吴丽华1

 , 陈燕飞1, 仪慧兰2
, 陈燕飞1, 仪慧兰2 1. 太原师范学院生物系, 山西 晋中 030619;
2. 山西大学生命科学学院, 山西 太原 030006
收稿日期:2018-07-25;修回日期:2018-12-18;网络出版日期:2019-03-12
基金项目:国家自然科学基金(21307087);山西省应用基础研究计划青年科技研究基金(201601D021104)
*通信作者:吴丽华, E-mail:wlh1981622_510@163.com.
摘要:[目的] 本研究探讨了HOG1MAPK在亚砷酸钠诱导酵母细胞凋亡中的作用。[方法] 以酵母野生株BY4741及其HOG1突变株(ΔHOG1)为材料,研究了亚砷酸钠对酵母细胞生长、相对存活率和氧化损伤的影响,并采用流式细胞术检测了亚砷酸钠胁迫下酵母细胞凋亡率、ROS水平和线粒体膜电位的变化。[结果] 亚砷酸钠可抑制酵母细胞生长,诱导细胞凋亡。在相同处理组中,ΔHOG1对亚砷酸钠更为敏感,表现为细胞存活率降低,凋亡率升高。在亚砷酸钠胁迫过程中,ΔHOG1胞内ROS水平和MDA含量显著高于野生株BY4741,而线粒体膜电位显著低于野生株。[结论] HOG1 MAPK可能通过影响胞内ROS水平和线粒体膜电位的变化调控亚砷酸钠诱导的酵母细胞凋亡。
关键词:亚砷酸钠酵母凋亡活性氧线粒体膜电位HOG1
Involvement of HOG1 MAPK in the regulation of sodium arsenite-induced apoptosis in yeast cells
Lihua Wu1

 , Yanfei Chen1, Huilan Yi2
, Yanfei Chen1, Huilan Yi2 1. Department of Biology, Taiyuan Normal University, Jinzhong 030619, Shanxi Province, China;
2. School of Life Sciences, Shanxi University, Taiyuan 030006, Shanxi Province, China
*Corresponding author: Lihua Wu, E-mail: wlh1981622_510@163.com.
Foundation item: Supported by the National Natural Science Foundation of China (21307087) and by the Applied Basic Research Programs of Shanxi Province (201601D021104)
Abstract: [Objective] In this study, we studied the role of HOG1 MAPK in sodium arsenite-induced apoptosis in yeast cells. [Methods] Yeast wild-type (BY4741) and HOG1 mutant (ΔHOG1) strains were used to study the effects of sodium arsenite on the growth and relative survival rate and oxidative damages of yeast cells. Further, the apoptotic rate, intracellular reactive oxygen species (ROS) level, and mitochondrial membrane potential of the yeast cells under sodium arsenite-induced stress were determined by flow cytometry. [Results] Sodium arsenite inhibited the growth of yeast cells and induced their apoptosis. Compared to the wild-type strain in the same treatment group, ΔHOG1 strain showed higher sensitivity to sodium arsenite with a lower cell survival rate and higher apoptotic rate. Under sodium arsenite-induced stress, ΔHOG1 strain showed significantly higher intracellular ROS and malondialdehyde (MDA) levels than the wild-type BY4741 strain. On the contrary, the mitochondrial membrane potential of ΔHOG1 strain was significantly lower than that of the wild-type BY4741 strain. [Conclusion] These results indicated that HOG1 MAPK gene was involved in the regulation of sodium arsenite-induced apoptosis by affecting intracellular ROS level and changing △ψM in yeast cells.
Keywords: sodium arseniteyeastapoptosisreactive oxygen speciesmitochondrial membrane potentialmitogen-activated protein kinase HOG1 (HOG1)
砷是广泛分布于自然界中的一种毒性较强的、具有致癌作用的重金属[1],它可以通过多种途径,如饮用或食用砷污染的水和食物、或者由于工业活动经皮肤或消化道进入人体[2-4]。流行病学研究表明,长期暴露于砷污染的环境中可以引起人体内活性氧(reactive oxygen species,ROS)水平显著升高,并增加心血管疾病、糖尿病、皮肤病等多种疾病及癌症的发病率[5-6]。
丝分裂原活化蛋白激酶(MAPKs)级联反应是真核生物控制细胞增殖、分化和死亡的重要信号通路之一,而p38 MAPK可被胞内较高水平的ROS激活,并导致细胞凋亡[7-8]。在模式生物酵母中,迄今为止已有5条MAPK通路被发现[9]。其中,HOG1 MAPK与哺乳动物中的p38 MAPK高度同源,对调控细胞的存活至关重要[10]。大量研究结果显示,多种外界刺激如高温[11]、低温[12]和柠檬酸[13]均可激活高渗透压(high-osmolarity and glycerol,HOG)信号通路。Thorsen等[14]研究发现,砷可激活酵母细胞中HOG信号通路,而HOG1 MAPK缺失后可显著增加酵母细胞对砷化物的敏感性。本课题组研究发现,亚砷酸钠处理诱发酵母细胞ROS增高的同时,可诱导细胞凋亡[15],使1991个基因差异表达,差异表达基因的功能涉及代谢过程、结合、转运等,其中HOG1表达显著上调。但是,HOG1 MAPK是否参与砷诱导的酵母细胞凋亡未见报道。因此,本实验选用酵母野生株BY4741及其突变体ΔHOG1为研究材料,研究HOG1在亚砷酸钠诱导酵母细胞凋亡中的作用,以期为砷的毒性机理提供实验依据。
1 材料和方法 1.1 实验材料 酵母菌(Saccharomyces cerevisiae)野生株BY4741 (MATa,his3Δ1,leu2Δ0,met15Δ0,ura3Δ0)及其突变体ΔHOG1 (hog1::KanMX4)由天津大学张丽琳老师惠赠[16]。酵母细胞接种于酵母浸出粉胨葡萄糖(yeast extract peptone dextrose,YPD)液体培养基中,28 ℃、180 r/min恒温振荡培养至对数期后用于毒性处理。
1.2 药物处理及细胞生长测定 取适量对数期酵母细胞接种于含有不同浓度亚砷酸钠(0、0.5、1.0、1.5、2.0 mmol/L)的液体培养基中,28 ℃、180 r/min恒温振荡培养24 h,取培养液在600 nm测其光密度值(OD600),并计算相对生长率(OD600处理组/OD600对照组×100%)。收集对照组和0.5 mmol/L处理组细胞用于胞内ROS水平、线粒体膜电位、细胞凋亡率以及丙二醛(Malondialdehyde,MDA)含量的检测。所有实验均设置3次重复,其中流式细胞仪检测的每个样品的细胞数不少于50000个。
1.3 细胞相对存活率检测 将相同浓度的对数期细胞涂布至含有不同浓度亚砷酸钠的平板上,使每个平板含有细胞数约200个,28 ℃恒温培养48 h后观察计数单菌落数,并计算细胞相对存活率(处理组单菌落数/对照组单菌落数×100%)。
1.4 细胞耐受性检测 收集对数期细胞,洗涤并用无菌磷酸盐缓冲溶液(phosphate buffer solution,PBS)将细胞悬液调整至OD600为2;然后用PBS将菌液以10倍梯度逐级稀释,每个梯度取5 μL并依次点样至固体培养基上(浓度由高到低);28 ℃恒温培养48 h后观察并拍照。
1.5 细胞凋亡率检测 收集酵母细胞并用PBS洗涤,用适量异硫氰酸荧光素标记的膜联蛋白V (fluorescein isothiocyanate-labeled annexin V,Annexin V-FITC)结合液重悬细胞后加入Annexin V-FITC和碘化丙啶(propidium iodide,PI)染液,混匀,室温避光孵育20 min后,流式细胞仪检测细胞凋亡率。
1.6 胞内ROS水平和MDA含量检测 胞内ROS检测参照文献[17]方法;丙二醛(MDA)含量检测参照文献[18]方法。
1.7 线粒体膜电位检测 利用荧光探针罗丹明123 (Rhodamine 123,Rho123)检测酵母细胞线粒体膜电位。将酵母细胞在2 μmol/L Rho 123中暗孵育15 min后,流式细胞仪检测细胞线粒体膜电位。
1.8 数据统计分析 采用SPSS 18.0对结果进行统计分析,处理组与对照组间的差异显著性用“*”表示(*P < 0.05,**P < 0.01),ΔHOG1突变株与野生株BY4741间的差异显著性用“ & ”表示( & P < 0.05,& & P < 0.01)。
2 结果和分析 2.1 HOG1对亚砷酸钠抑制酵母细胞生长和相对存活率的影响 亚砷酸钠对酵母细胞生长和相对存活率的影响如图 1所示。从图中可以看出,随着亚砷酸钠浓度的升高,野生株BY4741相对生长率和相对存活率逐渐下降;在相同浓度砷处理组中,ΔHOG1突变株相对生长率和存活率均显著低于野生株BY4741。在0.5 mmol/L处理组中,ΔHOG1突变株的相对生长率和存活率仅为野生株BY4741的4.08%和6.41%。结果表明,亚砷酸钠可抑制酵母细胞生长和分裂,HOG1在砷引起的酵母细胞生长抑制中起了重要作用。
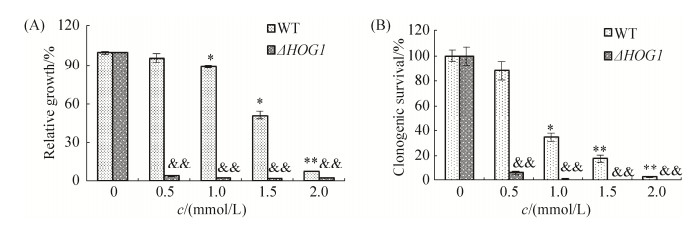 |
| 图 1 亚砷酸钠对WT与ΔHOG1突变株生长(A)和相对存活率(B)的影响 Figure 1 Effects of sodium arsnite on cell growth (A) and clonogenic survival rate (B) in yeast WT and ΔHOG1 strains. *: P < 0.05; **: P < 0.01; & & :P < 0.01 |
| 图选项 |
2.2 亚砷酸钠胁迫下WT与ΔHOG1突变株耐受性研究 从图 2可以看出,在不含亚砷酸钠的培养基中,野生株BY4741和ΔHOG1突变株生长状况无明显差异,均在稀释倍数为104处长出了浓密的菌落。当培养基中含有0.5 mmol/L亚砷酸钠时,ΔHOG1突变株生长状况显著差于野生株BY4741,仅在稀释倍数为100和101的地方长出了少量的菌落。结果说明,ΔHOG1突变株对亚砷酸钠的敏感性较高,即HOG1在提高酵母细胞对砷的抗性中起重要作用。
 |
| 图 2 WT与ΔHOG1对亚砷酸钠的耐受性分析 Figure 2 Evaluation of the sodium arsenite tolerance of yeast WT and ΔHOG1 strains |
| 图选项 |
2.3 HOG1对亚砷酸钠诱导酵母细胞凋亡的影响 采用Annexin/PI双染法检测亚砷酸钠对酵母细胞凋亡的影响(图 3)。结果显示,酵母细胞经0.5 mmol/L亚砷酸钠胁迫24 h后,野生株BY4741和ΔHOG1突变株早期凋亡率分别为对照组的2.30倍和4.21倍,且ΔHOG1突变株早期凋亡率显著高于野生株BY4741凋亡率。结果表明,亚砷酸钠可诱导酵母细胞发生凋亡,且HOG1可能与酵母细胞凋亡的抑制有关。
 |
| 图 3 亚砷酸钠对WT与ΔHOG1突变株细胞凋亡的影响 Figure 3 Effect of sodium arsenite on cell apoptosis in yeast WT and ΔHOG1 strains |
| 图选项 |
2.4 HOG1对亚砷酸钠引起酵母细胞氧化损伤的影响 经0.5 mmol/L处理24 h后,酵母细胞用ROS荧光探针标记,野生株BY4741胞内荧光强度与对照组间无显著差异,而ΔHOG1突变株平均荧光强度为45.54,约为野生株BY4741的2.4倍(图 4-A);同期,ΔHOG1突变株MDA含量比野生株BY4741增加了17.43倍,两者间具有极显著差异(图 4-B)。结果表明,亚砷酸钠可诱导酵母细胞内ROS水平升高,并引起氧化损伤,HOG1对抵抗亚砷酸钠引起的酵母细胞氧化损伤起重要作用。
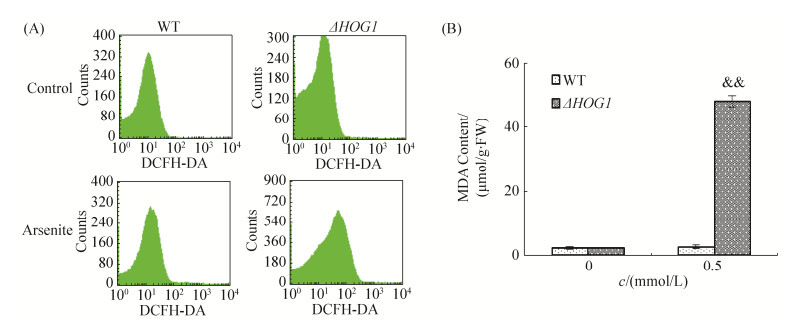 |
| 图 4 HOG1对亚砷酸钠引起酵母细胞氧化损伤的影响 Figure 4 Effect of HOG1 on sodium arsenite-induced oxidative damage in yeast cells. & & : P < 0.01 |
| 图选项 |
2.5 HOG1对亚砷酸钠致酵母细胞线粒体膜电位下降的影响 经0.5 mmol/L亚砷酸钠胁迫24 h后,用Rho 123荧光探针标记细胞,检测发现野生株BY4741细胞平均荧光强度略高于对照组,但两者之间无显著差异;ΔHOG1突变株细胞平均荧光强度为173.51,显著高于对照组,且与野生株BY4741之间具有极显著差异(图 5)。结果表明,亚砷酸钠可引起酵母细胞线粒体膜电位下降,HOG1对亚砷酸钠胁迫下维持酵母细胞线粒体膜电位起重要作用。
 |
| 图 5 亚砷酸钠对酵母细胞膜电位的影响 Figure 5 Effect of sodium arsenite on Δψ in yeast cells |
| 图选项 |
3 讨论 MAPK是一类广泛存在于真核生物中由脯氨酸依赖型的丝氨酸/苏氨酸组成的蛋白激酶,在进化途径上高度保守[19]。p38是MAPK家族中参与生物体生理过程调节的重要成员之一,在调控细胞周期和凋亡中起着至关重要的作用[20]。廖朝霞等[21]研究发现,异氟醚可激活p38 MAPK通路,诱导海马神经细胞凋亡;而p38抑制剂能减轻异氟醚麻醉引起的神经细胞凋亡。将p38 MAPK基因敲除后,小鼠胚胎成纤维细胞对亚砷酸盐的耐受性显著下降,并伴随着细胞凋亡的发生[22]。
HOG-MAPK途径是酵母细胞中发现的MAPK途径之一,与p38 MAPK高度同源,在高渗应激环境下可控制信号转导和基因表达[23]。Thorsen等[14]研究证明,亚砷酸盐可导致酵母细胞HOG1磷酸化水平增加,而ΔHOG1突变体对亚砷酸盐的敏感性增加。本研究中,我们以HOG1基因缺失的酵母细胞为材料,研究了亚砷酸钠对酵母细胞凋亡的影响。结果显示,酵母细胞经亚砷酸钠胁迫后,ΔHOG1突变体相对生长率、存活率和耐受性均低于野生株BY4741,HOG1基因缺失后,细胞凋亡率显著升高。细胞凋亡是存在于酵母细胞中主动结束生命的死亡过程,可被多种外源性刺激诱导。大量研究表明,酵母细胞的凋亡依赖于胞内ROS水平的升高和线粒体膜电位的变化[24-25]。
ROS是机体受到外界刺激后产生的一种代谢产物,在正常的生命活动过程中起着重要作用。现有研究证明,砷可引起植物、动物、微生物等多种细胞内ROS产生[26-29]。过量的ROS可导致DNA、蛋白质和脂类等生物大分子功能的破坏,引起细胞凋亡或坏死[30-33]。课题组在前期实验中发现,亚砷酸钠可改变酵母细胞抗氧化酶活性,导致胞内ROS升高,MDA含量升高,进而引起氧化损伤[17]。本实验中,在相同浓度的砷处理组中,ΔHOG1突变株胞内ROS水平和MDA含量均显著高于野生株酵母细胞,说明HOG1基因的缺失使酵母细胞的抗氧化能力下降,细胞发生氧化胁迫。用0.5 mmol/L的亚砷酸钠刺激酵母细胞后,ΔHOG1突变株线粒体膜电位显著下降,与野生株间具有显著差异,有可能是因为线粒体作为真核生物进行呼吸作用的细胞器,对氧化应激更加敏感,胞内ROS水平的升高引起了线粒体膜电位的改变,进而调控细胞凋亡的发生[34]。
综上所述,HOG1基因缺失菌株在亚砷酸钠处理后,细胞活性显著下降,ROS水平和MDA含量升高,更容易发生依赖于线粒体途径的细胞凋亡。结果说明,HOG1 MAPK可能通过对胞内ROS水平和线粒体膜电位的影响调控亚砷酸钠诱导的酵母细胞凋亡。
References
| [1] | Bhattacharjee H, Rosen BP, Mukhopadhyay R. Aquaglyceroporins and metalloid transport: implications in human diseases//Beitz E. Aquaporins. Berlin, Heidelberg: Springer, 2009,190: 309–325. |
| [2] | Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(4): 1643-1648. DOI:10.1073/pnas.98.4.1643 |
| [3] | Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. The Oncologist, 2001, 6(S2): 3-10. |
| [4] | Azizur Rahman M, Hasegawa H, Mahfuzur Rahman M, Mazid Miah MA, Tasmin A. Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicology and Environmental Safety, 2008, 69(2): 317-324. DOI:10.1016/j.ecoenv.2007.01.005 |
| [5] | Chayapong J, Madhyastha H, Madhyastha R, Nurrahmah QI, Nakajima Y, Choijookhuu N, Hishikawa Y, Maruyama M. Arsenic trioxide induces ROS activity and DNA damage, leading to G0/G1 extension in skin fibroblasts through the ATM-ATR-associated Chk pathway. Environmental Science and Pollution Research, 2017, 24(6): 5316-5325. DOI:10.1007/s11356-016-8215-7 |
| [6] | Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. Arsenic: toxicity, oxidative stress and human disease. Journal of Applied Toxicology, 2011, 31(2): 95-107. |
| [7] | Guo N, Peng ZL. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia-Pacific Journal of Clinical Oncology, 2013, 9(1): 6-11. DOI:10.1111/ajco.2013.9.issue-1 |
| [8] | Kang YH, Lee SJ. The role of p38 MAPK and JNK in arsenic trioxide-induced mitochondrial cell death in human cervical cancer cells. Journal of Cellular Physiology, 2008, 217(1): 23-33. |
| [9] | O'Rourke SM, Herskowitz I, O'Shea EK. Yeast go the whole HOG for the hyperosmotic response. Trends in Genetics, 2002, 18(8): 405-412. DOI:10.1016/S0168-9525(02)02723-3 |
| [10] | Azad GK, Singh V, Thakare MJ, Baranwal S, Tomar RS. Mitogen-activated protein kinase Hog1 is activated in response to curcumin exposure in the budding yeast Saccharomyces cerevisiae. BMC Microbiology, 2014, 14: 317. DOI:10.1186/s12866-014-0317-0 |
| [11] | Winkler A, Arkind C, Mattison CP, Burkholder A, Knoche K, Ota I. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryotic Cell, 2002, 1(2): 163-173. DOI:10.1128/EC.1.2.163-173.2002 |
| [12] | Panadero J, Pallotti C, Rodríguez-Vargas S, Randez-Gil F, Prieto JA. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. Journal of Biological Chemistry, 2006, 281(8): 4638-4645. DOI:10.1074/jbc.M512736200 |
| [13] | Lawrence CL, Botting CH, Antrobus R, Coote PJ. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Molecular and Cellular Biology, 2004, 24(8): 3307-3323. DOI:10.1128/MCB.24.8.3307-3323.2004 |
| [14] | Thorsen M, Di YJ, T?ngemo C, Morillas M, Ahmadpour D, Van der Does C, Wagner A, Johansson E, Boman J, Posas F, Wysocki R, Tamás MJ. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Molecular Biology of the Cell, 2006, 17(10): 4400-4410. DOI:10.1091/mbc.e06-04-0315 |
| [15] | Wu LH, Yi HL, Zhang HF. Involvement of NO in sodium arsenite-induced yeast cell death. Acta Scientiae Circumstantiae, 2012, 32(10): 2612-2616. (in Chinese) 吴丽华, 仪慧兰, 张虎芳. NO参与亚砷酸钠诱导酵母细胞死亡的调控. 环境科学学报, 2012, 32(10): 2612-2616. |
| [16] | Xiong B, Zhang LL, Xu HH, Yang Y, Jiang LH. Cadmium induces the activation of cell wall integrity pathway in budding yeast. Chemico-Biological Interactions, 2015, 240: 316-323. DOI:10.1016/j.cbi.2015.09.007 |
| [17] | Wu LH, Chen YF, Chen P, Yi HL. Sodium arsenite exposure affects the levels of antioxidant enzymes and lipid peroxidation in Saccharomyces cerevisiae. Asian Journal of Ecotoxicology, 2016, 11(3): 302-307. (in Chinese) 吴丽华, 陈燕飞, 陈鹏, 仪慧兰. 亚砷酸钠对酵母细胞抗氧化酶活性和脂质过氧化的影响. 生态毒理学报, 2016, 11(3): 302-307. |
| [18] | 潘军航.酿酒酵母耐铝机制中可能包含抗氧化作用.浙江大学硕士学位论文, 2005. |
| [19] | Campbell JS, Seger R, Graves JD, Graves LM, Jensen AM, Krebs EG. The MAP kinase cascade. Recent Progress in Hormone Research, 1995, 50: 131-159. |
| [20] | Bu SZ, Huang Q, Jiang YM, Min HB, Hou Y, Guo ZY, Wei JF, Wang JW, Ni X, Zheng SS. P38 mitogen-activated protein kinases is required for counteraction of 2-methoxyestradiol to estradiol-stimulated cell proliferation and induction of apoptosis in ovarian carcinoma cells via phosphorylation Bcl-2. Apoptosis, 2006, 11(3): 413-425. DOI:10.1007/s10495-006-4064-z |
| [21] | Liao ZX, Wang F, Cao DX, Liu CL, Li YJ. Effect of p38 MAPK pathway on isoflurane-induced neuronal apoptosis in hippocampus of neonatal rat. Chinese Pharmacological Bulletin, 2014, 30(12): 1661-1666. (in Chinese) 廖朝霞, 王飞, 曹德雄, 柳垂亮, 李玉娟. p38 MAPK信号通路在异氟醚诱导新生大鼠海马神经细胞凋亡的作用. 中国药理学通报, 2014, 30(12): 1661-1666. DOI:10.3969/j.issn.1001-1978.2014.12.009 |
| [22] | Liu AH, Gong XW, Wei J, Ming XY, Wang DA, Deng P, Luo SQ, Jiang Y. Effects of p38 MAPK gene knockout on arsenite-induced cell apoptosis. Chinese Journal of Pathophysiology, 2008, 24(5): 892-895. (in Chinese) 刘爱华, 龚小卫, 魏洁, 明小燕, 王达安, 邓鹏, 罗深秋, 姜勇. p38 MAPK基因敲除对亚砷酸盐诱导细胞凋亡的影响. 中国病理生理杂志, 2008, 24(5): 892-895. DOI:10.3321/j.issn:1000-4718.2008.05.012 |
| [23] | Wu XC, Hu SJ, Qian KX. HOG-MAPK pathway in yeast. Chinese Journal of Cell Biology, 2005, 27(3): 247-252. (in Chinese) 吴雪昌, 胡森杰, 钱凯先. 酵母HOG-MAPK途径. 细胞生物学杂志, 2005, 27(3): 247-252. |
| [24] | Du L, Yu Y, Chen JS, Liu Y, Xia YJ, Chen Q, Liu XJ. Arsenic induces caspase- and mitochondria-mediated apoptosis in Saccharomyces cerevisiae. FEMS Yeast Research, 2007, 7(6): 860-865. DOI:10.1111/fyr.2007.7.issue-6 |
| [25] | Zheng K, Pan JW, Ye L, Fu Y, Peng HZ, Wan BY, Gu Q, Bian HW, Han N, Wang JH, Kang B, Pan JH, Shao HH, Wang WZ, Zhu MY. Programmed cell death-involved aluminum toxicity in yeast alleviated by antiapoptotic members with decreased calcium signals. Plant Physiology, 2007, 143(1): 38-49. |
| [26] | Lee CH, Yu HS. Role of mitochondria, ROS, and DNA damage in arsenic induced carcinogenesis. Frontiers in Bioscience, 2016, 8: 312-320. DOI:10.2741/s465 |
| [27] | Wang GY, Zhang T, Sun W, Wang HS, Yin F, Wang ZY, Zuo DQ, Sun MX, Zhou ZF, Lin BH, Xu J, Hua YQ, Li HQ, Cai ZD. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free Radical Biology and Medicine, 2017, 106: 24-37. DOI:10.1016/j.freeradbiomed.2017.02.015 |
| [28] | Wu LH, Yi HL, Yi M. Assessment of arsenic toxicity using Allium/Vicia root tip micronucleus assays. Journal of Hazardous Materials, 2010, 176(1/3): 952-956. |
| [29] | Wu LH, Yi HL, Zhang HF. Reactive oxygen species and Ca2+ are involved in sodium arsenite-induced cell killing in yeast cells. FEMS Microbiology Letters, 2013, 343(1): 57-63. DOI:10.1111/femsle.2013.343.issue-1 |
| [30] | Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH. Heavy metals, islet function and diabetes development. Islets, 2009, 1(3): 169-176. DOI:10.4161/isl.1.3.9262 |
| [31] | Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology, 2011, 283(2/3): 65-87. |
| [32] | Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Molecular and Cellular Biochemistry, 2010, 345(1/2): 91-104. |
| [33] | Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Huang DZ, Chen YW. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicology Letters, 2014, 224(1): 130-140. DOI:10.1016/j.toxlet.2013.10.013 |
| [34] | 李雅楠. MAPK/线粒体凋亡途径在绵羊肺炎支原体致支气管上皮细胞氧化损伤中的作用机制研究.宁夏大学博士学位论文, 2017. |
