全文HTML
--> --> --> 随着我国工业化的快速发展,工业废水的排放量在显著增长[1]。尤其是煤化工、石化、制药等废水处理不当,将会对环境造成严重的危害。在煤化工、石化和制药废水中,酚类化合物是一类常见的有机污染物,其中的间甲酚因其具有较大的毒性和强烈的腐蚀性,对生物体具有直接或潜在的危害,其直接进入人体会引起蛋白质的团聚和变性,同时抑制环氧化酶的活性和血小板的凝结,进而影响中枢神经系统[2],间甲酚已被多国环保机构列入优先控制污染物的名单中。因此,对含有间甲酚废水的处理、转化和降解的研究已引起广泛的关注[3-6]。常用的间甲酚废水处理方法有生化法[7]、吸附法[8]和高级氧化法(催化臭氧氧化[9]、电催化氧化[10]、催化过氧化氢氧化[11])。由于传统方法对间甲酚的去除效果并不理想,高级氧化方法(advanced oxidation processes)被认为是处理间甲酚废水最常用的方法。众所周知,臭氧对有机污染物具有较强的降解和矿化能力[12]。催化臭氧氧化技术的核心为催化剂,目前,在催化臭氧氧化中常用的非均相催化剂有活性炭类、活性金属铁、金属氧化物、分子筛和天然矿物等[13-14]。在这些非均相催化剂的作用下,臭氧可以更有效地和有机物发生反应,以实现污染物的降解和矿化。然而上述非均相催化剂在应用于催化臭氧氧化反应时,仍存在一定问题,如催化剂稳定性不够、活性组分易流失、催化效率不高等[15]。
钙钛矿类混合金属氧化物具有明确的晶体结构,通用单元分子式为ABO3,式中的A表示稀有或碱土金属,B表示过渡金属,因其结构的复杂性和多样性,可作为催化剂应用于各类催化反应中,如光催化、燃料电池、三效催化剂、VOCs的治理和催化湿式过氧化物的氧化等[16]。这种催化剂在高温和腐蚀性介质中是稳定的,同时B位元素位于晶体结构的中心,可以防止活性组分的流失,这对于钙钛矿催化剂的活性和结构稳定性至关重要。笔者前期的研究发现,钙钛矿材料也可被用于催化臭氧氧化技术中,许多****也已经证明了钙钛矿在催化臭氧氧化反应中有着较好的活性[15, 17-20]。RIVAS等[21]将LaTi0.15Cu0.85O3用于催化臭氧氧化丙酮酸的实验中,在重复使用3次后,其活性甚至有所提高。因此,有必要进一步研究钙钛矿型催化剂在降解污染物中的应用情况。锆酸钙(CaZrO3)复合材料是一种重要的钙钛矿材料,常应用于发光材料[22-23]、湿度传感器[24]、陶瓷电容器[25]和超高温下的保护材料[26]等。与其他钙钛矿氧化物不同,有关其在催化臭氧氧化反应中的研究较少。因此,有必要对CaZrO3复合材料在废水处理中的应用及催化机制进行详细研究,以便更深入地了解其催化性能。
本研究采用共沉淀法制备了一系列Ca-Zr复合材料,在不同焙烧温度下,对复合材料进行了焙烧。鉴于制备方法对钙钛矿的结构性质和相纯度的决定性影响,考察了合成条件(主要是焙烧温度)对降解间甲酚催化性能的影响,且将使用XRD、SEM、TEM等表征手段对不同焙烧温度下制备的Ca-Zr复合材料催化剂的结构、形貌和组分进行了分析,对其在催化臭氧氧化中的催化活性、机理和稳定性进行阐释。
1.1. 实验原料
所用实验药品为ZrOCl·8H2O、CaCl2、(NH4)2C2O4·H2O、NH3·H2O(25%~28%)。钙锆比例设置为1.1∶1,称取3.631 g ZrOCl·8H2O和1.418 5 g CaCl2溶于100 mL超纯水中,将 (NH4)2C2O4·H2O溶于5 mL氨水中,同时,添加0.1 g聚乙二醇(PEG)。将上述2种溶液混合后,可产生白色乳胶状沉淀,充分反应后,在磁力搅拌器上搅拌1 h,将反应后的溶液在100 ℃下进行24 h老化,再进行真空抽滤,并用去离子水洗涤3次。将过滤后的沉淀于120 ℃下干燥12 h,之后在700~1 200 ℃下焙烧4 h,制备得到Ca-Zr复合材料。1.2. 催化剂表征
X-射线衍射测试采用德国布鲁克X射线衍射仪(Bruker D8 Focus型),CuKa辐射源,宽角度扫描范围2θ=10°~90°;使用Quante400F(FEI)场发射扫描电镜仪表征催化剂的样品形貌,工作电压为20 kV;使用WRT-1D微机热天平进行样品热重分析,温度为25~1 200 ℃;采用美国麦克仪器公司生产的Chemisorb 2720型脉冲化学吸附仪对催化剂的还原特性进行表征,称取0.10 g的催化剂样品于石英管中,在Ar气流中预处理15 min,由室温升至300 ℃后,保温30 min,然后降至室温,切换为10% H2/Ar混合气体,混合气流速为20 m2·min?1,待基线平稳后,以10 ℃·min?1升温至1 000 ℃,由热导池检测器检测出耗氢信号;采用高分辨透射电镜(Jeol 2100F HRTEM)进行样品的形貌表征;采用VG Scientific ESCA-3000光谱仪上进行X射线光电子能谱(XPS)表征。1.3. 催化剂评价
在250 mL的间歇反应器中进行催化臭氧氧化反应。反应过程中每隔2 min取样1.5 mL,连续取样10次,使用0.45 μm滤膜过滤后,测定出水的间甲酚浓度。间甲酚浓度采用大连依利特分析仪器有限公司生产的HPLC-P1201型高效液相色谱进行分析,所用色谱柱为C18色谱柱(4.6 mm×250 mm,5 μm),流动相为甲醇∶水=80∶20(体积比),流速为1.0 mL·min?1,检测波长为272 nm。2.1. 物相分析(XRD)
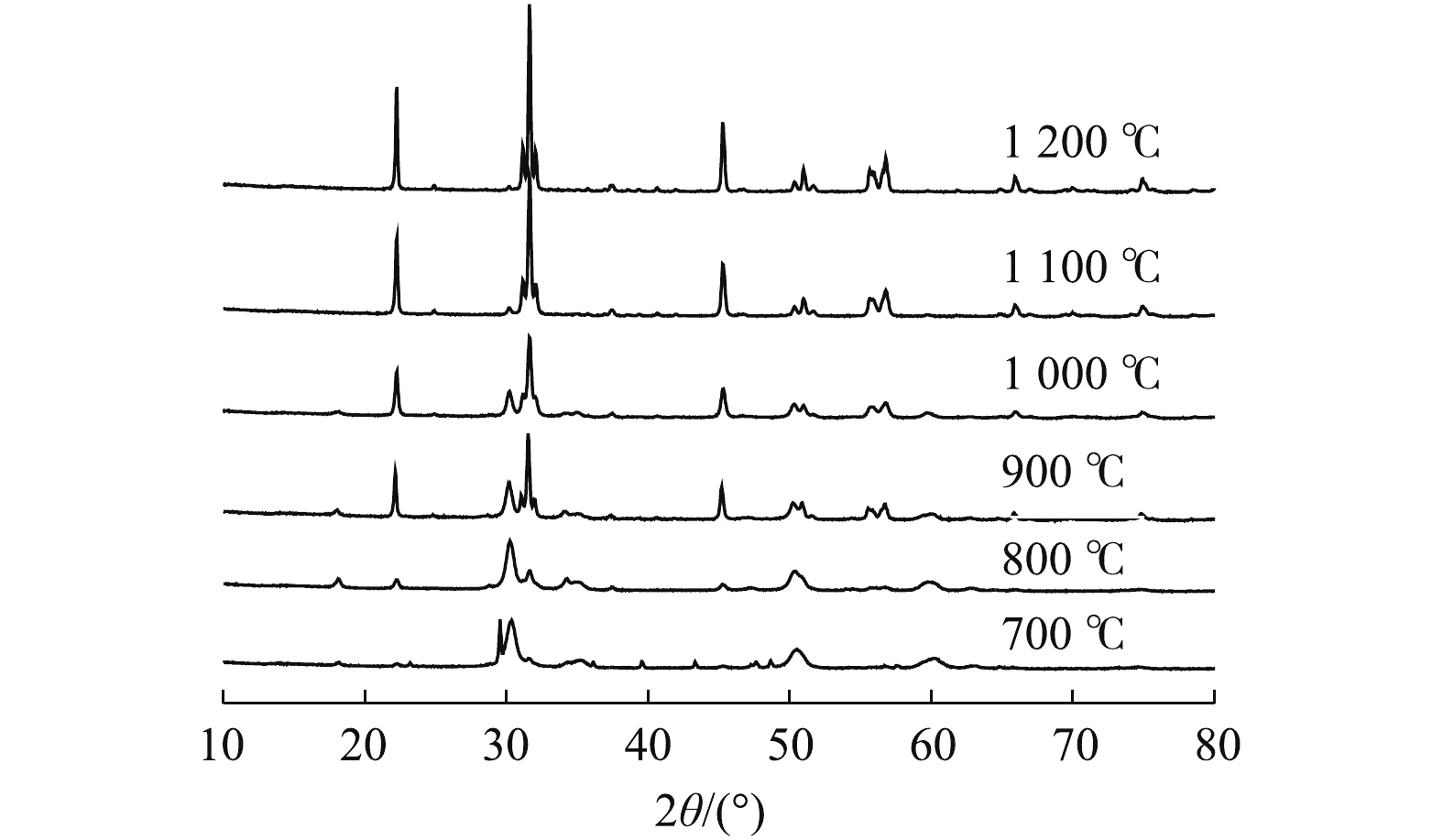
图1为在不同温度下制备出的样品的XRD表征结果。由图1可知,所合成的催化剂为典型的斜方晶系锆酸钙材料,且锆酸钙衍射峰强度随着焙烧温度的升高不断增强。此外,材料中还残存少量立方晶系的ZrO2晶体和六方晶系的Ca(OH)2晶体。结果表明,在不同焙烧温度下所得到的样品晶型有显著的变化,当焙烧温度升高到1 000 ℃以上时,所制备出的样品晶型较纯,为斜方晶系CaZrO3(PDF#35-0790)。2.2. 形貌(SEM)结果分析
图2为在不同焙烧温度下所制得样品的形貌表征结果。由图2(a)可知,当焙烧温度为700 ℃时,样品颗粒近球形,大小不均匀,粒径尺寸较大,结合图1可知,这些大的颗粒为ZrO2;当焙烧温度达到800 ℃时,样品颗粒有变小的趋势(图2(b));当升高焙烧温度达到1 100 ℃以上时(图2(e)),颗粒形状大小变得更加均匀。结合物相分析结果,当焙烧温度在1 000 ℃以上时,样品组分主要为CaZrO3,由此可知,所制备的催化剂大小均一,且分散均匀。2.3. 热重分析(TG-DTA)
图3为干燥后未焙烧的样品的热重-差热分析结果。由图3可知,TG曲线上有3次较为明显的失重,对应的DTA曲线在100 ℃左右出现1个小的吸热峰,这主要是由于样品中水的脱附引起的[27- 28]。在200 ℃左右出现的峰可以归因为样品失掉结晶水和CaC2O4转化成CaCO3所引起的;在490 ℃左右出现的峰可能是由无定形的ZrO2转化成介稳态的ZrO2导致的;在850 ℃左右出现的峰是稳定态的ZrO2与CaCO3在高温下反应生成CaZrO3。综上所述,在焙烧过程中温度的控制尤为重要,样品焙烧温度应不能低于800 ℃,才能合成结晶良好的CaZrO3,这与XRD谱图中所显示的物相变化趋势相一致。2.4. 程序升温还原(H2-TPR)分析
为了探究在催化臭氧氧化过程中臭氧对样品的影响,使用O3对样品进行了预处理(吹扫3 h),并对其进行H2-TPR分析(图4)。由图4所知,在700 ℃和800 ℃制备的样品所对应的还原峰在700~800 ℃,这可能是由于样品中的CaCO3还原导致的;当焙烧温度大于900 ℃时,样品还原峰位置均在600~700 ℃,这可能由于样品中的CaO还原导致的。经臭氧预处理后的样品与未经预处理的样品在被还原时,虽然在耗氢量上有一定的差别,但其还原峰位置并未发生改变。由此可以推测,所制备样品结构具有较强的稳定性。由BRIK等[29]所做的第一性原理计算结果也证实了此结论。2.5. 焙烧温度对催化臭氧氧化间甲酚的影响
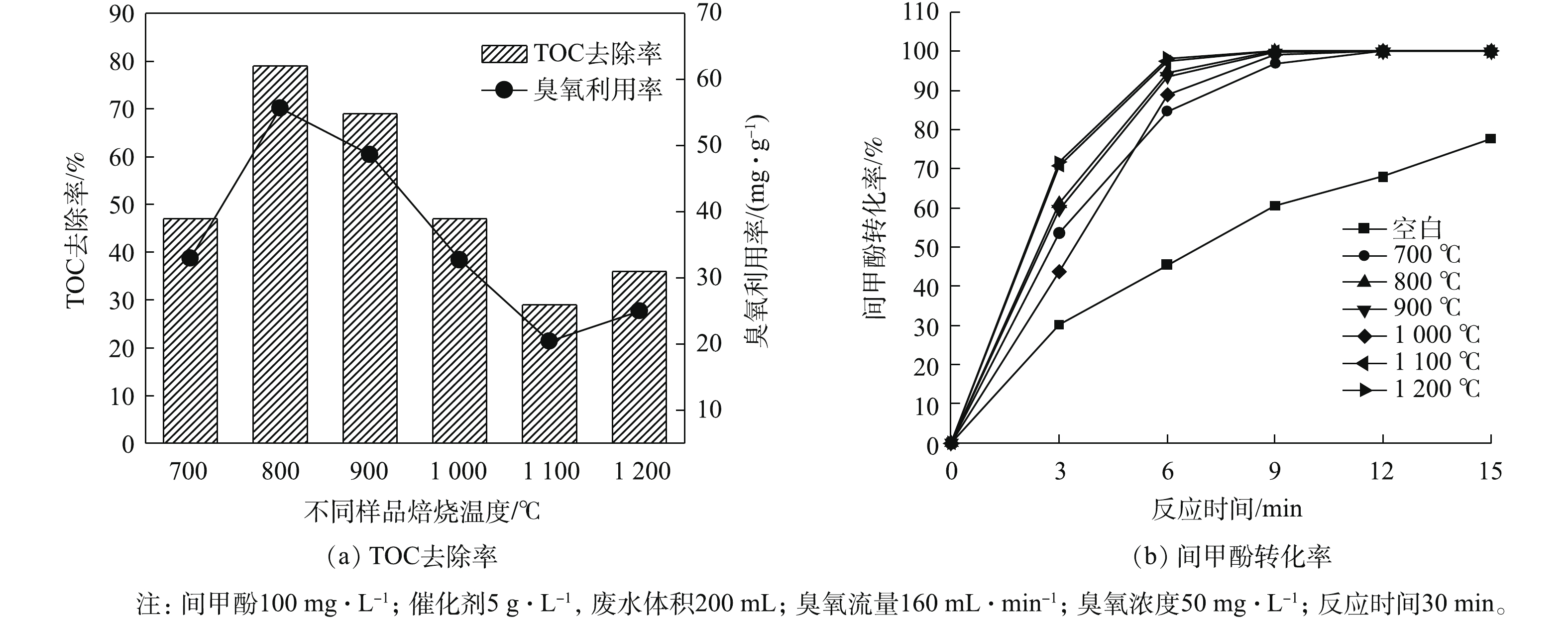
通过对样品的一系列表征发现,焙烧温度对催化剂的表面结构和性质具有显著的影响。为探究焙烧温度对催化剂催化臭氧氧化活性的影响,以间甲酚为底物,使用催化臭氧氧化方法对催化效果进行了评价,结果如图5所示。由图5可知,所合成的样品具有明显的催化活性,且随焙烧温度的升高,催化剂活性呈现出先升高后降低的趋势。其中单独臭氧反应TOC去除率为15%,臭氧利用率在10%左右。在焙烧温度为800 ℃时,TOC的去除率达到了79%,同时臭氧的利用率与TOC的去除率变化趋势相一致。即使对TOC去除率最低的样品也优于单独臭氧反应体系所对应的去除率。结合图1可知,当焙烧温度为800 ℃时,认为样品为ZrO2、Ca(OH)2和CaZrO3的复合材料,可能是三者的共同作用导致材料具有较高的催化活性。当焙烧温度升高到1 100 ℃时,CaZrO3晶型占比升高,Ca(OH)2晶型消失。通过催化臭氧氧化间甲酚结果可知其TOC的去除率有所下降,说明ZrO2和CaZrO3的复合材料不利于催化臭氧氧化反应。在1 200 ℃下焙烧的样品相较于1 100 ℃焙烧样品TOC去除率有所上升,可能样品中ZrO2含量的降低有利于臭氧反应。在催化臭氧氧化反应过程中,间甲酚转化率结果如图5(b)所示。由图5(b)可知,相较于单独的臭氧反应,加入样品均有利于间甲酚的催化氧化,效果最佳的为在1 100 ℃ 和1 200 ℃下焙烧的样品,这说明CaZrO3在间甲酚转化方面表现优异。在800 ℃和900 ℃下焙烧出的复合材料对间甲酚的转化率仅次于以上2种条件下所制备的样品,说明Ca-Zr复合材料对间甲酚的转化表现出较好的性能。2.6. 高分辨透射电镜(TEM)
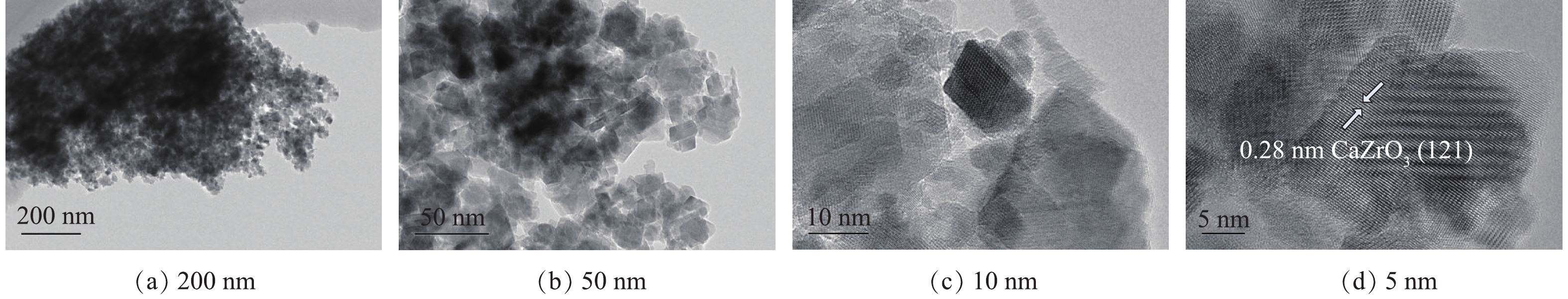
由于在800 ℃焙烧出的样品催化臭氧氧化活性最佳,为进一步确定该材料的表面形貌,使用高分辨透射电镜(HRTEM)对该条件下的样品进行表征,结果见图6。由图6(a)和6(b)可知,所制备复合材料样品由许多不规则的纳米颗粒组成。通过对选定的区域进行观察,发现了纳米颗粒的晶格图像(图6(c)和图6(d)),经过分析计算,样品具有0.29 nm的特征晶格间距,这说明样品的高暴露晶面为CaZrO3的(121)晶面。2.7. X射线光电子能谱分析(XPS)
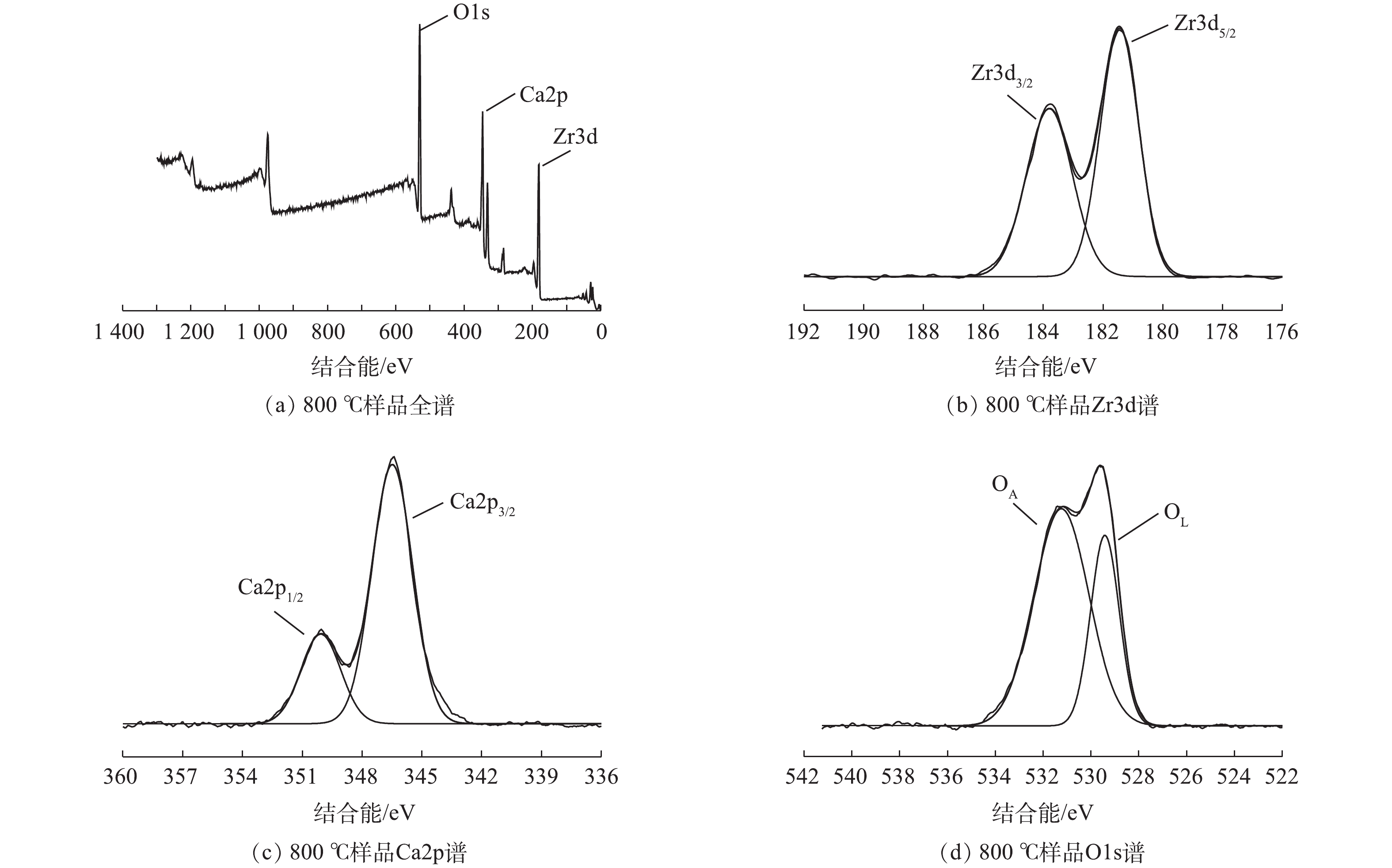
为了进一步探究所制备样品表面的元素状态,对其进行XPS表征,结果见图7。将Zr3d图谱解卷积成2个峰,其结合能分别为183.7 eV和181.4 eV,其分别对应Zr3d3/2和Zr3d5/2,这是由CaZrO3中的阳离子Zr4+引起的。将Ca2p图谱解卷积成2个峰,其结合能分别为350.0 eV和346.5 eV,其分别对应Ca2p1/2和Ca2p3/2。这些峰是由于Ca2+氧化态所导致的[30]。样品O1s图谱分别在结合能为529.4 eV和531.1 eV处出现峰值,分别归因于表面晶格氧(OL)、表面吸附氧(OA)或表面羟基(OH)物种[15, 31]。晶格氧和表面羟基在氧化反应中起重要作用。晶格氧失去电子,被氧化成O2并形成氧空穴,在富氧状态下,氧空穴导电被还原成晶格氧,两者之间的循环转变确保了催化活性[32]。另一方面,可能是由于表面羟基基团在起重要作用。2)以间甲酚的催化臭氧氧化降解反应作为探针反应,对合成出的催化剂性能进行评价。结果表明,所制备催化剂显示出较好的催化活性,焙烧温度在800 ℃时,所得样品TOC的去效果最优,去除率达到79%以上。
3)对800 ℃焙烧的样品进行TEM分析,结果显示,样品由纳米颗粒组成,其晶格间距为0.29 nm,即高暴露晶面为CaZrO3(121)晶面。对样品进行XPS表征,分析其表面的化学组成,结果显示,在催化臭氧氧化过程中,晶格氧与表面羟基物种起到重要的作用。
参考文献

 下载:
下载: 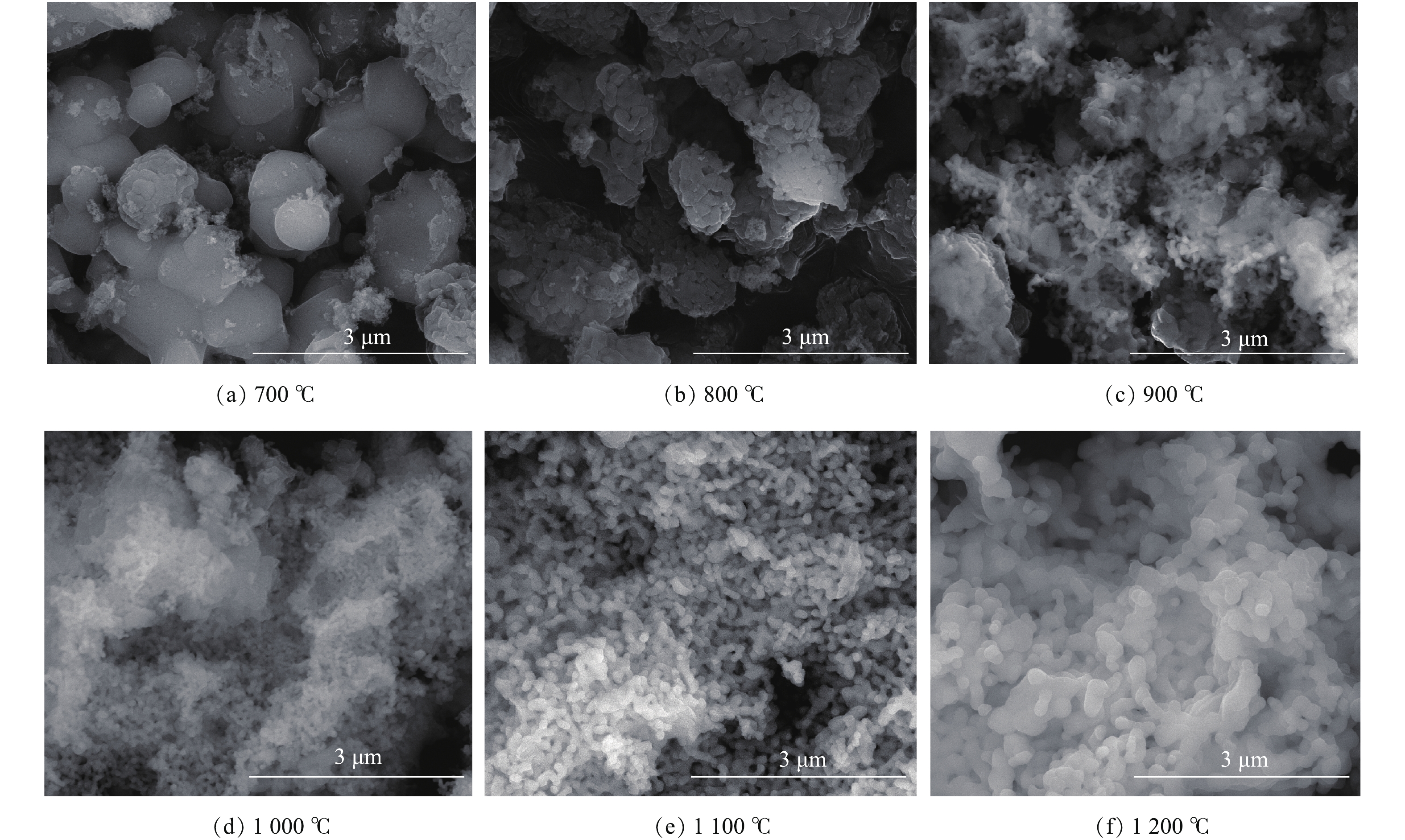


 点击查看大图
点击查看大图