis LGM培养液上清显著上调Caco-2细胞内ROR-γt与Foxp3 mRNA表达(P < 0.05),显著下调GATA3、IL-4、IL-17和TGF-β mRNA表达(P < 0.05)。L. animalis LGM灌胃显著上调小鼠结肠内ROR-γt和Foxp3的表达(P < 0.05),显著降低了促炎因子IL-4和IL-17的表达(P < 0.05),阻止了小鼠结肠长度缩短(P < 0.05)。[结论] 猪肠道分离L. animalis LGM表现出对Th细胞分化转录因子的选择性调节,显著上调Caco-2细胞及结肠炎小鼠ROR-γt与Foxp3 mRNA表达。降低DSS诱导结肠炎小鼠炎症水平,对DSS诱导结肠炎起保护作用,有助于维护肠道环境稳态。
关键词:Lactobacillus animalis肠道免疫猪DSS诱导结肠炎
Effects of Lactobacillus animalis LGM on transcription factors gene expressions in DSS-induced colitis mice
Yingzhou Cheng, Chunlong Mu

 , Weiyun Zhu
, Weiyun Zhu National Center for International Research on Animal Gut Nutrition, Laboratory of Gastrointestinal Microbiology, Jiangsu Key Laboratory of Gastrointestinal Nutrition and Animal Health, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China
Received: 24 February 2019; Revised: 11 May 2019; Published online: 14 June 2019
*Corresponding author: Chunlong Mu, E-mail: muchunlong@njau.edu.cn .
Foundation item: Supported by the Key Program of National Natural Science Foundation of China (31430082)
Abstract: [Objective] Investigating the relationship between Lactobacillus animalis LGM and transcription factors (T-bet, GATA3, ROR-γt and Foxp3) gene expressions in vivo or in vitro and observing the effects of L. animalis LGM gavage on DSS-induced colitis. [Methods] L. animalis LGM was isolated from pig colon contents using Hungate rolling tube technique. Bacterial supernatants were extracted and incubated Caco-2 cells with LPS (2 μg/mL) simultaneously. Bacterial suspension was adjusted to 1×109 CFU/0.2 mL and gavaged to DSS-induced mice. Gene expressions of transcription factors (T-bet, GATA-3, ROR-γt and Foxp3) and intracellular cytokines (IL-4, IL-17, INF-γ and IL-10) were analyzed by real-time PCR. [Results] In Caco-2 cells culture experiment, compared to control and LPS groups, L. animalis LGM supernatants treatment significantly downregulated the GATA-3, IL-4, IL-17 and TGF-β gene expressions and upregulated Foxp3 and ROR-γt gene expressions (P < 0.05). In DSS-induced colitis mice, compared to the DSS group, L. animalis LGM administration restored the colonic length and increased ROR-γt and Foxp3 gene expressions (P < 0.05). [Conclusion] L. animalis LGM isolated from pig colon contents in the present study have distinctive effects on transcription factors. ROR-γt and Foxp3 expressions were significantly upregulated both in Caco-2 cells and DSS-induced colitis mice. L. animalis LGM showed protection on DSS-induced colitis and gut inflammation.
Keywords: Lactobacillus animalisgut immunitypigsDSS-induced colitis
猪肠道内细菌资源十分丰富,已有的研究发现肠道细菌在宿主营养物质代谢及免疫屏障系统中发挥重要作用。肠道内乳酸菌因其数量种类丰富,维护肠道健康而备受关注,其代谢细菌素、乳酸能够维持肠道环境稳态,抑制病原菌生长与增殖,因此,乳酸菌成为动物生产中常考虑使用的益生菌[1-2]。其中,猪肠道分离的Lactobacillus animalis T12对金黄色葡萄球菌(Staphylococcus aureus)、大肠杆菌(Escherichia coli)和鼠伤寒沙门氏菌(Salmonella typhimurium)均有良好的抑制作用[3]。因此,L. animalis很早之前就被发现具有成为益生菌添加剂的可能,并在动物生产中有所应用[4]。然而,关于L. animalis参与肠道免疫的研究并不多。
肠道细菌与肠道免疫联系密切,能够诱导宿主肠道淋巴组织发育,调节肠道免疫应答,在维持肠道正常免疫功能中必不可少[5-6]。与此同时,宿主肠道免疫反应受自身辅助性T(Th)细胞调节,在Th细胞分化及发展过程中,T盒子转录因子(T-box expressed in T cells,T-bet)、GATA结合蛋白3(GATA binding protein 3,GATA3)、RAR相关孤儿受体(RAR-related orphan receptor gamma t,ROR-γt)和叉状头转录因子3 (Forkhead box P3,Foxp3)四种特异性转录因子发挥重要作用。T-bet与GATA3在Th1和Th2分化过程中不可或缺,影响体内Th1/Th2细胞平衡及其介导的免疫途径[7-8],ROR-γt促进Th17细胞分化,同时在抑制细菌介导肠道炎症方面起重要作用[9],Foxp3能够诱导调节性T (Treg)细胞分化,具有抗炎症免疫调节功能[10]。因此,T-bet、GATA3、ROR-γt和Foxp3或成为免疫调节策略中重要靶点。断奶仔猪饲喂Enterococcus faecium NCIMB 10415后,回肠淋巴组织中GATA3和Foxp3 mRNA表达显著降低[11]。因此,推测肠道细菌可能通过调节T-bet、GATA3、ROR-γt和Foxp3的表达影响肠道免疫反应。
本试验从猪结肠内容物中分离一株L. animalis LGM,对其基本性质进行初步探索,研究其对T-bet、GATA3、ROR-γt和Foxp3表达的影响。并通过探索L. animalis LGM培养液上清对Caco-2细胞转录因子mRNA表达的影响,以及L. animalis LGM灌胃对结肠炎小鼠症状及结肠内转录因子和细胞因子mRNA表达的调控,反映猪肠道分离L. animalis LGM对Th细胞分化转录因子表达的调节作用,揭示L. animalis LGM在肠道免疫中发挥的作用,为L. animalis LGM的肠道益生作用提供参考。
1 材料和方法 1.1 培养基 细菌培养基:改良M2GSC培养基(g/L):酪蛋白胨10,牛肉膏10,酵母浸出物2.5,葡萄糖5,可溶性淀粉1,纤维二糖1[12],CO2通气3–4 h,115 ℃高压灭菌20 min。
细胞培养基:DMEM培养基(吉诺生物医药技术有限公司),胎牛血清(FBS,Gen-view公司)。
1.2 试验动物与主要试剂 SPF级雌性C57BL/6J小鼠15只,体重18–22 g,购于南京江宁区青龙山动物繁殖场[SCXK(苏)2017-0001]。室温25±2 ℃,自由饮水采食。葡聚糖硫酸钠(DSS,上海翊圣生物科技有限公司);乳酸测定试剂盒、CCK-8细胞活力检测试剂盒(南京建成生物工程研究所);细菌基因组DNA快速抽提试剂盒、总RNA提取试剂盒(生工生物工程(上海)股份有限公司);普通PCR,反转录,荧光定量试剂(TaKaRa公司)。
1.3 细菌分离与纯化 购买新鲜杜×长×大三元杂交商品猪大肠。取新鲜商品猪结肠内容物10 g,溶于灭菌90 mL PBS溶液中,轻轻振荡混匀,经4层纱布过滤至血清瓶中作为接种液,做3个重复。抽取10 mL接种液,接种于150 mL密封血清瓶中(含90 mL M2GSC培养基),37 ℃厌氧培养48 h,重复接种、培养过程,依此传3代。取第三代培养液,利用密封Hungate滚管(含9 mL M2GSC培养基)梯度稀释至10–10,取10–8–10–10三个梯度,接种于未凝固的M2GSC固体培养基中,利用Hungate滚管技术均匀涂布于管壁,37 ℃厌氧培养24 h。在厌氧装置下,用接种环挑取单菌落接种于密封Hungate滚管中,37 ℃厌氧培养24 h。重复稀释、滚管、挑菌等纯化过程3次。
1.4 菌株鉴定 根据菌株16S rRNA序列鉴定其生物学分类。采用细菌基因组DNA快速抽提试剂盒法提取分离菌株基因组DNA,以多功能酶标仪(Tecan,Spark,Austria)检测提取基因组DNA浓度与质量。20 μL基础PCR体系:200 ng基因组DNA,10 μL Premix Taq酶,1 μL上、下游引物,ddH2O补足。16S rDNA扩增引物:8f (5′-CACGGATCCAGAG TTTGA(C/T)(A/C)TGGCTCAG-3′)和1510r (5′-GTG AAGCTTACGGCTACCTTGTTACGACTT-3′)[13]。PCR扩增程序:94 ℃ 5 min;94 ℃ 30 s,58 ℃ 30 s,68 ℃ 60 s,35个循环;68 ℃ 10 min;4 ℃终止反应。PCR产物交由上海英骏(Invitrogen)生物技术有限公司完成测序。根据其16S rRNA序列,在NCBI内进行BLAST (NCBI https://blast.ncbi.nlm.nih.gov/Blast.cgi)分析,获取同源性较高的相关序列,在MAGA 7.0内进行比对,用Maximum Likelihood法建立系统进化树。
1.5 L. animalis LGM生长曲线及培养液乳酸和丁酸含量测定 利用1 mol/L HCl溶液和1 mol/L NaOH溶液将Hungate滚管中细菌培养基pH分别调至3.5–8.5,按照1/10比例接种同一培养液,37 ℃培养,使用分光光度计分别在2、4、6、12、24 h时间点测定培养液OD600值,绘制分离菌株生长曲线。乳酸及丁酸是肠道细菌常见代谢产物,是肠道细菌维护肠道环境稳态、为宿主提供能量的重要体现,因此对L. animalis LGM乳酸及丁酸产量进行检测。选取细菌生长平台期,取细菌培养液,用乳酸测定试剂盒测定24 h内培养液乳酸积累量。丁酸测定:配制2.278 mmol/L丁酸溶液,依次稀释2、4、8、16倍,在岛津气相色谱仪中构建丁酸浓度标准曲线;取1 mL细菌培养液,加0.2 mL 25% (W/V)偏磷酸溶液,–20 ℃冷冻过夜,4 ℃、12000×g离心10 min,经0.22 μm过滤器至新离心管中,取500 μL上清,加等体积乙醚萃取后经气相色谱仪(Shimadzu,GC-14B,Japan)测定丁酸含量。
1.6 L. animalis LGM培养液上清对Caco-2细胞内转录因子基因表达的影响 待细菌培养至OD600为1时,取L. animalis LGM培养液,4 ℃、12000×g离心10 min,经0.22 μm过滤器过滤即得L. animalis LGM培养液上清。Caco-2细胞在含10% (V/V)胎牛血清(FBS)的DMEM完全培养基中,37 ℃、5% CO2条件下恒温培养,待细胞在细胞培养瓶中分布率达80%–90%时传代,细胞试验在20–50代内完成。试验分为3组,对照组、细菌脂多糖(Lipopolysaccharides,LPS)处理组、细菌脂多糖(LPS)+10% (V/V) L. animalis LGM培养液上清处理组,每组6个重复。24孔板每孔加1 mL完全培养基,接种1×105个细胞,37 ℃、5% CO2条件下培养至分布率达80%–90%。更换不含FBS的DMEM培养基,静默12 h。更换新鲜培养基,除对照组外,其余2组均由LPS (2 μg/mL)诱导细胞炎症,同时添加10% (V/V) L. animalis LGM培养液上清处理,37 ℃、5% CO2条件下培养24 h。用总RNA提取试剂盒提取处理细胞内RNA,以多功能酶标仪(Tecan,Spark,Austria)检测RNA浓度与质量,将RNA浓度调至500 ng/μL,利用反转录试剂将mRNA反转录为cDNA。Caco-2细胞内转录因子表达量采用荧光定量PCR技术检测分析,所用引物见表 1,细胞活性检测依据CCK-8细胞活力检测试剂盒。
表 1. 荧光定量所采用的引物 Table 1. Primers for Real-time PCR analysis
| Samples | Genes | Primer sequences (5′→3′) | Product size/bp | Annealing temperature/℃ | References |
| Caco-2 cell | T-bet | F: CCCCTTGGTGTGGACTGAGA | 87 | 61.13 | [8] |
| R: ACGCGCCTCCTCTTAGAGTC | 61.38 | ||||
| GATA3 | F: GTCCTCCCTGAGCCACATCT | 98 | 60.98 | ||
| R: GTGGTCCAAAGGACAGGCTG | 60.89 | ||||
| ROR-γt | F: GGCTCCCTGGATGAATAGAATG | 190 | 58.32 | [14] | |
| R: AGGCAGAGGCAGAAAATGTAAAG | 59.24 | ||||
| FOXP3 | F: TCCCAGAGTTCCTCCACAAC | 122 | 58.94 | [15] | |
| R: ATTGAGTGTCCGCTGCTTCT | 59.68 | ||||
| IL-4 | F: CACAAGTGCGATATCACCTT | 386 | 55.85 | [16] | |
| R: GCTCGAACACTTTGAATATT | 51.90 | ||||
| IL-17 | F: GGTTTGACTGAGTACCAATTTGC | 172 | 58.45 | [17] | |
| R: AAATTCCCAAGCCCAGAATC | 55.95 | ||||
| TGF-β | F: GGGACTATCCACCTGCAAGA | 239 | 58.80 | [18] | |
| R: CCTCCTTGGCGTAGTAGTCG | 59.62 | ||||
| β-actin | F: CACTGTGCCCATCTACGAGG | 154 | 60.18 | [19] | |
| R: AATGTCACGCACGATTTCC | 56.64 | ||||
| Mice | T-bet | GCCAGGGAACCGCTTATATG | 136 | 58.48 | [20] |
| GACGATCATCTGGGTCACATTGT | 60.68 | ||||
| GATA3 | CCTTAAAACTCTTGGCGTCC | 533 | 56.73 | [21] | |
| AGACACATGTCATCCCTGAG | 56.63 | ||||
| ROR-γt | TGTTTTATGGGGTTTGGGTATG | 122 | 56.56 | [22] | |
| CTGTGTGGATGTGTGTCTCTGATTA | 60.57 | ||||
| FOXP3 | CTCATGATAGTGCCTGTGTCCTCAA | 93 | 62.36 | [23] | |
| AGGGCCAGCATAGGTGCAAG | 62.56 | ||||
| IFN-γ | CACTGCATCTTGGCTTTGCA | 252 | 59.68 | [24] | |
| GCTGATGGCCTGATTGTCTTTC | 59.90 | ||||
| IL-4 | CACGGATGCGACAAAAATCAC | 251 | 58.76 | [25] | |
| CGAAAAGCCCGAAAGAGTCTCT | 60.61 | ||||
| IL-17 | TATCCCTCTGTGATCTGGGAAG | 161 | 58.42 | [26] | |
| ATCTTCTCGACCCTGAAAGTGA | 58.83 | ||||
| IL-10 | CTTACTGACTGGCATGAGGATCA | 101 | 59.87 | [27] | |
| GCAGCTCTAGGAGCATGTGG | 60.53 | ||||
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | 171 | 61.48 | [28] | |
| ATGGAGCCACCGATCCACA | 61.00 |
表选项
1.7 小鼠结肠炎诱导及L. animalis LGM灌胃对结肠内转录因子表达的影响 待L. animalis LGM培养至OD600为1时,取细菌悬液,梯度稀释至10–10,分别取10–8–10–10梯度滚管培养,数单菌落数计算细菌浓度。在4 ℃、10000×g条件下离心10 min,重悬于PBS溶液并将细菌浓度调至5×109 CFU/mL,用于灌胃试验。15只SPF级C57BL/6J雌性小鼠随机分为3组:对照组、DSS组和L. animalis LGM灌胃组,适应性饲养1周后,以DSS组与L. animalis LGM灌胃组小鼠构建急性结肠炎模型。将葡聚糖硫酸钠(DSS)溶于灭菌日常饮用水中,浓度为3% (W/V),小鼠自由饮水,隔1 d更换新鲜DSS饮用水,连续饮用7 d,每只小鼠饮水量大约5 mL/d。第8天更换正常饮水,开始灌胃阶段,对照组不进行处理;DSS组每天灌胃0.2 mL PBS溶液;L. animalis LGM灌胃组每天灌胃0.2 mL L. animalis LGM菌液(1×109 CFU),连续灌胃4 d。禁食1晚,第12天乙醚麻醉处死,分离小鼠结肠组织,测量长度后保存于–80 ℃冰箱。提取结肠组织RNA,采用荧光定量PCR技术检测分析结肠内转录因子和细胞因子的表达,所用引物见表 1。
1.8 统计学方法 试验数据使用SPSS 25.0 (SPSS Inc.,Chicago,USA)软件分析,利用单因素方差分析进行统计学分析,采用LSD-Turkey法进行多重比较。结果表示为mean±SEM,P < 0.05表示有显著性差异。文章图例均由Graphad Prism 7.0软件完成。
2 结果和分析 2.1 猪结肠Lactobacillus animalis LGM分离与鉴定 根据16S rRNA序列比对结果,确定分离菌株与Lactobacillus animalis KCTC 3501最为相似,相似性为99.79%,并将其命名为L. animalis LGM。此外,该菌株与Lactobacillus murinus LMG 14189及Lactobacillus apodemi DSM 16634也具有较高相似性。利用MAGA 7.0软件将分离菌株与相关菌株归纳在系统进化树中(图 1),更清晰地反映已分离L. animalis LGM的系统发生关系。
 |
| 图 1 本研究分离到的L. animalis LGM与相关菌株基于16S rRNA序列的Maximum Likelihood法系统进化树 Figure 1 A maximum-likelihood phylogenetic tree of L. animalis LGM and reference strains based on 16S rRNA gene sequences. Numbers in bracket refer to the number of bacterial 16S rRNA sequences in NCBI database. Bar 0.01 at the bottom is the sequence divergence. |
| 图选项 |
2.2 L. animalis LGM生长曲线及培养液乳酸、丁酸含量 不同pH条件下L. animalis LGM的生长曲线表明,L. animalis LGM在中性或略碱性环境下繁殖速度较快,但在酸性条件下受到抑制(图 2-A)。体外发酵pH为6.5,37 ℃恒温培养24 h内其乳酸累积浓度为46.16±1.19 mmol/L,但丁酸浓度较低,为1.28±0.16 mmol/L (图 2-B)。
 |
| 图 2 L. animalis LGM不同pH条件下生长曲线(A)及其乳酸、丁酸产量(B) Figure 2 L. animalis LGM growth curves in different pH conditions (A), and lactic acid and butyric acid production (B). Lactic acid and butyric acid accumulation was detected after 24 h cultivation. |
| 图选项 |
2.3 L. animalis LGM培养液上清对Caco-2细胞内转录因子mRNA表达的影响 LPS (2 μg/mL)处理导致Caco-2细胞活性显著降低(P < 0.05),显著上调IL-4和IL-17基因表达,同时显著下调细胞内TGF-β、GATA3和ROR-γt基因表达(P < 0.05)。与对照组及LPS组相比,L. animalis LGM培养液上清处理组显著下调细胞内GATA3和IL-4、IL-17、TGF-β基因表达(P < 0.05),同时显著上调Fxop3基因表达(P < 0.05)。与LPS组相比,L. animalis LGM培养液上清处理组显著上调ROR-γt基因表达(P < 0.05),恢复至对照组水平。从细胞活性方面看,L. animalis LGM培养液上清处理组细胞活性显著低于对照组与LPS组(P < 0.05,图 3)。
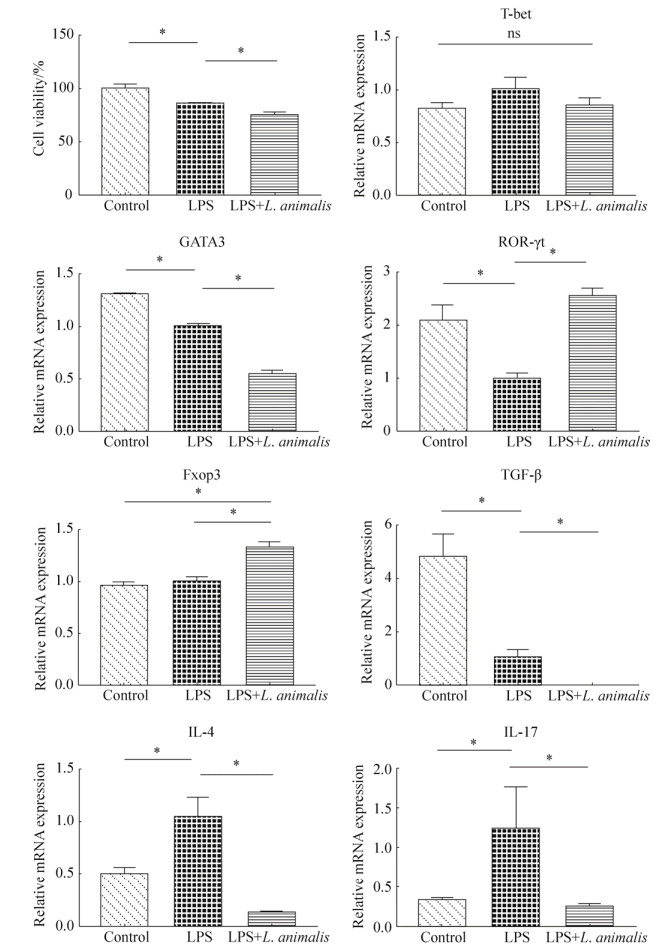 |
| 图 3 L. animalis LGM培养液上清对Caco-2细胞活性、转录因子和细胞因子基因表达的影响 Figure 3 The effects of L. animalis LGM supernatants (10%, V/V) on cell viability, transcription factors and intracellular cytokines gene expressions in Caco-2 cells. Control group: without LPS and bacterial supernatants; LPS group: treated with LPS (2 μg/mL); LPS+L. animalis group: treated with LPS (2 μg/mL) and L. animalis LGM supernatants simultaneously. * represent significant difference (P < 0.05). |
| 图选项 |
2.4 小鼠结肠炎诱导及L. animalis LGM灌胃对DSS结肠炎小鼠表型的影响 小鼠结肠炎诱导阶段,与对照组相比,DSS诱导小鼠体重逐日递减,到第6–7天时,DSS组和L. animalis LGM培养液上清处理组小鼠体重显著下降(P < 0.05,图 4-A),并伴有拉稀、易惊的症状。灌胃阶段,L. animalis LGM灌胃并没有改善小鼠体重的降低。整个试验过程,DSS组和L. animalis LGM灌胃组小鼠体增重无显著差异,灌胃结束后,与DSS组相比,L. animalis LGM灌胃小鼠体重增加6.41%。与对照组相比,DSS组小鼠结肠长度显著缩短(P < 0.05),L. animalis LGM灌胃组则显著高于DSS组(P < 0.05,图 4-B)。
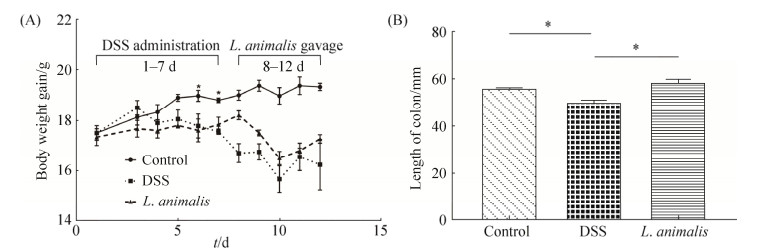 |
| 图 4 L. animalis LGM灌胃对DSS诱导结肠炎小鼠体重和结肠长度的影响 Figure 4 Effects of L. animalis LGM suspension oral administration on DSS-induced colitis mice body weight gain and colon length. Control group: without DSS and bacterial suspension administration; DSS group: treated with 3% (V/V) DSS solution; L. animalis group: treated with 3% (V/V) DSS solution and gavaged L. animalis LGM suspension (5×109 CFU/ mL) for 4 days. A: Body weight gain of mice through the whole experiment; B: Colon length of various groups. * represent significant difference (P < 0.05). |
| 图选项 |
2.5 L. animalis LGM灌胃对DSS结肠炎小鼠结肠内转录因子表达的影响 与对照组相比,DSS组小鼠结肠内T-bet、GATA3、ROR-γt和Foxp3 mRNA表达显著降低(P < 0.05);L. animalis LGM灌胃改变了结肠炎小鼠结肠内Th细胞分化转录因子的表达,与DSS组相比,L. animalis LGM灌胃显著上调了ROR-γt与Foxp3的表达(P < 0.05),其中Foxp3表达恢复至对照组水平(图 5-A)。与对照组相比,DSS组小鼠结肠内促炎因子IL-4、IL-17表达显著增加(P < 0.05);L. animalis LGM灌胃显著降低了结肠炎小鼠结肠内IL-4、IL-17的表达(P < 0.05),同时显著上调IFN-γ的表达(P < 0.05),DSS组与L. animalis LGM灌胃组小鼠结肠内IL-10表达均显著降低(P < 0.05,图 5-B)。
 |
| 图 5 L. animalis LGM灌胃对小鼠结肠内Th细胞分化转录因子和细胞因子基因表达的影响 Figure 5 Effects of L. animalis LGM suspension oral administration on transcription factors and intracellular cytokines gene expressions in mice colon. Control group: without DSS and bacterial suspension administration; DSS group: treated with 3% (V/V) DSS solution; L. animalis group: treated with 3% (V/V) DSS solution and gavaged L. animalis LGM suspension (5×109 CFU/mL) for 4 days. A: Transcription factors (T-bet, GATA3, ROR-γt and Foxp3) gene expressions; B: Intracellular cytokines (IFN-γ, IL-4, IL-17 and IL-10) gene expressions. * represent significant difference (P < 0.05). |
| 图选项 |
3 讨论 乳酸菌在猪生长发育过程中扮演重要角色,高通量测序发现乳酸菌是猪肠道中最丰富的几类细菌之一[29]。研究发现,饲喂Lactobacillus fermentum I5007、Lactobacillus reuteri BSA131、Lactobacillus plantarum ZJ316、Lactobacillus rhamnosus GG、Lactobacillus amylovorus和Enterococcus faecium等乳酸菌在猪生长发育过程中均表现出益生作用[30]。本试验从猪肠道内分离出一株L. animalis LGM,发现其对Foxp3和ROR-γt表达有调节作用,对结肠炎小鼠表现出益生效果。
L. animalis是一种常见乳酸菌,本研究中L. animalis LGM最适pH为6.5,与L. animalis TMW 1.971最适pH 6.0相似,与Lactobacillus curvatus TMW 1.624、Lactobacillus reuteri TMW 1.106最适pH 4.4具有明显区别[31]。有研究报道,L. animalis LA4与新鲜粪便悬液共同培养,显著增加粪便菌群中肠球菌(Enterococcus)和乳酸杆菌(Lactobacillus)数量[32]。乳酸是肠道细菌常见代谢产物,是肠道细菌维护肠道环境稳态的重要体现,本研究中检测到L. animalis LGM培养液中具有较高水平乳酸产量。然而,关于L. animalis的益生作用鲜有报道。
已有研究表明,乳酸菌能够通过影响细胞活性和细胞因子表达,调节炎症反应。在LPS诱导的细胞炎症模型中,Lactobacillus rhamnosus GG能够有效抑制LPS诱导的炎症反应,降低小鼠巨噬细胞促炎细胞因子TNF-α的表达[33]。本研究中,L. animalis LGM培养液上清能够显著降低LPS致炎Caco-2细胞内促炎因子IL-4和IL-17 mRNA表达,表现出抗炎作用。另外,乳酸菌可能通过抑制NF-κB信号下调细胞活性。研究发现,Lactobacillus rhamnosus GG、Lactobacillus acidophilus和Streptococcus salivarius JIM8772 Sn均对LPS或TNF-α诱导的NF-κB活化产生抑制作用[34-35]。与LPS组相比,L. animalis LGM培养液上清显著降低Caco-2细胞活性。这可能与乳酸菌抑制NF-κB信号相关,研究发现,NF-κB信号途径与肿瘤生长联系密切,抑制NF-κB活化将会抑制肿瘤细胞生长[36-38]。另外,细胞活性的降低也可能受L. animalis LGM产生的乳酸等代谢物及其营造的低pH环境影响[39]。因此,乳酸菌L. animalis LGM能够影响Caco-2细胞活性。
肠道菌群稳定和肠道结构完整是肠道健康的基础[40]。乳酸菌作为宿主共生菌,具有维护肠道健康的能力。研究发现,Lactobacillus reuteri R2LC灌胃使DSS诱导结肠炎小鼠结肠黏膜厚度恢复,同时增加了结肠末端紧密连接蛋白ZO-1的表达,缓解了肠道炎症[41]。本研究的小鼠灌胃试验中,L. animalis LGM灌胃组小鼠结肠长度显著高于DSS组,接近正常小鼠,说明L. animalis LGM灌胃对小鼠结肠有保护作用。
乳酸菌在结肠炎小鼠免疫调节中发挥重要作用,Lactobacillus curvatus WiKim38灌胃显著提高DSS诱导结肠炎小鼠树突细胞IL-10表达,改善小鼠结肠炎症水平[42]。Faecalibacterium prausnitzii分泌抗菌肽在结肠炎小鼠体内降低促炎因子IL-5和IL-17 mRNA表达,抑制了Th1、Th2和Th17介导的免疫反应[43]。本试验中,在LPS诱导的Caco-2细胞炎症和DSS诱导的小鼠结肠炎中,L. animalis LGM均能够显著上调Th细胞分化转录因子ROR-γt与Foxp3的表达。研究表明,ROR-γt与Foxp3在维护肠道稳态方面发挥重要作用。ROR-γt在肠道细菌免疫耐受机制中发挥重要作用,机体内ROR-γt+固有淋巴样细胞的存在能够缓解正常肠道菌群引起的炎症反应,但ROR-γt基因敲出后,小鼠脾脏表现增大,内环境IgG显著增加[9]。Foxp3+调节性T细胞能够产生抗炎症因子,调节宿主免疫反应水平,并可通过与B、T淋巴细胞弱化因子削弱淋巴细胞效应[10, 44]。除此之外,本试验中,L. animalis LGM培养液上清显著下调Caco-2细胞内GATA3表达,L. animalis LGM灌胃显著下调小鼠结肠内促炎因子IL-4与IL-17表达。IL4和IL17表达的下调可能参与缓解Th2细胞介导的免疫反应[45]。因此我们推测,L. animalis LGM具有调节肠道免疫、缓解肠道炎症、抑制T淋巴细胞效应等作用,在结肠炎小鼠肠道内发挥益生作用。
4 结论 本研究从猪结肠食糜中分离出一株乳杆菌L. animalis LGM,该菌株表现出对Th细胞分化转录因子的选择性调节,显著上调LPS诱导的Caco-2细胞及DSS诱导结肠炎小鼠结肠黏膜的ROR-γt与Foxp3 mRNA表达,降低DSS诱导结肠炎小鼠炎症水平,对DSS诱导结肠炎起保护作用,能够作为潜在的益生菌调节肠道健康。
References
| [1] | Arqués JL, Rodriguez E, Langa S, Landete JM, Medina M. Antimicrobial activity of lactic acid bacteria in dairy products and gut:effect on pathogens. BioMed Research International, 2015, 2015: 584183. |
| [2] | Wang SM, Zhang LW, Shan YJ. Lactobacilli and colon carcinoma-A review. Acta Microbiologica Sinica, 2015, 55(6): 667-674. (in Chinese) 王淑梅, 张兰威, 单毓娟. 乳酸菌与结肠癌. 微生物学报, 2015, 55(6): 667-674. |
| [3] | Li MX, Li N, Li Z, Xiang WL, Yang ZR, Luo F. Screening and identification of Lactobacillus animalis strain and its inhibitory protein characteristics. Microbiology China, 2009, 36(7): 1001-1007. (in Chinese) 李明雄, 李妮, 李征, 向文良, 杨志荣, 罗璠. 动物乳杆菌的分离鉴定及其抑菌蛋白的特性分析. 微生物学通报, 2009, 36(7): 1001-1007. |
| [4] | Ayala DI, Chen JC, Bugarel M, Loneragan GH, den Bakker HC, Kottapalli KR, Brashears MM, Nightingale KK. Molecular detection and quantification of viable probiotic strains in animal feedstuffs using the commercial direct fed microbial Lactobacillus animalis NP51 as a model. Journal of Microbiological Methods, 2018, 149: 36-43. DOI:10.1016/j.mimet.2018.04.012 |
| [5] | Zhang YM, Tian F, Guo XK. Intestinal lymphoid tissue and commensal bacteria work together to maintain intestinal homeostasis. Chinese Journal of Microecology, 2010, 22(9): 854-856. (in Chinese) 张羽萌, 田菲, 郭晓奎. 肠道淋巴组织与共生细菌对肠道环境稳态的共同维持. 中国微生态学杂志, 2010, 22(9): 854-856. |
| [6] | Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Current Opinion in Gastroenterology, 2015, 31(1): 69-75. DOI:10.1097/MOG.0000000000000139 |
| [7] | Szabo SJ, Kim ST, Costa GL, Zhang XK, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell, 2000, 100(6): 655-669. DOI:10.1016/S0092-8674(00)80702-3 |
| [8] | Bahria-Sediki IB, Yousfi N, Paul C, Chebil M, Cherif M, Zermani R, El Gaaied ABA, Bettaieb A. Clinical significance of T-bet, GATA-3, and Bcl-6 transcription factor expression in bladder carcinoma. Journal of Translational Medicine, 2016, 14(1): 144. DOI:10.1186/s12967-016-0891-z |
| [9] | Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, Wherry EJ, Koni PA, Bushman FD, Elson CO, Eberl G, Artis D, Sonnenberg GF. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature, 2013, 498(7452): 113-117. DOI:10.1038/nature12240 |
| [10] | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology, 2003, 4(4): 330-336. DOI:10.1038/ni904 |
| [11] | Kreuzer S, Rieger J, Strucken EM, Thaben N, Hünigen H, N ckler K, Janczyk P, Plendl J, Brockmann GA. Characterization of CD4+ subpopulations and CD25+ cells in ileal lymphatic tissue of weaned piglets infected with Salmonella typhimurium with or without Enterococus faecium feeding. Veterinary Immunology and Immunopathology, 2014, 158(3/4): 143-155. |
| [12] | Miyazaki K, Martin JC, Marinsek-Logar R, Flint HJ. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B14. Anaerobe, 1997, 3(6): 373-381. DOI:10.1006/anae.1997.0125 |
| [13] | Zhang DY, Ji HF, Wang SX, Liu H, Wang YM. Advances in of lactobacillus micro-ecology in pig intestine. Feeding and Husbandry, 2010(10): 42-44. (in Chinese) 张董燕, 季海峰, 王四新, 刘辉, 王雅民. 猪肠道乳酸菌的微生态学研究进展. 饲料与畜牧, 2010(10): 42-44. |
| [14] | Wang LL, Tang PH, Shi CG, Wan YH, Tang W, Hou XX, Pan NL, Shi YB, Tao QL. Expression of CD39 mRNA is altered in the peripheral blood of patients with allergic asthma. Biomedical Reports, 2014, 2(1): 75-78. DOI:10.3892/br.2013.196 |
| [15] | Liu YY, Xia TT, Jin CH, Gu DM, Yu J, Shi WQ, Zhang K, Zhang LP, Ye JX, Li L. FOXP3 and CEACAM6 expression and T cell infiltration in the occurrence and development of colon cancer. Oncology Letters, 2016, 11(6): 3693-3701. DOI:10.3892/ol.2016.4439 |
| [16] | Nilsen EM, Lundin KEA, Kraj?i P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon γ. Gut, 1995, 37(6): 766-776. DOI:10.1136/gut.37.6.766 |
| [17] | Mardegan GP, Shibli JA, Roth LA, Faveri M, Giro G, Bastos MF. Transforming growth factor-β, interleukin-17, and IL-23 gene expression profiles associated with human peri-implantitis. Clinical Oral Implants Research, 2017, 28(7): e10-e15. DOI:10.1111/clr.12846 |
| [18] | Shibata SI, Marushima H, Asakura T, Matsuura T, Eda H, Aoki K, Matsudaira H, Ueda K, Ohkawa K. Three-dimensional culture using a radial flow bioreactor induces matrix metalloprotease 7-mediated EMT-like process in tumor cells via TGFβ1/Smad pathway. International Journal of Oncology, 2009, 34(5): 1433-1448. |
| [19] | Glare EM, Divjak M, Bailey MJ, Walters EH. β-actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax, 2002, 57(9): 765-770. DOI:10.1136/thorax.57.9.765 |
| [20] | Liu NS, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nature Immunology, 2003, 4(7): 687-693. DOI:10.1038/ni941 |
| [21] | Zhang CL, Gui L, Xu YJ, Wu T, Liu D. Preventive effects of andrographolide on the development of diabetes in autoimmune diabetic NOD mice by inducing immune tolerance. International Immunopharmacology, 2013, 16(4): 451-456. DOI:10.1016/j.intimp.2013.05.002 |
| [22] | Zhu M, Xu Q, Li XL, He Q, Wang WF. Modulating effects of leflunomide on the balance of Th17/Treg cells in collageninduced arthritis DBA/1 mice. Central European Journal of Immunology, 2014, 39(2): 152-158. |
| [23] | Zhong YC, Wang X, Ji QW, Mao XB, Tang HX, Yi GW, Meng K, Yang XF, Zeng QT. CD4+LAP+ and CD4+CD25+Foxp3+ regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE-/- mice. Journal of Clinical Immunology, 2012, 32(5): 1104-1117. DOI:10.1007/s10875-012-9699-7 |
| [24] | Bay-Richter C, Janelidze S, Sauro A, Bucala R, Lipton J, Deierborg T, Brundin L. Behavioural and neurobiological consequences of macrophage migration inhibitory factor gene deletion in mice. Journal of Neuroinflammation, 2015, 12: 163. DOI:10.1186/s12974-015-0387-4 |
| [25] | Jin WY, Huang W, Chen LQ, Jin MJ, Wang QM, Gao ZG, Jin ZH. Topical application of JAK1/JAK2 inhibitor momelotinib exhibits significant anti-inflammatory responses in DNCB-induced atopic dermatitis model mice. International Journal of Molecular Sciences, 2018, 19(12): 3973. DOI:10.3390/ijms19123973 |
| [26] | Zhao SH, Yang YY, Liu W, Xuan ZQ, Wu SM, Yu SF, Mei K, Huang YJ, Zhang P, Cai JM, Ni J, Zhao YX. Protective effect of hydrogen-rich saline against radiation-induced immune dysfunction. Journal of Cellular and Molecular Medicine, 2014, 18(5): 938-946. DOI:10.1111/jcmm.12245 |
| [27] | Li HD, Zhang ZR, Zhang QX, Qin ZC, He DM, Chen JS. Treatment with exogenous hydrogen sulfide attenuates hyperoxia-induced acute lung injury in mice. European Journal of Applied Physiology, 2013, 113(6): 1555-1563. DOI:10.1007/s00421-012-2584-5 |
| [28] | Zhao JY, Zhuang FF, Wang HY, Wu D, Zhang JS. Msx2 plays a critical role in lens epithelium cell cycle control. International Journal of Ophthalmology, 2013, 6(3): 276-279. |
| [29] | Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, Isaacson RE. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Veterinary Microbiology, 2011, 153(1/2): 124-133. |
| [30] | Yang FJ, Hou CL, Zeng XF, Qiao SY. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens, 2015, 4(1): 34-45. DOI:10.3390/pathogens4010034 |
| [31] | Rühmkorf C, Bork C, Mischnick P, Rübsam H, Becker T, Vogel RF. Identification of Lactobacillus curvatus TMW 1.624 dextransucrase and comparative characterization with Lactobacillus reuteri TMW 1.106 and Lactobacillus animalis TMW 1.971 dextransucrases. Food Microbiology, 2013, 34(1): 52-61. |
| [32] | Biagi G, Cipollini I, Pompei A, Zaghini G, Matteuzzi D. Effect of a Lactobacillus animalis strain on composition and metabolism of the intestinal microflora in adult dogs. Veterinary Microbiology, 2007, 124(1/2): 160-165. |
| [33] | Pe a JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cellular Microbiology, 2003, 5(4): 277-285. DOI:10.1046/j.1462-5822.2003.t01-1-00275.x |
| [34] | Lee SK, Yang KM, Cheon JH, Kim TI, Kim WH. Anti-inflammatory mechanism of Lactobacillus rhamnosus GG in lipopolysaccharide-stimulated HT-29 Cell. The Korean Journal of Gastroenterology, 2012, 60(2): 86-93. DOI:10.4166/kjg.2012.60.2.86 |
| [35] | Kaci G, Lakhdari O, Doré J, Ehrlich SD, Renault P, Blottière HM, Delorme C. Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Applied and Environmental Microbiology, 2011, 77(13): 4681-4684. DOI:10.1128/AEM.03021-10 |
| [36] | Lee SI, Kim HS, Koo JM, Kim IH. Lactobacillus acidophilus modulates inflammatory activity by regulating the TLR4 and NF-κB expression in porcine peripheral blood mononuclear cells after lipopolysaccharide challenge. British Journal of Nutrition, 2016, 115(4): 567-575. DOI:10.1017/S0007114515004857 |
| [37] | Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells, 2016, 5(2): 15. DOI:10.3390/cells5020015 |
| [38] | Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Archiv, 2005, 446(5): 475-482. DOI:10.1007/s00428-005-1264-9 |
| [39] | van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. Stress responses in lactic acid bacteria. Antonie van Leeuwenhoek, 2002, 82(1/4): 187-216. DOI:10.1023/A:1020631532202 |
| [40] | Shen YJ, Bian ZL, Shao JG. Research progress of on the mechanism of impaired intestinal mucosal barrier function and repair therapy in inflammatory bowel disease. Shandong Medical Journal, 2019, 59(2): 90-93. (in Chinese) 沈羽嘉, 卞兆连, 邵建国. 炎症性肠病肠黏膜屏障功能受损机制与修复治疗的研究进展. 山东医药, 2019, 59(2): 90-93. DOI:10.3969/j.issn.1002-266X.2019.02.027 |
| [41] | Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiologica, 2016, 217(4): 300-310. DOI:10.1111/apha.12695 |
| [42] | Jo SG, Noh EJ, Lee JY, Kim G, Choi JH, Lee ME, Song JH, Chang JY, Park JH. Lactobacillus curvatus WiKim38 isolated from kimchi induces IL-10 production in dendritic cells and alleviates DSS-induced colitis in mice. Journal of Microbiology, 2016, 54(7): 503-509. DOI:10.1007/s12275-016-6160-2 |
| [43] | Breyner NM, Michon C, de Sousa CS, Vilas Boas PB, Chain F, Azevedo VA, Langella P, Chatel JM. Microbial anti-Inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Frontiers in Microbiology, 2017, 8: 114. |
| [44] | Zhang HX, Zhu B, Fu XX, Zeng JC, Zhang JA, Wang WD, Kong B, Xiang WY, Zhong JX, Wang CY, Zheng XB, Xu JF. BTLA associates with increased Foxp3 expression in CD4+ T cells in dextran sulfate sodium-induced colitis. International Journal of Clinical & Experimental Pathology, 2015, 8(2): 1259-1269. |
| [45] | Lan F, Zhang N, Gevaert E, Zhang L, Bachert C. Viruses and bacteria in Th2-biased allergic airway disease. Allergy, 2016, 71(10): 1381-1392. DOI:10.1111/all.12934 |
