全文HTML
--> --> --> 三氯乙烯(TCE)作为氯代烃的一种,具有水溶性差、易吸附于土壤以及化学结构稳定等特点,因此,其很难从低渗透土壤介质中被去除。长期存在于土壤介质中的TCE作为污染源,会持续污染土壤和地下水,并严重威胁着人类健康[1-2]。近年来,表面活性剂由于对氯代烃污染物具有增溶作用,故其受到了越来越多的关注。表面活性剂可分为阳离子型表面活性剂、阴离子型表面活性剂和非离子型表面活性剂。大量研究[3-6]表明,阳离子型表面活性剂较容易吸附到土壤颗粒上,而阴离子型表面活性剂容易与土壤中的阳离子共沉淀,相比于阳离子型和阴离子型表面活性剂,非离子型表面活性剂由于其临界胶束浓度低,具有更好的增溶能力和经济效益。作为非离子型表面活性剂,吐温-80(Tween-80)具有非离子型表面活性剂的许多优点,如水溶性好、稳定性高、受电解质和酸碱等外部因素影响较小等。此外,与其他非离子型表面活性剂相比,Tween-80具有成本低、对土壤和地下水中微生物的毒性弱等优点[7-8]。因此,Tween-80已广泛应用于土壤和地下水中氯代烃污染物的增溶过程中。然而,为了防止二次污染,Tween-80增溶后的TCE溶液需要进一步处理,但含有表面活性剂的TCE会被Tween-80形成的胶束包裹,从而影响TCE的处理效果[9]。因此,如何高效去除含表面活性剂Tween-80的水相中的TCE是当前亟待解决的一个技术难题。高级氧化技术(AOPs)因具有高效快速的特性,常被用于处理含有表面活性剂水溶液中的污染物[10-11]。在高级氧化技术中,常用的氧化剂主要有臭氧[12]、过氧化氢[13-14]、过硫酸盐[15-16]和过碳酸钠[17-18]等。过碳酸钠(Na2CO3·1.5H2O2,SPC)被称为固体过氧化氢(H2O2),是一种实用价值较高的绿色氧化剂。作为H2O2的载体,SPC与水混合时可以释放出H2O2[19]。其分解产物通常为水、二氧化碳和碳酸钠,故其具有无毒性及无二次污染的优势。与传统的H2O2相比,SPC具有价格低廉、操作安全、方便储存运输和适用pH范围广等优点[20]。与过硫酸盐相比,SPC可以通过引入碳酸根,以保证水环境pH不致过低,进而降低氧化过程酸性条件对水环境中微生物的影响[21]。基于上述优点,近年来SPC在污染场地修复中受到了越来越多的关注[22-23]。
与传统Fenton反应[24]相似,Fe(Ⅱ)可以活化SPC用于降解各种污染物。然而,该体系反应速度过快,Fe(Ⅱ)迅速转化为Fe(Ⅲ),不能持续降解污染物,制约了其在实际中的应用。纳米零价铁(nZVI)作为一种环境友好型催化材料,与普通铁粉相比,具有更强的还原能力和更好的迁移能力,近年来常用于污染场地修复[25-26]。但是该过程耗时长、成本高。采用nZVI活化SPC需要降低溶液的pH,因为只有在酸性条件下nZVI表面才被腐蚀且释放出Fe(Ⅱ)。
迄今为止,将nZVI与Fe(Ⅱ)协同活化SPC应用于降解有机污染物(含表面活性剂)的研究尚鲜有报道。本研究基于Fe(Ⅱ)与SPC释放的H2O2反应产生强氧化性的羟基自由基(·OH)的过程中,也同时降低溶液pH的特点,通过nZVI自身腐蚀逐步释放Fe(Ⅱ),及时地将体系中产生的Fe(III)转化为Fe(Ⅱ),以期达到持续强化的协同效果。nZVI协同Fe(Ⅱ)活化SPC体系既能克服单独的Fe(Ⅱ)活化SPC时不能持续高效降解TCE的缺点,也能克服单独的nZVI不能活化SPC的不足,具有较高的潜在应用价值。鉴于此,本研究以含Tween-80的水相中TCE为研究对象,SPC/Fe(Ⅱ)/nZVI为反应体系,主要探究了在Tween-80存在下该反应体系降解TCE的有效性;分别考察了Tween-80浓度、溶液初始pH以及无机阴离子对TCE降解效果的影响;最后考察了该体系中活性氧自由基的类型及其对TCE降解的作用机制。
1.1. 试剂与仪器
试剂:SPC购于Acros Organics上海公司;TCE、七水合硫酸亚铁(FeSO4·7H2O)、硫氰酸铵和硝酸钴购于阿拉丁试剂(上海)有限公司;Tween-80、正己烷、氢氧化钠(NaOH)、碳酸氢钠(NaHCO3)和氯化钠(NaCl)购于上海泰坦科技股份有限公司;硝基苯(NB)、四氯化碳(CT)、异丙醇(IPA)、氯仿(CF)和硫酸(H2SO4)购于上海凌峰化学试剂有限公司。以上试剂均为分析纯,直接使用。纳米零价铁(纯度>99%,平均粒径50 nm)购于上海超威纳米科技有限公司。所有实验用水均为超纯水。实验仪器:Agilent 7890A型气相色谱仪(安捷伦科技有限公司);LC-20AT型高效液相色谱仪(日本岛津公司);85-2型恒温磁力搅拌器(上海闵行虹浦仪器厂);SDC-6型低温恒温槽(宁波新芝生物科技股份有限公司);DR-6000型紫外分光光度计(哈希水质分析仪器有限公司);P8-10型pH测定仪(德国Sartorius公司);XW-80A型涡旋振荡器(上海青浦沪西仪器厂);Classic UV MK2型超纯水机(英国ELGA公司);AL204型电子分析天平(瑞士Metter-Toledo集团);SK2200LH型超声波清洗器(上海科导超声仪器有限公司)。
1.2. 实验方法
首先用超纯水配制TCE和Tween-80混合母液,之后将母液稀释至所需浓度(TCE=0.15 mmol·L?1,Tween-80=13 mg·L?1),置于带夹层的实验反应器中(有效体积250 mL,内径6 cm,高度9 cm),采用低温恒温槽使反应温度控制在20 ℃。为确保反应溶液混合均匀,将反应器置于磁力搅拌器上(转速为600 r·min?1)。实验过程中依次加入所需量的FeSO4·7H2O、nZVI和SPC,开始反应计时,在即定时间点取样,并与正己烷快速混合,以终止反应,样品通过气相色谱仪分析。除考察溶液初始pH的影响外,其他实验均不调整溶液pH。所有实验设2个平行样,结果取平均值。1.3. 分析方法
溶液中TCE和CT浓度均采用气相色谱仪分析测定。其中TCE分析条件:电子俘获检测器(ECD),自动进样器(Agilent 7693),DB-VRX色谱柱(长60 m、内径250 μm、膜厚1.4 μm),进样口和检测器温度分别为240 ℃和260 ℃,色谱柱温度为75 ℃,载气为氮气,进样量为1.0 μL,分流比20∶1。在分析CT时,除色谱柱温度为100 ℃外,其余条件与TCE一致。溶液中NB浓度采用高效液相色谱仪分析测定。分析条件:紫外可见光检测器(UV),C18反相色谱柱(250 mm×4.6 mm,5 μm),流动相由40%超纯水和60%色谱级甲醇组成,进样量为10 μL,检测器温度为35 ℃,流量为1.0 mL·min?1。
溶液中Tween-80浓度采用紫外分光光度计(波长623 nm,显色剂(NH4)2Co(SCN)4)测定[27]。
2.1. nZVI的表征
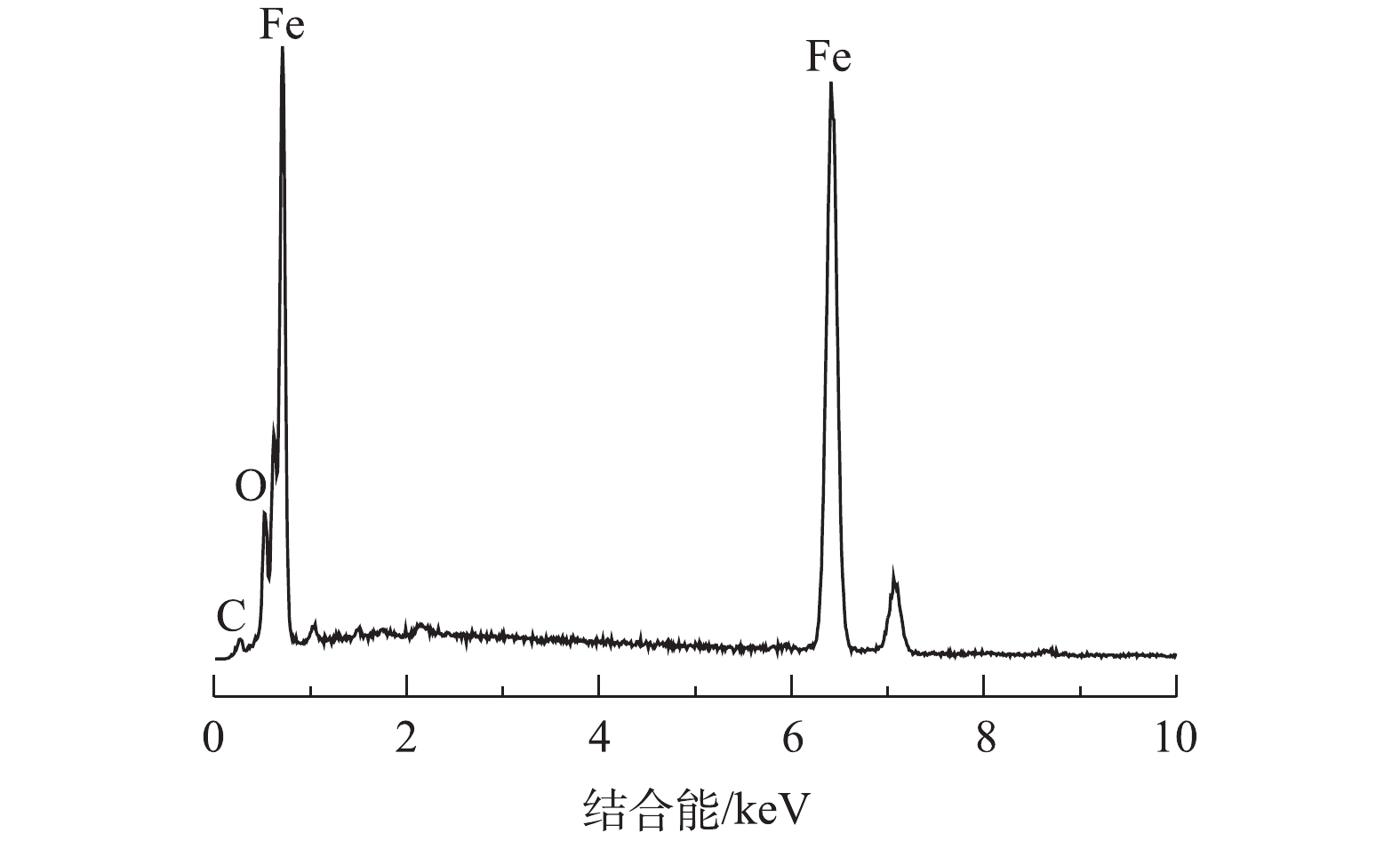
nZVI的TEM结果如图1所示,单一的nZVI颗粒具有典型的核壳结构,内部为新鲜的纳米零价铁,外部为氧化物薄膜,颗粒的平均粒径为50~100 nm。颗粒与颗粒之间有团聚现象,呈球形链状排列聚集在一起,与已有研究报道的典型纳米零价铁的形貌特征[28]一致。nZVI的XRD特征图谱如图2所示。与JCPDS粉末衍射标准卡数据[JCPDS 06-0696]比对后可以看出,在2θ=44.6°处有很强的α-Fe0特征衍射峰。为了分析nZVI材料的化学元素组成,本研究对nZVI进行了EDS分析,结果如图3所示。EDS分析显示,nZVI材料中C、O和Fe元素的质量分数分别为1.21%、2.48%和92.59%,表明在nZVI表面形成了铁氧化物,这与nZVI的TEM分析得出的铁的氧化物外壳组成结果相符合。2.2. nZVI/Fe(Ⅱ)协同活化SPC降解TCE(含Tween-80)的有效性
图4为TCE分别在nZVI、SPC/nZVI、Fe(Ⅱ)/nZVI、SPC/Fe(Ⅱ)和SPC/Fe(Ⅱ)/nZVI等体系中的降解效果。其中,SPC、Fe(Ⅱ)和TCE初始浓度分别为0.6、0.6和0.15 mmol·L?1,nZVI和Tween-80初始浓度分别为25 mg·L?1和13 mg·L?1。空白组的实验结果表明,当体系中未投加任何药剂时,60 min内,因挥发损失的TCE量小于5%,可以忽略不计。虽然有研究[29]报道,nZVI可以通过还原作用有效去除TCE,但是通常需要较大的nZVI投加量和较长的反应时间,且在单独投加nZVI时,TCE在60 min内的去除率仅为8.5%,因此,在本研究中少量的nZVI在短时间内无法有效去除TCE。在Fe(Ⅱ)/nZVI体系中,TCE的去除率也仅有7.7%,这与单独投加nZVI时所得到的结果相似。在SPC/nZVI体系中,TCE的去除率为8.3%,这说明单独的nZVI不能有效活化SPC。这是因为在SPC/nZVI体系中,反应过程中溶液的pH始终维持在10左右,在此条件下,nZVI难以腐蚀释放Fe(Ⅱ),进而阻碍了活性氧自由基的产生,且少部分腐蚀产生的Fe(Ⅱ)也会因为pH升高导致Fe(Ⅱ)沉淀,从而失去催化活性。而在SPC/Fe(Ⅱ)体系中,虽然TCE的降解率达到64.5%,但反应过于迅速,在3 min时,反应即终止,不能持续高效降解TCE。ZANG等[30-31]探究了SPC/Fe(Ⅱ)体系对水溶液中以及泥浆系统中TCE的降解效果,结果显示TCE最终去除率均可达到95%以上,然而SPC/Fe(Ⅱ)体系的反应过于迅速,导致SPC和Fe(Ⅱ)的利用率降低,不能持续降解污染物。臧学轲[32]还探究了羟胺促进柠檬酸-Fe(Ⅱ)活化过碳酸钠降解TCE,发现盐酸羟胺能有效地将Fe(Ⅲ)还原为Fe(Ⅱ),强化降解TCE,然而该体系中加入的药剂种类较多,且加入盐酸羟胺以及柠檬酸会导致地下水中化学需氧量的升高,从而容易导致二次污染和修复成本的增高。而在SPC/Fe(Ⅱ)/nZVI体系中,3 min时TCE的降解率为73.9%,60 min时TCE基本完全去除(97.3%),既克服了单独的Fe(Ⅱ)活化SPC时不能持续高效降解TCE的缺点,也克服了单独的nZVI不能活化SPC的不足,表明Fe(Ⅱ)与nZVI起到了良好的协同活化SPC的作用,达到了预期的目的。nZVI和Fe(Ⅱ)协同活化SPC能够持续高效降解TCE。这主要是因为:在该体系中,Fe(Ⅱ)先活化SPC释放的H2O2,产生羟基自由基(·OH)(如式(1)和式(2)所示),TCE被快速氧化降解;同时Fe(Ⅱ)会被快速氧化成Fe(Ⅲ),投加的Fe(Ⅱ)被迅速消耗完,经检测,反应3 min时Fe(Ⅱ)的量与最初投加的0.6 mmol·L?1的Fe(Ⅱ)量相比较,Fe(Ⅱ)量迅速下降为0.03 mmol·L?1。而此后TCE仍继续缓慢降解,一方面这是由于Fe(Ⅱ)活化SPC降解TCE后溶液的pH降低(pH由5.20降为3.74),促使nZVI腐蚀释放Fe(Ⅱ)[33](如式(3)所示),在nZVI表面产生活性氧自由基,TCE被进一步缓慢降解;另一方面,nZVI也可以将体系中产生的Fe(III)部分还原为Fe(Ⅱ)(如式(4)所示)。综上可知,nZVI和Fe(Ⅱ)可以协同活化SPC降解TCE(含Tween-80)。
2.3. Tween-80浓度对TCE降解的影响
为了探究Tween-80浓度对TCE降解效果的影响,固定SPC、Fe(Ⅱ)、nZVI和TCE的初始浓度分别为0.6 mmol·L?1、0.6 mmol·L?1、25 mg·L?1和0.15 mmol·L?1,分别投加不同浓度的Tween-80(0、7、13、130、260 mg·L?1),结果如图5所示。在SPC/Fe(Ⅱ)/nZVI体系中,TCE的降解率分别为98.6%、98.5%、97.3%、50.2%和19.4%。这表明随着Tween-80浓度的增加,TCE的降解率逐渐降低,Tween-80的加入会抑制TCE的降解,Tween-80浓度越高,抑制效果越明显。这是因为当表面活性剂Tween-80达到临界胶束浓度(CMC,13 mg·L?1)时,Tween-80分子会形成胶束,将TCE包裹在内,由于该体系中产生的·OH不具有选择性,·OH只有先破坏包覆TCE的Tween-80胶束后[34],才能与TCE进行反应。综上可知,SPC/Fe(Ⅱ)/nZVI体系中过多的Tween-80会与TCE竞争·OH,进而不利于TCE的降解。为了验证Tween-80是否会在反应过程中与TCE竞争·OH,表1呈现了初始浓度为260 mg·L?1的Tween-80在SPC/Fe(Ⅱ)/nZVI和SPC/Fe(Ⅱ)/nZVI/TCE 2体系中浓度的变化结果。结果表明,在60 min内,2个体系中Tween-80的浓度分别下降至205 mg·L?1和199 mg·L?1,下降幅度基本相同,说明在SPC/Fe(Ⅱ)/nZVI体系中(无论有无TCE),Tween-80均会被部分去除。该结果进一步证实了Tween-80可以与·OH发生反应,Tween-80和TCE在反应过程中均会被降解,因此,Tween-80的存在会影响TCE的去除。
2.4. SPC/Fe(Ⅱ)/nZVI体系中TCE(含Tween-80)的降解机制
目前已经有许多研究证实,在Fenton反应过程中,起主要作用的是·OH,而化学探针实验已经证实了SPC/Fe(Ⅱ)/nZVI体系(Tween-80存在的情况下)中·OH和
2.5. 溶液初始pH对TCE(含Tween-80)降解的影响
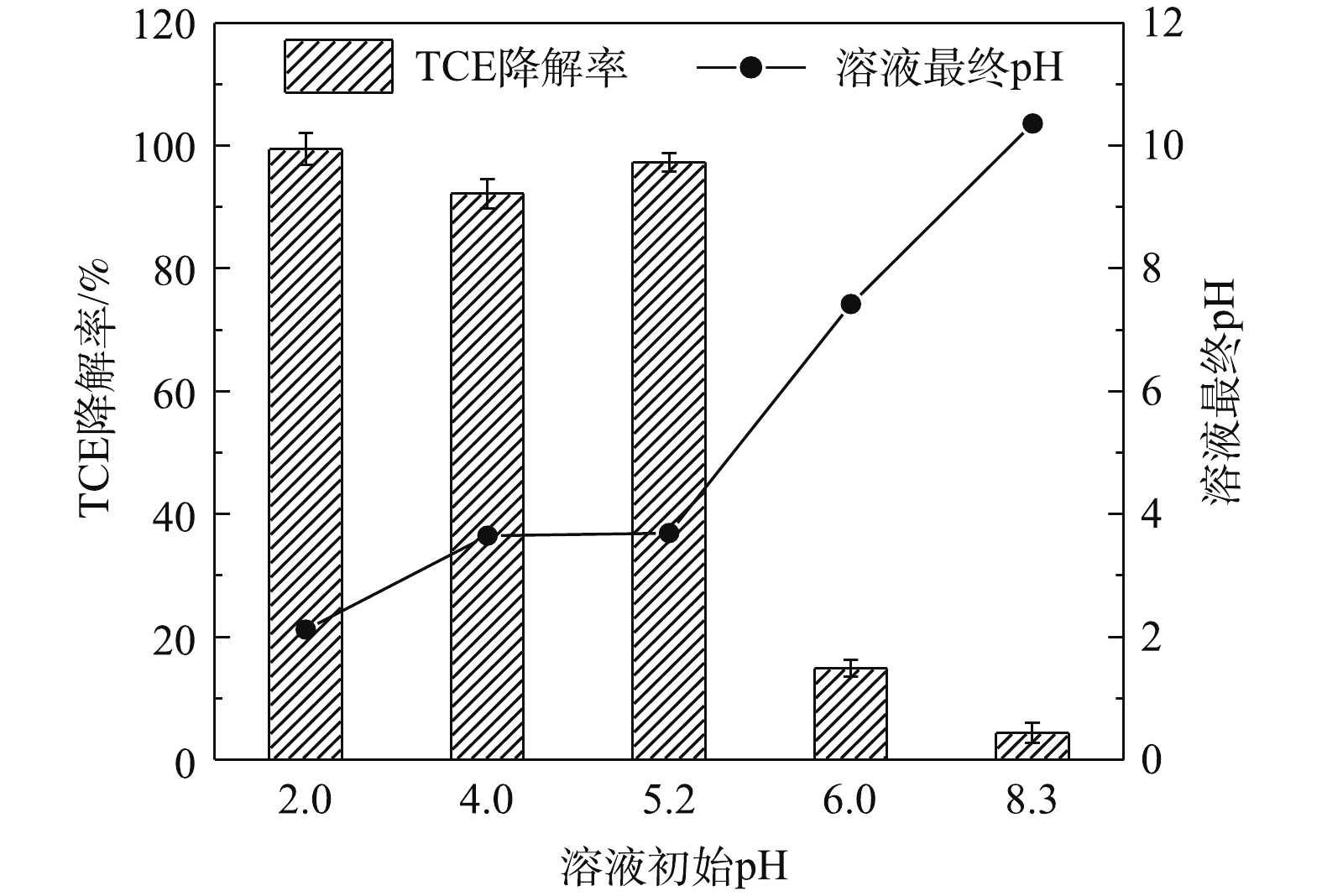
为探究在Tween-80存在下溶液初始pH对SPC/Fe(Ⅱ)/nZVI体系降解TCE的影响,实验选用0.1 mol·L?1的NaOH和H2SO4溶液将溶液初始pH调整为2.0、4.0、5.20(未调整)、6.0和8.3。在本次实验中,SPC、Fe(Ⅱ)、nZVI、TCE、Tween-80的投加量分别为0.6 mmol·L?1、0.6 mmol·L?1、25 mg·L?1、0.15 mmol·L?1、13 mg·L?1。由图8可知,反应溶液在未调整时的初始pH为5.20,60 min时溶液的pH为3.69,TCE的降解率为97.3%。用H2SO4调整溶液初始pH至2.0和4.0时,对TCE的降解效果影响比较小,且反应后溶液的最终pH分别为2.12和3.65。用NaOH调整初始pH至6.0和8.3时,体系中TCE的降解率有明显的下降,60 min时TCE的降解率分别为14.9%和4.4%,最终溶液pH分别升高至7.42和10.36,以上结果表明弱酸性或碱性条件不利于该体系中TCE的去除。由于溶液pH对Fenton反应的影响很大,通常取最佳pH为3左右[40],在此条件下·OH的产生量最高。溶液初始pH的提高,会导致Fe(Ⅱ)沉淀,减少了Fe(Ⅱ)的有效浓度,影响体系中·OH的产生。而溶液初始pH为2.0、4.0和5.2时,其相应的终点pH分别为2.12、3.65和3.69,这说明在溶液初始pH为2.0~5.2时,不存在由于反应过程中pH升高导致Fe(Ⅱ)沉淀而失去催化活性的情况。但是,在碱性条件下,nZVI不易转化为Fe(Ⅱ),·OH的氧化能力也比较弱[41],因此,TCE的降解率明显下降。综上可知,在Tween-80存在下,溶液初始pH为2.0~5.2时,SPC/Fe(Ⅱ)/nZVI体系可有效去除TCE。2.6. 无机阴离子对TCE(含Tween-80)降解的影响
为探究在Tween-80存在下无机阴离子对SPC/Fe(Ⅱ)/nZVI体系降解TCE的影响,实验选用水中最常见的Cl?和2)在SPC/Fe(Ⅱ)/nZVI体系中,Tween-80的存在会抑制TCE的降解,Tween-80浓度越高,抑制效果越明显。
3)在SPC/Fe(Ⅱ)/nZVI体系(Tween-80存在的情况下)中,产生·OH和
4)在Tween-80存在下,溶液初始pH为2.0~5.2时,SPC/Fe(Ⅱ)/nZVI体系可有效去除TCE;溶液中Cl?的存在对TCE的抑制效果不明显,而
参考文献






 下载:
下载: 








 点击查看大图
点击查看大图