 )
) 1河南大学认知、脑与健康研究所, 教育科学学院, 河南省心理与行为研究所, 开封 475004
2新乡医学院第二附属医院, 河南省精神病医院, 新乡 453002
3河南省生物精神病学重点实验室, 新乡医学院, 新乡 453002
收稿日期:2020-11-09发布日期:2021-04-25通讯作者:王慧颖E-mail:wanghuiyingwing@foxmail.com基金资助:教育部人文社会科学研究项目(20YJC190019);河南省生物精神病学重点实验室开放课题(ZDSYS2019009);河南省医学科技攻关计划联合共建项目(2018020375)Propranolol rescued secondary trauma induced by immediate extinction
WANG Hongbo1, XING Xiaoli1, WANG Huiying2,3( )
) 1Institute of Cognition, Brain and Health; School of Educational Science; Henan Key Lab of Psychology and Behavior, Henan University, Kaifeng 475004, China
2Henan Mental Hospital, the Second Affiliated Hospital of Xinxiang Medical University, Xinxiang 453002, China
3Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang 453002, China
Received:2020-11-09Published:2021-04-25Contact:WANG Huiying E-mail:wanghuiyingwing@foxmail.com摘要/Abstract
摘要: 恐惧消退能力的受损是创伤后应激障碍(posttraumatic stress disorder, PTSD)的标志之一。以往的研究表明, 相较于延迟消退, 恐惧获得后短时间内进行的消退训练不能形成长时程的消退记忆, 这一现象被称为即刻消退缺损。然而, 目前并不清楚这种缺损是一次性的, 还是会继续影响其后的重消退。实验1中, 大鼠在恐惧习得后1小时(即刻消退)或24小时(延迟消退)开始进行消退训练, 24小时后进行重消退, 再24小时后进行消退记忆的测试。结果显示, 与延迟消退相比, 即刻消退效果缺损, 并引起第二天的重消退也出现效果缺损。实验2中, 恐惧获得后立即给予大鼠盐水或β-肾上腺素受体阻断剂普萘洛尔(10 mg/kg, i.p.), 然后测试即刻消退和重消退的效果。结果显示, 普萘洛尔虽没有阻止即刻消退的缺损, 但避免了重消退出现缺损。总之, 严重创伤后的即刻消退不但无法有效抑制恐惧反应, 还可能造成二次创伤, 会损害恐惧消退能力, 而普萘洛尔可修复即刻消退引起的重消退缺损。这些结果将有助于我们加深对PTSD病理机制和早期干预后果的认识。
图/表 2

图1即刻消退引发重消退效果缺损 注: **p < 0.01, #p = 0.06误差线为标准误(SE)
 图1即刻消退引发重消退效果缺损 注: **p < 0.01, #p = 0.06误差线为标准误(SE)
图1即刻消退引发重消退效果缺损 注: **p < 0.01, #p = 0.06误差线为标准误(SE) 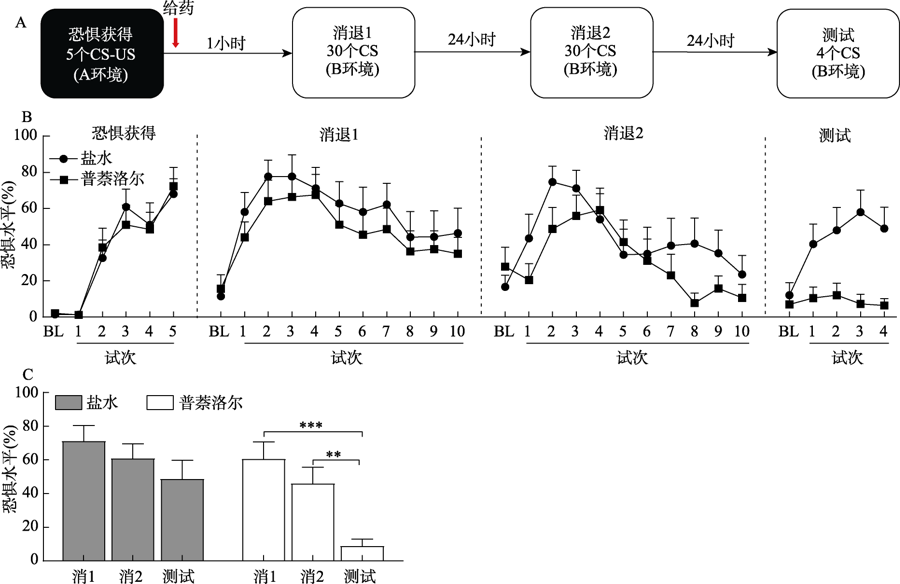
图2普萘洛尔修复即刻消退引发的重消退缺损 注: **p < 0.01, ***p < 0.001, 误差线为标准误( SE)
 图2普萘洛尔修复即刻消退引发的重消退缺损 注: **p < 0.01, ***p < 0.001, 误差线为标准误( SE)
图2普萘洛尔修复即刻消退引发的重消退缺损 注: **p < 0.01, ***p < 0.001, 误差线为标准误( SE) 参考文献 46
| [1] | Argolo, F.C., Cavalcanti-Ribeiro, P., Netto, L.R., & Quarantini, L.C. (2015). Prevention of posttraumatic stress disorder with propranolol: A meta-analytic review. Journal of Psychosomatic Research, 79(2),89-93. doi: 10.1016/j.jpsychores.2015.04.006URL |
| [2] | American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders(DSM-5?). American Psychiatric Pub. |
| [3] | Borodovitsyna, O., Joshi, N., & Chandler, D. (2018). Persistent stress-induced neuroplastic changes in the locus coeruleus/ norepinephrine system. Neural Plasticity, 2018,1892570. doi: 10.1155/2018/1892570pmid: 30008741 |
| [4] | Cain, C.K., Blouin, A.M., & Barad, M. (2004). Adrenergic transmission facilitates extinction of conditional fear in mice. Learning & Memory, 11(2),179-187. |
| [5] | Careaga, M.B.L., Girardi, C.E.N., & Suchecki, D. (2016). Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neuroscience & Biobehavioral Reviews, 71,48-57. doi: 10.1016/j.neubiorev.2016.08.023URL |
| [6] | Chalkia, A., Weermeijer, J., van Oudenhove, L., & Beckers, T. (2019). Acute but not permanent effects of propranolol on fear memory expression in humans. Frontiers in Human Neuroscience, 13,51. doi: 10.3389/fnhum.2019.00051URL |
| [7] | Chang, C.H., & Maren, S. (2009). Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learning & Memory, 16(1),62-68. |
| [8] | Chang, C.H., & Maren, S. (2011). Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learning & Memory, 18(4),221-225. |
| [9] | Cho, J.H., Deisseroth, K., & Bolshakov, V.Y. (2013). Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron, 80(6),1491-1507. doi: 10.1016/j.neuron.2013.09.025URL |
| [10] | Dunsmoor, J.E., Kroes, M.C.W., Moscatelli, C.M., Evans, M.D., Davachi, L., & Phelps, E.A. (2018). Event segmentation protects emotional memories from competing experiences encoded close in time. Nature Human Behaviour, 2(4),291-299. doi: 10.1038/s41562-018-0317-4pmid: 30221203 |
| [11] | Fan, S.J., Jiang, H., Yang, L.-J., Liu, X., Song, J., & Pan, F. (2011). Effects of adrenergic agents on stress-induced brain microstructural and immunochemical changes in adult male Wistar rats. Annals of Anatomy-Anatomischer Anzeiger, 193(5),418-424. doi: 10.1016/j.aanat.2011.06.001URL |
| [12] | Fitzgerald, P.J., Giustino, T.F., Seemann, J.R., & Maren, S. (2015). Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proceedings of the National Academy of Sciences of the United States of America, 112(28),E3729-3737. |
| [13] | Giustino, T.F., Fitzgerald, P.J., & Maren, S. (2016). Revisiting propranolol and PTSD: Memory erasure or extinction enhancement? Neurobiology of Learning and Memory, 130,26-33. doi: 10.1016/j.nlm.2016.01.009pmid: 26808441 |
| [14] | Giustino, T.F., Fitzgerald, P.J., Ressler, R.L., & Maren, S. (2019). Locus coeruleus toggles reciprocal prefrontal firing to reinstate fear. Proceedings of the National Academy of Sciences of the United States of America, 116(17),8570-8575. doi: 10.1073/pnas.1814278116pmid: 30971490 |
| [15] | Giustino, T.F., & Maren, S. (2018). Noradrenergic modulation of fear conditioning and extinction. Frontiers in Behavioral Neuroscience, 12,43. doi: 10.3389/fnbeh.2018.00043pmid: 29593511 |
| [16] | Giustino, T.F., Ramanathan, K.R., Totty, M.S., Miles, O.W., & Maren, S. (2020). Locus coeruleus norepinephrine drives stress-induced increases in basolateral amygdala firing and impairs extinction learning. Journal of Neuroscience, 40(4),907-916. doi: 10.1523/JNEUROSCI.1092-19.2019URL |
| [17] | Giustino, T.F., Seemann, J.R., Acca, G.M., Goode, T.D., Fitzgerald, P.J., & Maren, S. (2017). Beta-adrenoceptor blockade in the basolateral amygdala, but not the medial prefrontal cortex, rescues the immediate extinction deficit. Neuropsychopharmacology, 42(13),2537-2544. doi: 10.1038/npp.2017.89pmid: 28462941 |
| [18] | G?k?ek-Sara?, ?., Wesierska, M., & Jakubowska-Do?ru, E. (2015). Comparison of spatial learning in the partially baited radial-arm maze task between commonly used rat strains: Wistar, Spargue-Dawley, Long-Evans, and outcrossed Wistar/ Sprague-Dawley. Learning & Behavior, 43(1),83-94. |
| [19] | H?lscher, C. (2002). Different strains of rats show different sensitivity to block of long-term potentiation by nitric oxide synthase inhibitors. European Journal of Pharmacology, 457(2-3),99-106. |
| [20] | Huff, N.C., Hernandez, J.A., Blanding, N.Q., & LaBar, K.S. (2009). Delayed extinction attenuates conditioned fear renewal and spontaneous recovery in humans. Behavioral Neuroscience, 123(4),834-843. doi: 10.1037/a0016511URL |
| [21] | Khan, V., Sharma, S., Bhandari, U., Ali, S.M., & Haque, S.E. (2018). Raspberry ketone protects against isoproterenol- induced myocardial infarction in rats. Life Sciences, 194,205-212. doi: 10.1016/j.lfs.2017.12.013URL |
| [22] | Kyriazi, P., Headley, D.B., & Pare, D. (2018). Multi-dimensional coding by basolateral amygdala neurons. Neuron, 99(6),1315-1328.e1315. doi: 10.1016/j.neuron.2018.07.036URL |
| [23] | Maren, S. (2014). Nature and causes of the immediate extinction deficit: A brief review. Neurobiology of Learning and Memory, 113,19-24. doi: 10.1016/j.nlm.2013.10.012URL |
| [24] | Maren, S., & Chang, C.H. (2006). Recent fear is resistant to extinction. Proceedings of the National Academy of Sciences of the United States of America, 103(47),18020-18025. |
| [25] | McCall, J.G., Al-Hasani, R., Siuda, E.R., Hong, D.Y., Norris, A.J., Ford, C.P., & Bruchas, M.R. (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron, 87(3),605-620. doi: 10.1016/j.neuron.2015.07.002URL |
| [26] | McGaugh, J.L. (2000). Memory - a century of consolidation. Science, 287(5451),248-251. doi: 10.1126/science.287.5451.248URL |
| [27] | Merz, C.J., Hamacher-Dang, T.C., & Wolf, O.T. (2016). Immediate extinction promotes the return of fear. Neurobiology of Learning and Memory, 131,109-116. doi: 10.1016/j.nlm.2016.03.013URL |
| [28] | Muravieva, E.V., & Alberini, C.M. (2010). Limited efficacy of propranolol on the reconsolidation of fear memories. Learning & Memory, 17(6),306-313. |
| [29] | Przybyslawski, J., Roullet, P., & Sara, S.J. (1999). Attenuation of emotional and nonemotional memories after their reactivation: Role of beta adrenergic receptors. Journal of Neuroscience, 19(15),6623-6628. pmid: 10414990 |
| [30] | Robinson, M.J.F., & Franklin, K.B.J. (2010). Reconsolidation of a morphine place preference: Impact of the strength and age of memory on disruption by propranolol and midazolam. Behavioural Brain Research, 213(2),201-207. doi: 10.1016/j.bbr.2010.04.056pmid: 20457186 |
| [31] | Rodriguez-Romaguera, J., Sotres-Bayon, F., Mueller, D., & Quirk, G.J. (2009). Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biological Psychiatry, 65(10),887-892. doi: 10.1016/j.biopsych.2009.01.009pmid: 19246030 |
| [32] | Rothbaum, B.O., Kearns, M.C., Reiser, E., Davis, J.S., Kerley, K.A., Rothbaum, A.O.,… Ressler, K.J. (2014). Early intervention following trauma may mitigate genetic risk for PTSD in civilians: A pilot prospective emergency department study. Journal of Clinical Psychiatry, 75(12),1380-1387. |
| [33] | Sah, P. (2017). Fear, anxiety, and the amygdala. Neuron, 96(1),1-2. doi: 10.1016/j.neuron.2017.09.013URL |
| [34] | Sharp, B.M. (2017). Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Translational Psychiatry, 7(8),e1194. doi: 10.1038/tp.2017.161URL |
| [35] | Siddiqui, S.A., Singh, S., Ranjan, V., Ugale, R., Saha, S., & Prakash, A. (2017). Enhanced histone acetylation in the infralimbic prefrontal cortex is associated with fear extinction. Cellular and Molecular Neurobiology, 37(7),1287-1301. doi: 10.1007/s10571-017-0464-6pmid: 28097489 |
| [36] | Sierra-Mercado, D., Padilla-Coreano, N., & Quirk, G.J. (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36(2),529-538. doi: 10.1038/npp.2010.184pmid: 20962768 |
| [37] | Singewald, N., & Holmes, A. (2019). Rodent models of impaired fear extinction. Psychopharmacology, 236(1),21-32. doi: 10.1007/s00213-018-5054-xpmid: 30377749 |
| [38] | Singh, S., Siddiqui, S.A., Tripathy, S., Kumar, S., Saha, S., Ugale, R.,… Prakash, A. (2018). Decreased level of histone acetylation in the infralimbic prefrontal cortex following immediate extinction may result in deficit of extinction memory. Brain Research Bulletin, 140,355-364. doi: 10.1016/j.brainresbull.2018.06.004URL |
| [39] | Stafford, J.M., Maughan, D.K., Ilioi, E.C., & Lattal, K.M. (2013). Exposure to a fearful context during periods of memory plasticity impairs extinction via hyperactivation of frontal-amygdalar circuits. Learning & Memory, 20(3),156-163. |
| [40] | Taherian, F., Vafaei, A.A., Vaezi, G.H., Eskandarian, S., Kashef, A., & Rashidy-Pour, A. (2014). Propranolol-induced impairment of contextual fear memory reconsolidation in rats: A similar effect on weak and strong recent and remote memories. Basic & Clinical Neuroscience, 5(3),231-239. |
| [41] | Totty, M.S., Payne, M.R., & Maren, S. (2019). Event boundaries do not cause the immediate extinction deficit after Pavlovian fear conditioning in rats. Scientific Reports, 9(1),9459. doi: 10.1038/s41598-019-46010-4URL |
| [42] | van Marle, H. J. F. V., Hermans, E. J., Qin, S., & Fernández, G. (2009). From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66(7),649-655. doi: 10.1016/j.biopsych.2009.05.014pmid: 19596123 |
| [43] | Vervliet, B., Craske, M.G., & Hermans, D. (2013). Fear extinction and relapse: State of the art. Annual Review of Clinical Psychology, 9,215-248. doi: 10.1146/annurev-clinpsy-050212-185542pmid: 23537484 |
| [44] | Wicking, M., Steiger, F., Nees, F., Diener, S.J., Grimm, O., Ruttorf, M.… Flor, H. (2016). Deficient fear extinction memory in posttraumatic stress disorder. Neurobiology of Learning and Memory, 136,116-126. doi: S1074-7427(16)30202-7pmid: 27686278 |
| [45] | Woods, A.M., & Bouton, M.E. (2008). Immediate extinction causes a less durable loss of performance than delayed extinction following either fear or appetitive conditioning. Learning & Memory, 15(12),909-920. |
| [46] | Wright, L.A., Sijbrandij, M., Sinnerton, R., Lewis, C., Roberts, N.P., & Bisson, J.I. (2019). Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: A systematic review and meta-analysis. Translational Psychiatry, 9(1),334. doi: 10.1038/s41398-019-0673-5URL |
相关文章 6
| [1] | 李俊娇, 陈伟, 胡琰健, 曹杨婧文, 郑希付. 预期错误与急性应激对不同强度恐惧记忆提取消退的影响[J]. 心理学报, 2021, 53(6): 587-602. |
| [2] | 胡静初,张蔚欣,陈小婷,王文清,王子洁,庄楚群,冯彪,郑希付. 远期恐惧记忆再巩固更新机制的线索选择性特点[J]. 心理学报, 2019, 51(3): 316-323. |
| [3] | 廖素群;郑希付. 认知重评对负性效价的抑制促进条件性恐惧消退[J]. 心理学报, 2016, 48(4): 352-361. |
| [4] | 徐亮;区诵宜;郑希付;陈婷;冯彪;闫沛. 状态焦虑对条件性恐惧泛化的影响[J]. 心理学报, 2016, 48(12): 1507-1518. |
| [5] | 孙楠,魏艺铭,李倩,郑希付. 条件性恐惧记忆消退返回的性别差异[J]. 心理学报, 2012, 44(3): 314-321. |
| [6] | 李培培,张丽丽,韦美,李敏. 条件性恐惧大鼠边缘下区Cdk5激酶活性、caspase-3表达以及突触结构的变化[J]. 心理学报, 2011, 43(05): 544-552. |
PDF全文下载地址:
http://journal.psych.ac.cn/xlxb/CN/article/downloadArticleFile.do?attachType=PDF&id=4956
