全文HTML
--> --> --> 砷在地壳和天然矿物中广泛存在,也是世界各地水生态系统的主要污染物之一[1-2]。砷在地下水中主要以As(Ⅲ)和As(Ⅴ)形态存在,由于As(Ⅲ)更容易被细胞摄取,故其毒性比As(Ⅴ)更强[3]。砷污染地下水广泛分布在6大洲70多个国家,包括孟加拉国、印度、中国、越南、尼泊尔、墨西哥、匈牙利、阿根廷等[4]。部分地区地下水中砷的浓度可以达到数百甚至数千微克每升,远远高于世界卫生组织所推荐的饮用水中10 μg·L?1限值[5]。长期摄入砷可能引发皮肤病、心血管疾病以及其他各种癌症[6]。地下水砷污染是一个严重的环境问题,因此地下水砷污染的治理也一直是研究热点。吸附法以其高效、灵活、成本低、操作简便等优点被广泛应用于水体中砷的去除[7]。有研究表明,铁氧化物对As(Ⅴ)有较好的吸附作用,但地下水中的砷通常以As(Ⅲ)形态存在,ZHANG等[8]制备了一种铁锰二元氧化物吸附剂,利用锰氧化物氧化和氧化铁吸附作用机制实现了地下水中As(Ⅲ)的高效去除。近年来,纳米零价铁(nano zero-valent iron,nZVI)因其体积小、比表面积大、还原性强、吸附性能好、环境友好等特点,被广泛应用于水体中重金属和有机物污染物的控制[9-10]。此外,有研究[11]表明,nZVI材料在地下水砷污染控制中具有明显优势。
目前,关于nZVI除砷的研究主要是在实验室模拟地下水的严格厌氧环境中,或直接暴露在大气环境下进行的[12-13]。溶解氧的存在明显影响了nZVI在水溶液中的反应行为及对砷的去除作用[14]。但不同氧含量对nZVI除砷效果影响的研究尚未见报道。在自然的地下环境中,随着土壤向沉积物的转变,氧浓度呈现由高向低逐渐变化的趋势[15]。此外,由于地下水过度开采以及季节性变化等也会造成地下水位波动,导致地下水处于厌氧、好氧交替的环境之中[16-18],进而对nZVI除砷效果产生未知的影响。因此,研究不同氧含量条件下nZVI去除As(Ⅲ)和As(Ⅴ)的作用机制,通过人为调控氧含量强化nZVI除砷效果具有重要的环境意义。
本研究通过分析不同氧含量(厌氧、低氧、中氧和高氧)条件下nZVI分别去除As(Ⅲ)和As(Ⅴ)的作用机制,以探索氧含量对nZVI除砷效果的影响。此外,利用扫描电子显微镜(SEM)、X射线衍射(XRD)和X射线光电子能谱(XPS)等对nZVI-H2O-O2-As(Ⅴ)/As(Ⅲ)反应产物进行了表征,进而明确氧气促进nZVI除As(Ⅴ)/As(Ⅲ)的作用机理。
1.1. 实验原料
硫酸亚铁(FeSO4·7H2O)、硼氢化钠(NaBH4)、亚砷酸钠(NaAsO2)和砷酸钠(Na2HAsO4·7H2O)购自上海阿拉丁生化科技股份有限公司。氢氧化钠(NaOH)、氯化钠(NaCl)、盐酸(HCl)、无水乙醇(C2H6O)购于中国国药集团化学试剂有限公司。药品均为分析级或优级纯。NaAsO2和Na2HAsO4·7H2O分别用于制备10 g·L?1 As(Ⅲ)和As(Ⅴ)的储备溶液。实验用水均为超纯水(18.2 MΩ·cm)。1.2. nZVI的合成方法
根据SUN的方法[19],用硼氢化钠还原亚铁盐制得nZVI。将100 mL NaBH4溶液(0.2 mol·L?1)以每秒1~2滴的速度滴加到100 mL (V水∶V乙醇=4∶1) FeSO4·7H2O(0.05 mol·L?1)溶液中,滴定过程中持续机械搅拌,滴完后继续反应20 min,整个过程处于氮气保护气氛中。然后将生成的黑色nZVI分别用脱氧水和脱氧乙醇各清洗3次以去除杂质。最后将nZVI保存在乙醇溶液中备用。1.3. 不同氧含量下nZVI除砷实验
实验以0.01 mol·L?1NaCl为背景电解质,反应溶液体系为30 mL,nZVI投加量为200 mg·L?1,初始砷浓度为50 mg·L?1,反应在铝箔包覆的50 mL密封西林瓶中进行,置于转速为150 r·min?1的摇床上振荡。含砷溶液曝氮气(99.99%)以排除氧气,加入nZVI前用NaOH和HCl溶液调节体系pH至7.2。不同的氧气含量通过加入不同体积的氧气(99.99%)来实现。其中,氧含量为顶空与溶液两部分的氧气总和,低氧浓度定义为O2/nZVI的摩尔比为0.05、0.125、0.25和0.5,中氧浓度定义为O2/nZVI的摩尔比为1.0和2.0,高氧浓度定义为O2/nZVI的摩尔比为3.0、4.0和5.0。在温度为25 ℃,大气压为101.3 kPa的条件下,氧气在水中的亨利系数为1.28×10?8 mol·(L·Pa)?1,根据亨利定律和克拉佩龙方程,并对水蒸气的分压进行校正(水的蒸气压为0.031 67×105 Pa),经计算,低氧对应的溶解氧为0.21、0.51、1.03和2.05 mg·L?1,中氧对应的溶解氧为4.10 mg·L?1和8.20 mg·L?1,高氧对应的溶解氧为12.30、16.40和20.50 mg·L?1。所有实验均在室温(25 ℃)下重复进行。1.4. 分析方法及表征技术
反应过程中每隔一定时间进行取样,经0.22 μm的滤膜过滤后采用高效液相色谱(HPLC,LC-20A,岛津,日本)串联原子荧光光谱(AFS,AFS-2202E,海光,中国)测定溶液中砷的浓度。反应结束后,通过离心和真空冷冻干燥得到固体样品。采用扫描电镜(SEM,Gemini 300,蔡司,德国)对产物形貌进行扫描。固体的成分是由X射线衍射(XRD,X/Pert PRO MPD,帕纳科,荷兰)分析。反应固体中砷和铁的氧化价态用X射线光电子能谱(XPS,ESCALAB 250 xi,赛默飞世尔,美国)分析,结合能值均以C1s 284.8 eV作为参照。2.1. 氧气含量对nZVI除砷效果的影响
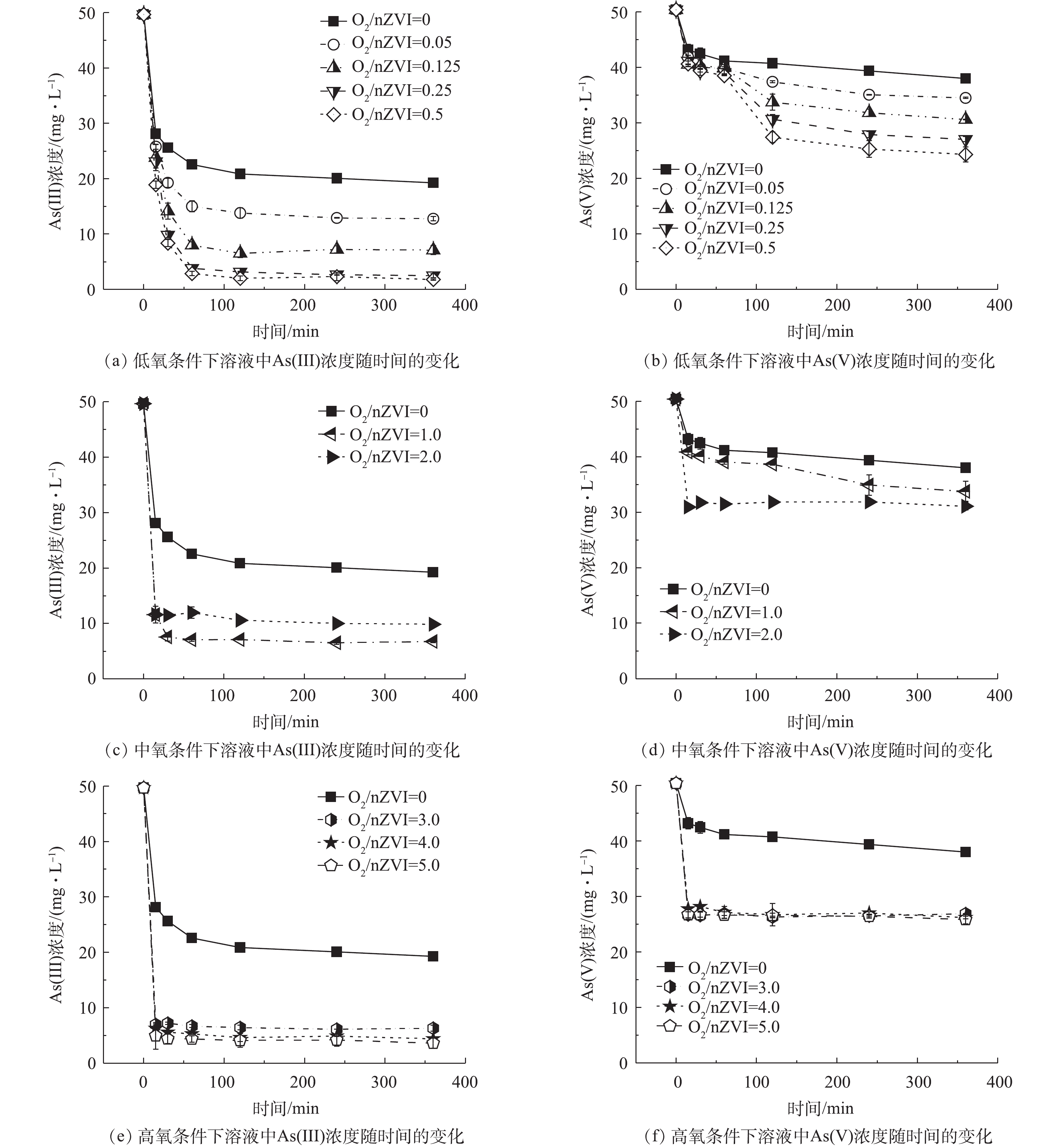
不同氧含量条件下As(Ⅲ)/As(Ⅴ)浓度随时间的变化见图1。1)氧气含量对nZVI除As(Ⅲ)效果的影响。nZVI去除As(Ⅲ)过程中As(Ⅲ)的浓度随时间变化结果如图1(a)、图1(c)、图1(e)所示,不同氧含量条件下,As(Ⅲ)浓度均随时间延长迅速降低,并逐渐趋于稳定达到反应平衡。厌氧条件下As(Ⅲ)去除率为61.23%;低氧条件下随着氧含量的增加As(Ⅲ)去除率有所升高,在O2/nZVI摩尔比为0.5时,As(Ⅲ)的去除率达到最大,较厌氧条件增加了35.04%。在中氧和高氧(O2/nZVI摩尔比>0.5)条件下,伴随着As(Ⅲ)氧化作用,溶液中可检测到少量As(Ⅴ)(0.26~4.38 mg·L?1)导致As(Ⅲ)去除率与总砷去除率存在差异。中氧条件下反应30 min达到平衡但As(Ⅲ)去除率低,在O2/nZVI摩尔比为2.0时,As(Ⅲ)和总砷的去除率分别为80.13%和74.17%,总砷去除率较厌氧条件只升高了12.94%;高氧条件下总砷的去除率有所升高且稳定在90%左右。
2)氧气含量对nZVI除As(Ⅴ)效果的影响。在nZVI去除As(Ⅴ)的过程中,溶液中未检测到As(Ⅲ),故As(Ⅴ)的去除率即总砷的去除率。不同氧含量条件下溶液中As(Ⅴ)的浓度随时间变化结果如图1(b)、图1(d)、图1(f)所示。厌氧条件下砷的去除率为24.61%;在低氧条件下As(Ⅴ)去除率随着氧含量的增加而升高,在O2/nZVI摩尔比为0.5时As(Ⅴ)去除率达到最大,为51.75%,较厌氧条件增加了27.14%;在中氧条件下砷的去除率下降,在O2/nZVI摩尔比为1.0时去除率为33.03%,较厌氧条件只增加了8.42%;在高氧条件下砷的去除率增大且稳定在48%左右。
对比反应达到平衡后nZVI对As(Ⅲ)和As(Ⅴ)去除(图2)结果表明,不同氧气含量对nZVI除砷的促进程度不同,且整体去除率随着氧含量的增加呈现先升高再下降又升高的趋势;nZVI对As(Ⅲ)的去除率大于As(Ⅴ),说明在As(Ⅲ)为主要污染物的地下水中,采用nZVI除砷具有明显优势;此外,不管是对As(Ⅲ)还是As(Ⅴ),有氧条件下砷的去除效果均好于厌氧条件,说明氧气的存在明显促进了砷的去除。
2.2. 反应后溶解态铁和pH随O2/nZVI摩尔比的变化
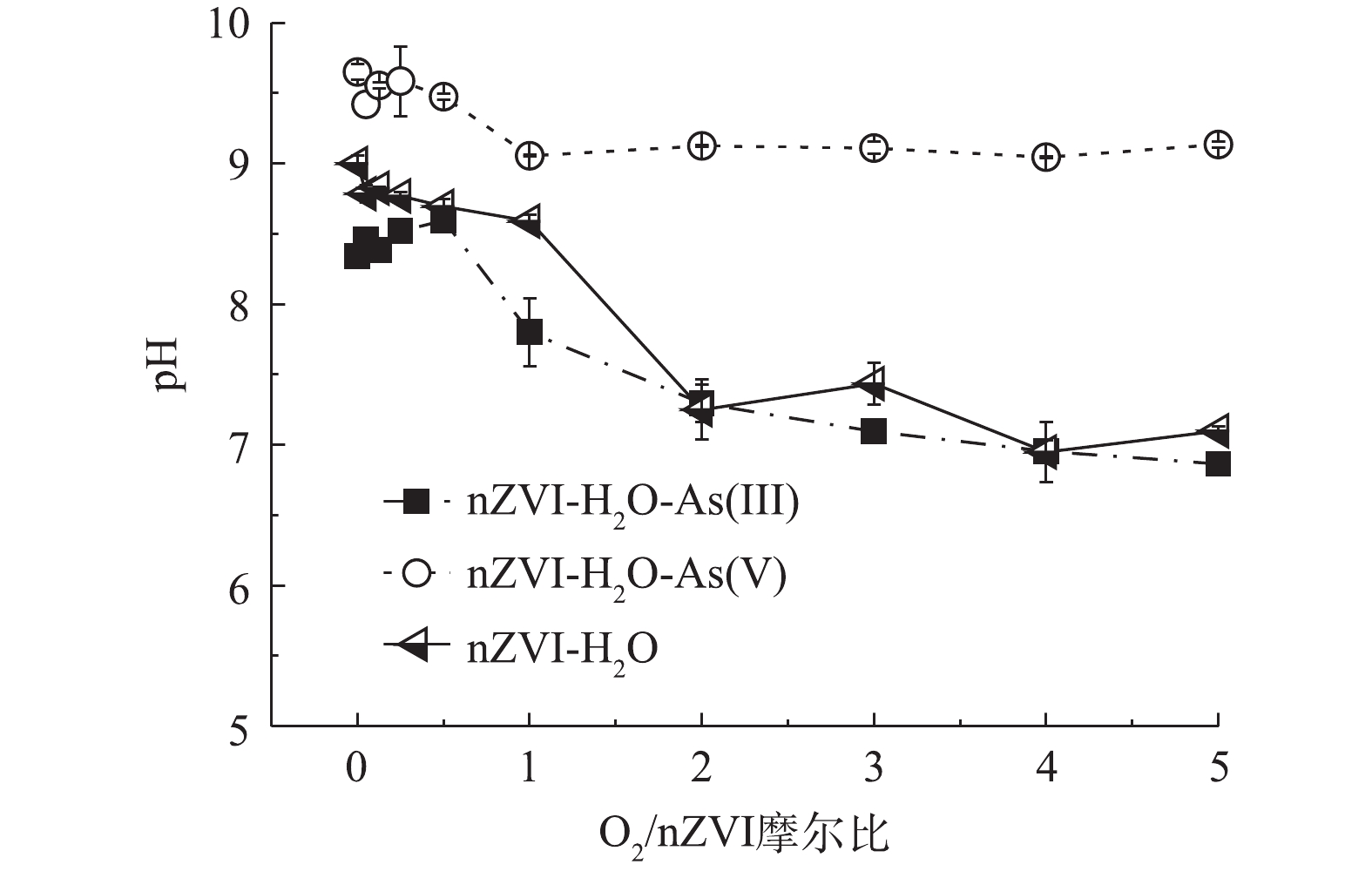
图3是反应达到平衡后不同氧含量条件对溶解态铁释放的影响。如图3(a)所示,As(Ⅲ)体系中,随着O2/nZVI摩尔比增加溶解态铁浓度呈现先上升后降低的趋势,在O2/nZVI摩尔比为0~0.5时主要溶出Fe(Ⅱ),且浓度较低(0.86~2.99 mg·L?1);在O2/nZVI摩尔比为1.0时总铁浓度达到最高(15.89 mg·L?1),且以Fe(Ⅲ)为主。从图3(b)可以看出,As(Ⅴ)体系与As(Ⅲ)体系的溶解态铁整体变化趋势一致,在O2/nZVI摩尔比为1.0时总铁浓度达到最高(9.87 mg·L?1)。化学反应式(1)~式(5)解释了溶解态铁浓度变化的原因[20-22]:在无氧条件下nZVI发生自发腐蚀反应(式(1));低氧条件下发生氧腐蚀溶出少量Fe(Ⅱ)(式(2)),且伴随反应式(3)和式(4)的发生,最终仍产生Fe(Ⅱ);中氧和高氧条件下溶解态铁浓度进一步增加,随着反应(式(4))的进行最终产生Fe(Ⅲ)为主的溶解态铁。其中在中氧条件下过多铁离子溶出,溶解态铁对砷无去除效果从而使除砷效果降低。但在高氧条件下溶出的铁离子浓度远低于中氧条件,推测在过量氧气的作用下铁离子进一步转化为(羟基)氧化铁,增强砷的去除效果[13, 23]。图4是不同氧含量条件下nZVI除砷时溶液pH的变化趋势。在整个氧含量范围内,As(Ⅲ)体系溶液pH在7.0~8.5,As(Ⅴ)体系溶液pH>9.0。砷的存在形态与pH有关,对于As(Ⅲ),
2.3. 产物表征结果
1) SEM与XRD表征结果。图5是新制备nZVI及不同氧含量条件下nZVI-H2O、nZVI-H2O-As(Ⅲ)和nZVI-H2O-As(Ⅴ)体系反应产物的SEM图。为进一步分析不同氧含量下各个体系所生成的产物类型,对其进行了XRD表征分析(图6)。结合SEM与XRD表征可知,新制备的nZVI颗粒呈球形,粒径在50~100 nm,整体呈链条状,存在团聚现象(图5(a)),在衍射角2θ为44.67°处有较宽的衍射峰,对应体心立方结构α-Fe的(110)晶面,这说明制备的是结晶性较差的零价铁(图6(a))。厌氧条件下各体系铁核发生不同程度的溶解,反应后的产物仍然以零价铁为主。对于nZVI-H2O体系随着氧含量的增加产物形貌由扁片状向粗糙粒状、片状及针状结构转化(图5(b))。在低氧和中氧条件下生成的主要产物为磁铁矿/磁赤铁矿[21, 26](式(5)~式(9)),在高氧条件下生成磁铁矿/磁赤铁矿、纤铁矿、针铁矿等多相混合物[27-28],反应见式(10)~式(12),结果见图6(a)。nZVI-H2O-As(Ⅲ)/As(Ⅴ)体系在有氧条件下的产物形貌和结构与nZVI-H2O体系存在明显差异。其中在低氧条件下均呈片状褶皱结构,在中氧和高氧条件下产物形貌为均为粗糙片状和颗粒絮状(图5(c)和图5(d)),形貌的变化与生成沉淀或新的物相有关,但XRD结果表明无明显晶型(羟基)氧化铁的峰(图6(b)和图6(c))。这是由于As(Ⅲ)/As(Ⅴ)与Fe(O, OH)6之间存在很强的亲和力,在(羟基)氧化铁生成过程中,砷会破坏Fe-O-Fe键从而抑制铁矿物例如针铁矿、纤铁矿和磁铁矿晶体的形成[29-30]。同时As(Ⅴ)/As(Ⅲ)可加速Fe(Ⅲ)水解形成无定型铁矿物如水铁矿,并干扰无定型铁矿物向其他晶态铁矿物转变过程中的晶体成核和生长[31]。有氧条件下氧气可促进nZVI氧化为Fe(Ⅱ)和Fe(Ⅲ)(式(2)和式(4))并进一步在氧化或水解作用下形成无定型(羟基)氧化铁从而促进砷的去除[23]。其中低氧条件下产物仍存在明显α-Fe衍射峰,且除砷效果比厌氧条件好,这说明nZVI经少量氧化生成的无定型铁矿物可促进砷的去除;而在中氧条件下α-Fe衍射峰变弱,且砷去除率降低,结合图3可知,由于溶液中大量铁离子溶出,且溶解态的铁对砷无去除效果所致;在高氧条件下α-Fe衍射峰消失,溶液中铁离子浓度减少,这说明溶出铁离子与氧气反应生成较多无定型铁矿物,再次增强了砷的去除率(图6(b)和图6(c))。
2) XPS表征结果。采用XPS手段进一步分析氧气对nZVI除砷的影响机制,利用分峰软件avantage5.52对表征结果进行分析(表1)。在nZVI-H2O-As(Ⅲ)/As(Ⅴ)体系中,反应产物仍然分别以As(Ⅲ)和As(Ⅴ)为主。随着氧含量的增大,固体表面As(Ⅲ)比例逐渐减小,As(Ⅴ)比例逐渐增大,As(0)比例逐渐减小,说明nZVI除砷的机理除吸附外,还同时存在砷的氧化和还原作用,且随着氧含量增加抑制了砷的还原,促进了砷的氧化。此外,随着氧含量的增加,固体表面Fe(Ⅱ)比例逐渐减小,Fe(Ⅲ)比例逐渐增大,Fe(0)比例逐渐减小,证实了氧气的存在促进了nZVI的氧化,并形成新的铁矿物,进而影响对砷的吸附行为。
2.4. 氧气促进nZVI除砷的作用机制
在厌氧和低氧条件下,nZVI-H2O-As(Ⅴ)/As(Ⅲ)体系中产生浓度较低的溶解态Fe(Ⅱ),XRD表征结果表明,反应产物只有α-Fe的衍射峰,说明固体中主要成分仍然以nZVI为主且溶解至游离态程度较低。XPS表征结果表明,固体表面砷的形态以As(Ⅴ)、As(Ⅲ)和As(0)这3种形式存在,这说明nZVI仍然保持着核壳结构所具有的特殊作用。铁核具有还原能力,nZVI氧化还原电位为?0.447 V(Fe(0)/Fe(Ⅱ)),As(Ⅴ)反应至As(0)所需电位为0.449 V,nZVI将As(Ⅴ)还原至As(0)在热力学上是可行的[32-33]。As(Ⅲ)和As(Ⅴ)反应体系产物固体表面分别以As(Ⅲ)和As(Ⅴ)为主,这说明nZVI除砷主要是以吸附作用为主,砷与铁氧化物表面吸附位点上的OH2/OH?进行配体交换,通常在表面形成以双齿双核为主的内球络合物[34-35]。XRD与XPS表征结果表明,在厌氧和低氧条件下的反应产物并无较大的差异,固体表面Fe(0)含量略微下降,Fe(Ⅲ)含量略微增加。但低氧条件下nZVI除砷速度(图1(a)和图1(b))明显比厌氧条件下快,且砷的去除率(图2)也明显比厌氧条件下高,这与nZVI少量氧化有关。综合SEM与XPS结果说明在低氧条件下,nZVI的少量氧化造成其微观形貌的改变,所形成的少量无定型(羟基)氧化铁提升了对砷的吸附作用,使砷的去除率增加。在中氧和高氧条件下,XPS结果显示固体表面砷和铁各价态含量所占比例大致相当(表1)。nZVI-H2O-As(Ⅲ)体系主要以As(Ⅲ)和As(Ⅴ)为主,nZVI-H2O-As(Ⅴ)体系主要以As(Ⅴ)为主,且无As(0),说明在有氧条件下nZVI除砷主要是以吸附和氧化为主。固体表面Fe(Ⅲ)明显增加,且XRD结果显示α-Fe的衍射峰变弱或消失且没有明显衍射峰,说明nZVI被大量氧化,氧气氧化nZVI为溶解态Fe(Ⅱ)/Fe(Ⅲ),铁离子在氧气的作用下进一步被氧化形成无定型或弱晶型铁矿物,这些铁矿物可起到除砷的作用,此外As(Ⅲ)/As(Ⅴ)也可在新的铁矿物形成的过程中被掺杂固定下来[36]。其中在中氧条件下,氧气促进nZVI氧化为大量溶解态Fe(Ⅱ)/Fe(Ⅲ)(图3),溶解态铁对砷无去除效果,从而降低砷的去除效果;而在高氧条件下溶解态铁Fe(Ⅱ)/Fe(Ⅲ)在足量氧气的作用下进一步被氧化为铁矿物,增强了对砷的去除效果。
高氧条件生成更多铁矿物促进砷的去除,但该条件下nZVI对砷的去除率比低氧条件的最大去除率低,原因可能为低氧条件下nZVI氧化在其表面生成无定型水铁矿,无定型水铁矿均匀分布在nZVI表面,比表面积较大,因此对砷具有较强的吸附能力。而高氧条件下nZVI大量氧化生成了结构更有序的铁矿物,研究表明,随着非晶铁矿物向晶态铁矿物的转变,产物比表面积和吸附位点密度降低,从而降低了其对砷的吸附量[37-38]。
2) nZVI除砷的作用机制包括砷的吸附、还原和氧化等,不同氧气含量对nZVI的氧化程度及除砷效果具有较大的影响。在低氧条件下,nZVI少量氧化生成的无定型铁矿物促进了砷的去除;在中氧条件下,nZVI氧化生成较多的溶解态铁,溶解态铁对砷无去除效果,从而造成砷的去除率的下降;在高氧条件下,溶解态铁被足量氧气进一步氧化为无定型铁矿物,增强了对砷的去除效果。
3)传统铁氧化物对As(Ⅴ)具有良好的去除效果,去除As(Ⅲ)时需先将其氧化为As(Ⅴ)再进行吸附处理。在相同的实验条件下,nZVI对As(Ⅲ)的去除效果好于As(Ⅴ),可实现As(Ⅲ)的直接高效去除,因此,将nZVI作为修复剂应用于主要以As(Ⅲ)污染为主的地下水修复中更具优势。
参考文献

 下载:
下载: 



 点击查看大图
点击查看大图