 ,, 莫晓婷, 张霞, 徐妙云, 赵军
,, 莫晓婷, 张霞, 徐妙云, 赵军 ,, 王磊
,, 王磊 ,中国农业科学院生物技术研究所,北京 100081
,中国农业科学院生物技术研究所,北京 100081Isolation and functional characterization of a stress-responsive transcription factor ZmC2H2-1 in Zea mays
Dezhou Wang ,, Xiaoting Mo, Xia Zhang, Miaoyun Xu, Jun Zhao
,, Xiaoting Mo, Xia Zhang, Miaoyun Xu, Jun Zhao ,, Lei Wang
,, Lei Wang ,Biotechnology Research Institute, Chinese Academy of Agricultural Science, Beijing 100081, China
,Biotechnology Research Institute, Chinese Academy of Agricultural Science, Beijing 100081, China通讯作者:
编委: 严建兵
收稿日期:2018-05-4修回日期:2018-06-28网络出版日期:2018-09-20
| 基金资助: |
Editorial board:
Received:2018-05-4Revised:2018-06-28Online:2018-09-20
| Fund supported: |
作者简介 About authors
汪德州,博士研究生,专业方向:生物化学与分子生物学E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1602KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
汪德州, 莫晓婷, 张霞, 徐妙云, 赵军, 王磊. 玉米逆境响应相关转录因子ZmC2H2-1基因克隆及功能验证 [J]. 遗传, 2018, 40(9): 767-778 doi:10.16288/j.yczz.18-119
Dezhou Wang, Xiaoting Mo, Xia Zhang, Miaoyun Xu, Jun Zhao, Lei Wang.
玉米(Zea mays L.)原产自中南美洲,目前世界各地均有栽培。2016年,我国玉米的种植面积达到3676万公顷,已成为第一大农作物,提高玉米的抗逆性对进一步提高玉米产量具有重要意义。逆境胁迫影响植物生长发育和作物产量,对相关基因的挖掘和鉴定有助于进一步研究植物抵御胁迫的机制[1]。目前在植物中已经鉴定了许多包含锌指结构域的蛋白,这些蛋白多数是能结合DNA的转录因子,并广泛参与基因的转录、翻译、蛋白质折叠及染色质修饰等过程,主要功能是调控基因的表达[2]。其中,C2H2型锌指蛋白与植物抗逆性密切相关[3]。
锌指蛋白(zinc finger protein,ZFP)因其具有指状结构域且能结合Zn2+而得名,主要包括C2H2与C3H家族。根据半胱氨酸(C)和组氨酸(H)残基的数目和位置,可将含锌指结构域的转录因子分为C2H2、C2C2、C3H、C3HC4和C3HC5等5个亚类[3,4]。锌指结构的保守序列为(CX2-4CX3FX5LX2HX3-5H),其中Cys和His与锌原子形成配位键结合Zn2+,可形成一种稳定指装的多肽构型。C2H2亚类的锌指基序为两个Cys和两个His结合一个Zn2+。植物C2H2锌指蛋白有两个结构特征:一是锌指与DNA接触面处有一段高度保守的氨基酸序列QALGGH;二是两个相邻锌指结构间的间隔较长。
Takatsuji等[5]首次在矮牵牛(Petunia hybrida Vilm L.)中发现了第一个C2H2编码基因—TAZ,该基因参与调控绒毡层的发育和花粉成熟,TAZ1功能缺失突变体绒毡层发育异常,花粉粒败育。拟南芥(Arabidopsis thaliana L.)ZFP1(zinc finger protein 1)在愈伤组织中的异位表达能抑制细胞分化[6]。Joseph等[7]研究发现,拟南芥ZFP3能抑制ABA的产生,影响种子的萌发和早期发育。Zhou等[8]发现ZFP5与表皮细胞的发育有关,ZFP5与ZFP6还参与赤霉素和细胞分裂素介导的腺毛发育。拟南芥中SAT10是第一个被证明与非生物胁迫相关的C2H2型转录因子编码基因,该基因可上调耐盐基因的表达[9]。拟南芥AZF1和AZF2可通过下调渗透胁迫和ABA信号途径的靶基因表达水平从而降低抗逆性[10]。Sakamoto等[11]研究发现,在高盐胁迫和冷诱导条件下,STZ、AZF1、AZF2和AZF3表达水平显著增加,AZF2相对变化较小;ABA诱导会增强AZF2的表达,但对其他的C2H2编码基因的表达没有影响。Sakamoto等[12]还发现,在逆境胁迫下STZ和AZF2在叶片中的表达量明显高于根部,STZ的转基因材料表现出较强的抗寒能力并伴随矮化现象。水稻(Oryza sativaL.)和棉花(Gossypium sppL.)的STZ同源基因在盐诱导条件下基因的表达显著增强[13,14]。水稻RZF71和RZF5基因参与高盐和渗透胁迫[15,16],大豆(Glycine max L.)SCOF-1和SCTF-1被证明与低温胁迫有关[17]。另有研究发现,矮牵牛中ZPT2-2基因的表达可提高植物的抗旱性;ZPT2-3可被低温、干旱、茉莉酸和重金属诱导,但不受ABA诱导[18]。大豆C2H2-ZFPs与根瘤的固氮机制有关[19],莴苣(Lactuca sativa Linn L.)的抽薹时间受C2H2家族基因表达调控[20],白杨(Populus tomentosa Carr L.)和山杨(Populus davidiana L.)C2H2家族基因不仅参与根和花器官的发育而且与抗旱相关,其调控方式可能是通过启动子区的激素受体或逆境响应因子来实现[21,22,23]。综上所述,C2H2家族中的转录因子基因广泛参与了植物中的逆境胁迫应答。
目前,对玉米C2H2家族基因的功能研究较少。Royo等[24]发现玉米ZmMRPI-1和ZmMRPI-2通过调控MYB转录因子基因ZmMRP-1与靶标启动子的结合能力,从而调控玉米胚乳转移细胞的分化及在该细胞中特异基因的表达。C2H2家族成员众多但绝大多数功能未知[25]。玉米C2H2类转录因子是否参与玉米的逆境应答仍然未知。为了探究玉米C2H2家族基因在玉米抗逆途径中的功能,本研究从玉米中分离出C2H2类转录因子基因ZmC2H2-1,结果发现ZmC2H2-1是一个逆境相关的负调控转录因子基因,该研究为玉米抗逆的研究及应用提供了新的数据和基因资源。
1 材料和方法
1.1 材料
试验所用玉米品种为齐319(Q319),种植于中国农业科学院实验田及温室。拟南芥(Columbia wild type)种植于温室,培养温度为18℃,光周期为12 h/12 h,相对湿度40%~60%。1.2 玉米ZmC2H2-1基因的克隆
选取饱满的玉米Q319种子用0.5%的H2O2浸泡消毒24 h,自来水冲洗后转移至滤纸上,于28℃培养箱中培养。待种子胚根长至5 mm后播种到湿润的蛭石中生长3周,三叶一心期时取幼嫩的叶片,用CTAB法[26, 27]提取基因组DNA,置于-20℃中保存备用。选取三叶一心期玉米幼嫩的叶片为材料,利用Trizol法[28]抽提玉米总RNA。用RNase free DNase(Promega公司,美国)消除残存的DNA,然后用Reverse Transcription System进行反转录获得玉米cDNA。反转录反应体系如下:DNase消化的RNA (1 μg/μL)13.5 μL,dNTP Mixture (10 mmol/L) 2.5 μL,5×RT Buffer 2.5 μL,Reverse Transcriptase 2.5 μL,RNase inhibitor 0.5 μL,用无RNase水补至50 μL。反应体系混匀后于25℃退火5 min,然后于42℃反应1 h,最后于70℃反应15 min终止反应。反转录产物稀释5~10倍,-70℃保存备用。
以Q319 cDNA为模板,根据ZmC2H2-1基因序列设计引物扩增该基因的全长序列,ZmC2H2引物信息见表1。PCR扩增体系如下:cDNA模板(200 ng/μL)1 μL,5×Hifi缓冲液4 μL,2.5 mmol/L dNTP 2 μL,引物各加0.5 μL(10 μmol/L),HiFi HotStart DNA 聚合酶0.5 μL,加ddH2O补至20 μL。PCR扩增条件:95℃预变性5 min;98℃变性20 s,60℃退火15 s,72℃延伸20 s,39个循环;最后再72℃延伸5 min。
Table 1
表1
表1 本研究使用的引物序列
Table 1
| 引物名称 | 引物序列(5′→3′) |
|---|---|
| ZmC2H2 | F:ATGGCGGGGCTGTCGCT |
| R: TCAAAGGACCCCAAAGAAAG | |
| BD | F:GAATTCATGGCGGGGCTGTCGCT |
| R: GGATCCTCAAAGGACCCCAAAGAAAG | |
| Check | F:AGGCTGAGGATGAGGGATTGC |
| R: TCGCTACCGAGCATTCCAG | |
| Q | F:AGGCTGAGAGGATGAGGGATTGC |
| R: GCTCCAGGAACTCTATGCCCAC | |
| Actin1 | F:CCAACAGAGAGAGAAGATGACT |
| R: ATGTCTCTTACAATTTCCCG |
新窗口打开|下载CSV
1.3 玉米ZmC2H2-1同源蛋白聚类分析
通过Gramene数据库(http://www.gramene.org/)和Uniprot蛋白质数据库(http://www.uniprot.org/)检索来源于不同物种的ZmC2H2-1相关蛋白序列,使用MEGA5.0对来源不同的ZmC2H2-1同源蛋白进行聚类分析。1.4 玉米ZmC2H2-1亚细胞定位分析
首先利用Gateway[29]系统的BP反应将玉米ZmC2H2-1基因的CDS序列构建到入门载体pDONRTM221上,再运用Gateway系统的LR反应将目的基因构建到pK7FWG2载体上,构建瞬时表达载体pK7FWG2-(35S::ZmC2H2-1::T35S)。以正常培养4周的拟南芥叶片为材料制备拟南芥原生质体。溶液配方:(1)Enzyme solution 10 mL,包括15% cellulase R10 1 mL、4% macerozyme R10 1 mL、0.8 mol/L mannitol 5 mL、2 mol/L KCl 0.1 mL、0.2 mol/L MES(用KOH调整pH至5.7) 1 mL,55℃溶解10 min后再加入1 mol/L CaCl2 0.1 mL、1.5% BSA 1 mL、H2O 0.8 mL;(2)PEG solution 10 mL(40%),包括PEG4000 4 g、0.8 mol/L mannitol 2.5 mL、1 mol/L CaCl2 1 mL、H2O 3 mL;(3)W5 500 mL,包括NaCl(154 mmol/L) 9.0 g、CaCl2(125 mmol/L) 18.4 g、2 mol/L KCl 1.25 mL、0.2 mol/L MES 5 mL;(4)MMG solution 50 mL,包括MgCl2(15 mmol/L) 0.152 g、0.2 mol/L MES 1 mL、0.8 mol/L mannitol 25 mL。新鲜配制的酶解液用0.45 μm的滤头过滤除
菌,转入无菌的100 mL三角瓶中;取4周后未抽薹的拟南芥叶片切成细条状置于酶解液中,于23℃黑暗中酶解3~4 h;酶解液经过100~200目的筛子过滤,将滤液置于50 mL离心管中,于4℃、100 g离心2 min;弃上清,沉淀用20 mL预冷的W5溶液轻柔洗涤,于4℃、100 g离心2 min;弃上清,沉淀用20 mL预冷的W5溶液轻柔重悬,冰上放置30 min;于23℃、100 g离心2 min,弃上清,加入1~2 mL MMG重悬后即为原生质体。取上述原生质体溶液200 μL放入2 mL离心管中,加入20 μL(1 μg/μL)质粒载体,用移液器轻柔混匀后加入220 μL PEG solution,混匀后室温放置10~15 min;加入0.8 mL W5溶液,轻轻颠倒混匀,于23℃、100 g离心2 min,弃上清;加入W5溶液重悬,于23℃、100 g离心2 min,弃上清;再次加入1 mL W5溶液重悬,室温黑暗条件下孵育16~18 h,用激光共聚焦显微镜观察蛋白的表达定位情况。
选取三叶一心期的Q319黄化苗,进行玉米原生质体的制备,方法参照上述拟南芥原生质体转化,酶解时间调整为5 h,PEG浓度由40%变为30%。利用PEG介导转化的方法将pK7FWG2-(35S::ZmC2H2- 1-GFP::T35S)质粒分别转化玉米和拟南芥原生质体,在LSM700激光共聚焦显微镜下观察细胞中GFP绿色荧光的位置。
1.5 玉米ZmC2H2-1转录激活特性分析
利用酵母系统验证ZmC2H2-1的转录激活特性。将玉米ZmC2H2-1基因的CDS序列构建到pGBKT7载体(简称BD载体)上,BD引物信息见表1。制备AH109酵母感受态细胞,将构建好的质粒载体转化酵母感受态细胞,转化成功后涂布在SD/-Trp缺陷固体培养基上,30℃倒置培养2~3 d。挑取单克隆酵母菌落接种于SD/-Trp液体缺陷培养基中,30℃振荡培养2~3 d,提取酵母质粒PCR验证阳性结果。将上一步所得菌液稀释到OD600=0.1,分别点接到含有X-gal的SD/-Trp、SD/-Trp/-His缺陷固体培养基上,30℃倒置培养2~3 d,阳性对照为pGBKT7- GAL4-SV40-T53,阴性对照为空载pGBKT7。钓饵蛋白如果无转录激活则转化子不能在SD/-Trp/-His培养基上生长,钓饵蛋白有转录激活则转化子可以在SD/-Trp/-His培养基上生长。以上载体和试剂均购自宝生物工程(大连)有限公司。1.6 玉米ZmC2H2-1基因过表达载体构建及转基因植株鉴定
首先用Gateway系统的BP反应将玉米ZmC2H2-1基因CDS序列构建到入门载体pDONRTM221上,再运用Gateway系统的LR反应将目的基因构建到pGWEAR/pGWVP64载体上。将构建好的终载体热激转化导入农杆菌GV3101,采用蘸花法[28]转化拟南芥。待种子成熟后收获T0代种子,筛选鉴定重复至T3代获得纯系转基因植株。转基因植株用Bar抗性筛选,基因组DNA提取方法采用CTAB法(分别选取地上部和地下部),PCR检测引物Check、qRT-PCR检测引物Q及内参引物Actin1信息见表1。1.7 玉米逆境诱导表达分析
选取健康饱满的Q319玉米种子于0.5%的H2O2浸泡消毒24 h,自来水冲洗后转移至滤纸上,于28℃培养箱中培养。待种子胚根长至5 mm后播种到湿润的蛭石中生长约3周,待幼苗长至三叶一心时,小心将其从蛭石中取出,尽量避免伤及根部,水中静置2~3 h消除转移造成的创伤刺激。取长势一致的材料分为6组,其中1组作为对照(每次每组10株),另外5组分别放入NaCl(250 mmol/L)、PEG6000 (20%)、ABA(100 μmol /L)、GA(100 μmol/L)和SA (100 μmol/L)溶液中进行处理,在0 h、0.5 h、1 h、3 h、6 h、9 h、24 h分别取地上和地下部冻存于液氮中。总RNA提取及反转录方法参照1.2。对照组材料的cDNA按照1、5、25、50倍稀释,每个样品进行3次重复。qRT-PCR扩增体系包括:SYBR Premix Ex TaqTM(2×)10.0 μL,PCR Forward Primer (10 μmol/L) 0.4 μL,PCR Reverse Primer (10 μmol/L) 0.4 Μl (宝生物工程(大连)有限公司),cDNA模板1.0 μL,补水至20 μL。qRT-PCR扩增程序:95℃ 30 s;95℃ 10 s,60℃ 34 s,循环40次;72℃延伸10 min。目的基因相对表达水平采用2-ΔΔCT法计算。1.8 转ZmC2H2-1基因拟南芥叶片失水率
分别选取野生型及过表达ZmC2H2-1转基因拟南芥各3个纯系,常规培养4周,选取每株拟南芥最宽的4片叶子,在室温条件下放于滤纸上,分别在0、5、25、45、65、85、105 min时称量重量,计算叶片失水率。每次实验每株系统计20株,实验独立重复3次。1.9 转ZmC2H2-1基因拟南芥生理(干旱、高 盐、ABA诱导生长)实验
分别选取野生型及过表达ZmC2H2-1转基因拟南芥各3个纯系,在1/2 MS培养基上生长一周后,再转移到土中生长一周,浇饱和水并称重,之后干旱至拟南芥出现萎蔫(连续控水3周,每次复水量化且保持一致),复水10 d后统计不同组拟南芥存活 率。每次每株系使用20株苗进行实验,重复3次独立实验。将野生型和纯系过表达ZmC2H2-1拟南芥的种子消毒后竖直播种于1/2 MS培养基上,在培养基中分别加入终浓度为200 mmol/L NaCl、15% PEG、1 μmol/L ABA,14℃暗处理3 d后再光照培养1周,拍照并统计各种处理情况下植株的存活率。每次 每株系使用不少于20株苗进行实验,重复3次独立实验。
2 结果与分析
2.1 玉米ZmC2H2-1基因克隆
为了探究玉米C2H2家族基因在玉米抗逆途径中的功能,本研究从玉米反转录cDNA中扩增得到玉米Zm00001d016552基因CDS,全长序列为1251 bp,命名为ZmC2H2-1。该基因由11个内含子、12个外显子组成,编码一个由416个氨基酸组成的多肽。氨基酸序列分析发现,ZmC2H2-1包含2个典型的锌指结构(图1A),属于锌指蛋白家族的C2H2亚家族。图1
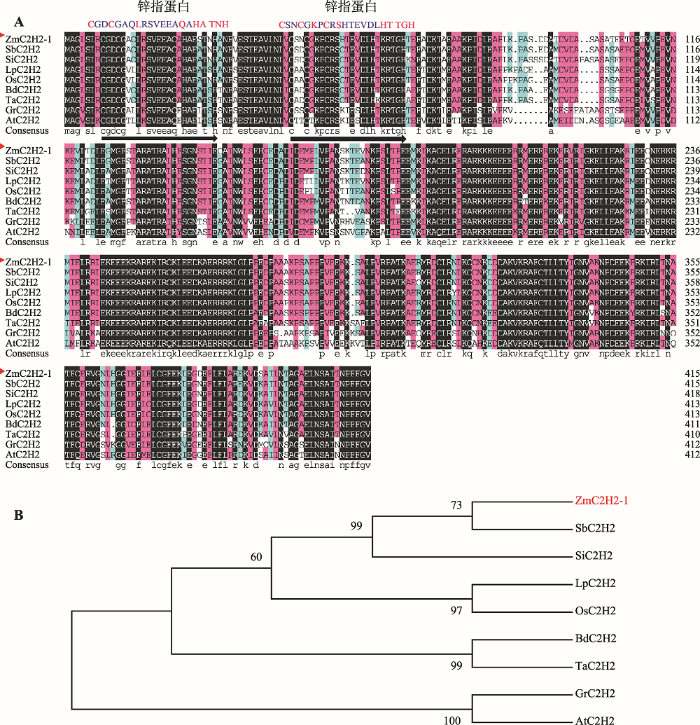 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1ZmC2H2-1同源蛋白氨基酸序列比对及系统进化分析
A:ZmC2H2-1同源蛋白氨基酸序列比对;B:ZmC2H2-1系统进化分析。ZmC2H2-1: Zea mays Zm00001d016552; SbC2H2: Sorghum bicolor SORBI_3004G153200; SiC2H2: Setaria italica Si017327m; LpC2H2: Leersia perrieri LPERR02G14040.1; OsC2H2: Oryza sativa Os02g0504500; BdC2H2: Brachypodium distachyon BRADI3G43900.1; TaC2H2: Triticum aestivum TRIAE_CS42_6AS_TGACv1_485676_ AA1549950.1; GrC2H2: Gossypium raimondii B456_005G130000; AtC2H2: Arabidopsis thaliana AT1G04850。序列来源于
Fig. 1Sequence alignment of animo acid sequence of ZmC2H2-1with its homologues and phylogenetic analysis
经序列相似性比对,发现C2H2亚家族在进化上相对保守,推测该蛋白的功能具有较高的相似性。系统进化分析发现,玉米、高粱(Sorghum bicolor L.)和小米(Setaria italica L.)的同源关系更近(图1B),但同源基因功能均未见报道[30]。
2.2 玉米ZmC2H2-1亚细胞定位
转录因子在细胞核中发挥功能。为了验证ZmC2H2-1的亚细胞定位,本研究构建了瞬时表达载体,观察绿色荧光蛋白表达情况。以转化空载体的拟南芥原生质体作为对照,绿色荧光蛋白主要分布于细胞质中,而转化ZmC2H2-1-GFP的原生质体,绿色荧光蛋白在细胞质和细胞核中均有分布(图2A);同时在以玉米黄化苗制备的原生质体中,ZmC2H2-1在细胞质、细胞核上均有分布,但在细胞核中的表达更强。上述结果表明ZmC2H2-1主要在细胞核中发挥功能(图2B)。图2
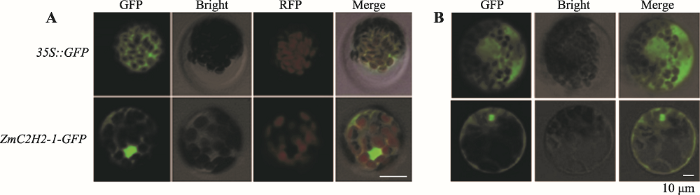 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2ZmC2H2-1在拟南芥和玉米中亚细胞定位分析
A:拟南芥中ZmC2H2-1亚细胞定位;B:玉米中ZmC2H2-1亚细胞定位。Bar=10 μm。Bright:明场;RFP:叶绿体自发荧光;GFP:绿色荧光;Merge:多通道叠加。
Fig. 2Subcellular localization of ZmC2H2-1 in Arabidopsis thaliana and Zea mays
2.3 玉米ZmC2H2-1转录激活验证
部分转录因子具有转录激活结构域,可以直接激活靶基因的表达,但也有部分转录因子不具有激活功能。为了验证ZmC2H2-1是否具有转录激活功能,本研究构建了酵母表达载体(构建流程见图3A),进行转录激活检验。结果表明,阳性对照pGBKT7- GAL4-SV40-T53在SD/-Trp/-His培养基上正常生长,阴性对照pGBKT7在SD/-Trp/-His培养基上不生长,pGBKT7-C2H2-1在SD/-Trp/-His培养基上也不生长,说明ZmC2H2-1不具有转录激活活性(图3B)。图3
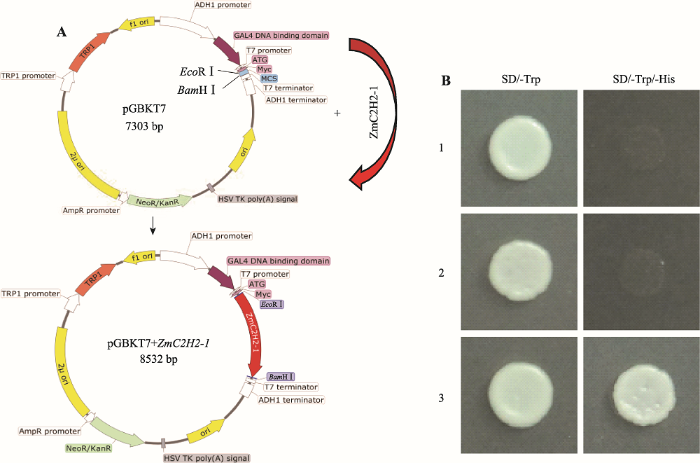 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3载体构建及ZmC2H2-1转录激活验证
A:酵母载体构建流程;B:转录激活验证。1:pGBKT7;2:pGBKT7-C2H2-1;3:pGBKT7-GAL4-SV40-T53。
Fig. 3Construction and transcriptional activation analysis of ZmC2H2-1
2.4 逆境诱导下玉米ZmC2H2-1基因表达分析
为了研究ZmC2H2-1在玉米抗逆中的功能,本研究对玉米幼苗进行了非生物胁迫逆境(PEG模拟干旱、高盐、ABA、GA和SA)处理,并对该基因的表达进行了定量分析(图4)。结果表明,在ABA和高盐胁迫处理下,玉米幼苗的地上部和地下部中ZmC2H2-1基因的表达显著下调,植株表现出对ABA和高盐的敏感性。对玉米幼苗进行高盐处理,在0.5 h和1 h时,地下部ZmC2H2-1基因的表达下调,此时地上部无显著变化;而随着处理时间的延长,地下部和地上部ZmC2H2-1基因的表达趋于一致性的下调,这说明ZmC2H2-1基因参与了盐胁迫信号传递过程。在ABA胁迫处理下,玉米幼苗地下部和地上部ZmC2H2-1基因的表达变化类似于高盐处理的结果,表明ZmC2H2-1基因表达也受到ABA的调控。在PEG模拟干旱处理下,玉米幼苗地上部ZmC2H2-1基因的表达受干旱胁迫显著下调,地下部变化相对不明显。用GA和SA处理玉米幼苗,地下部和地上部ZmC2H2-1基因的表达变化趋势不明显,可能不参与或者不是直接参与GA及SA的胁迫应答。以上结果表明,ABA、高盐、干旱等非生物逆境抑制ZmC2H2-1基因的表达,说明ZmC2H2-1是逆境胁迫的负调控因子。图4
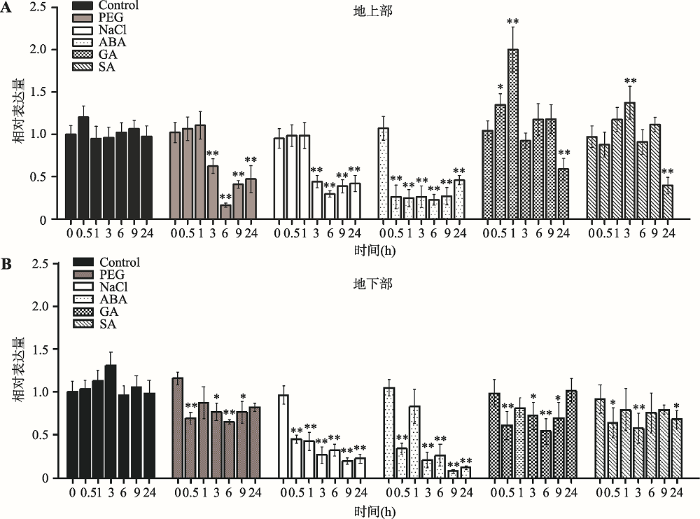 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4玉米转录因子ZmC2H2-1在PEG、高盐及ABA、GA、SA等激素诱导下的表达特性分析
A:地上部ZmC2H2-1表达分析;B:地下部ZmC2H2-1表达分析。以玉米Actin1为内参,未处理时目标基因的表达量设置为1。图中所示数据为3次重复平均值,误差线表示标准差(+SD)。*表示P< 0.05,差异显著;**表示P < 0.01,差异极显著。
Fig. 4Expression characteristics of ZmC2H2-1 in response to PEG, NaCI , ABA, GA and SA
2.5 转ZmC2H2-1基因拟南芥表达验证
为进一步阐明ZmC2H2-1基因功能,将ZmC2H2-1基因构建到pGWVP64载体,并转化到拟南芥中,获得转ZmC2H2-1拟南芥阳性植株。选3个不同的纯系转ZmC2H2-1基因拟南芥(分别为OE1、OE2和OE3)种子在温室培养4周,取幼嫩的叶片提取RNA后反转录成cDNA,利用qRT-PCR检测ZmC2H2-1基因的表达水平。结果发现,3个纯系转基因拟南芥植株中ZmC2H2-1基因表达显著高于野生型(图5)。图5
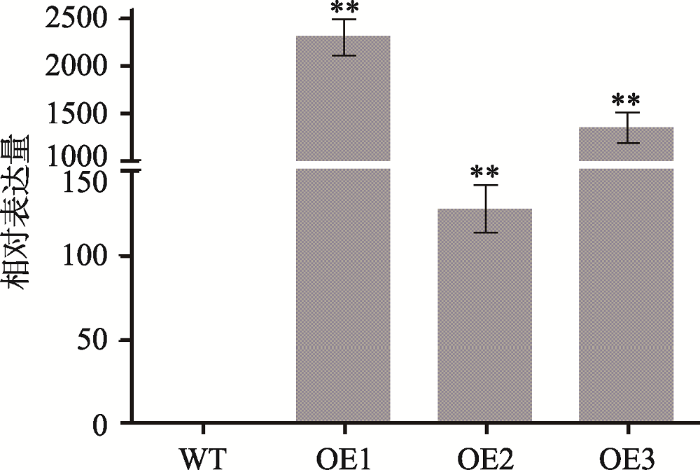 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5转基因拟南芥qRT-PCR检测
WT:野生型;OE1、OE2、OE3表示3个纯系转基因拟南芥植株。**表示P < 0.01,差异极显著。
Fig. 5qRT-PCR analysis of transgenics Arabidopsis thaliana
2.6 转ZmC2H2-1基因拟南芥叶片失水率测定
为了解析ZmC2H2-1基因在植物抗逆中的功能,本研究对转基因植株的失水率进行了测定。结果表明,3个纯系转基因拟南芥叶片失水率均高于野生型,其中OE1株系叶片失水率与野生型相比存在极显著差异(图6),这种显著性与ZmC2H2-1基因的表达水平直接相关,其表达量越高植株的抗逆性就越低,说明ZmC2H2-1基因在拟南芥中是逆境应答的负调控因子,这与ZmC2H2在逆境条件下的表达结果相一致。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6叶片失水率测定
* P< 0.05,表示野生型和3个转基因系之间差异显著;** P < 0.01,表示野生型和3个转基因系之间差异极显著。
Fig. 6Water loss rate of leves in WT and transgenic plants
2.7 转ZmC2H2-1基因拟南芥生理(干旱、高 盐、ABA诱导生长)实验
以上研究发现,ZmC2H2-1基因功能与非生物逆境胁迫相关。为进一步解析ZmC2H2-1基因与干旱、高盐等逆境的关系,本研究利用转基因拟南芥植株分别进行了干旱、高盐、ABA诱导生长等实验进行验证。转ZmC2H2-1基因拟南芥干旱实验结果表明,连续控水3周后,所有植株全部失绿,与野生型相比,过表达ZmC2H2-1拟南芥植株旱死亡率更高(图7)。复水10 d后统计存活率,野生型存活率约70%,其中ZmC2H2-1基因表达量最高的OE1株系存活率仅为10%,说明过表达ZmC2H2-1会降低植株的耐旱性,而这一结果与PEG模拟干旱处理及叶片失水率实验结果相一致。
图7
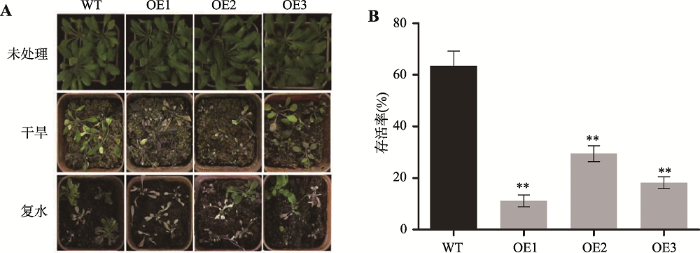 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转ZmC2H2-1基因拟南芥的耐旱性
A:干旱实验表型;B:存活率统计。**表示P < 0.01,差异极显著。
Fig. 7Drought tolerance of ZmC2H2-1 transgenics plants
转ZmC2H2-1基因拟南芥ABA诱导生长实验结果表明,终浓度为1 μmol/L的ABA会抑制转ZmC2H2-1基因拟南芥的生长。与野生型相比较,过表达ZmC2H2-1拟南芥ZmC2H2-1基因表达量越高的株系,其生长受ABA抑制越严重(图8)。
图8
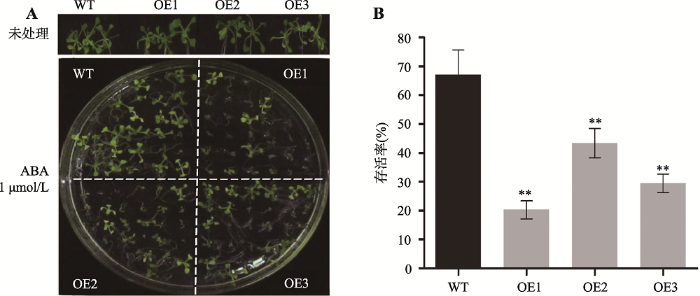 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8ABA处理对转ZmC2H2-1基因拟南芥的生长抑制
A:ABA抑制生长实验表型;B:存活率统计。**表示P < 0.01,差异极显著。
Fig. 8Growth inhibition of ZmC2H2-1 transgenic plants with ABA treatment
转ZmC2H2-1基因拟南芥高盐诱导生长实验结果表明,终浓度为200 mmol/L的NaCl严重影响转ZmC2H2-1基因拟南芥的生长(图9A)。与野生型相比,过表达ZmC2H2-1拟南芥植株死亡率升高,ZmC2H2-1基因表达量越高,致死率越高(图9B),说明ZmC2H2-1属于高盐胁迫的负调控因子。
图9
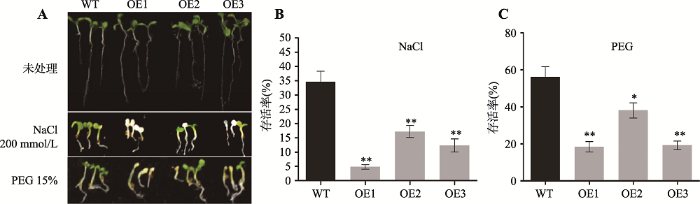 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9转ZmC2H2-1基因拟南芥对高盐和PEG渗透胁迫的抗性
A:盐胁迫及PEG渗透胁迫实验表型;B:盐胁迫实验存活率统计;C:渗透胁迫实验存活率统计。**表示P < 0.01,差异极显著。
Fig. 9Stress tolerance of ZmC2H2-1transgenic plants treated with high salt and PEG osmotic stress
转ZmC2H2-1基因拟南芥渗透胁迫耐受性实验结果表明,与野生型相比,过表达ZmC2H2-1拟南芥植株对PEG胁迫更为敏感,且与ZmC2H2-1基因表达量直接相关,ZmC2H2-1基因表达量越高,植株对PEG渗透胁迫耐受性越差,说明ZmC2H2-1基因是干旱胁迫的负调节因子(图9,A和C)。
3 讨 论
非生物胁迫已成为影响作物产量的主要因素,玉米作为全球第一大粮食作物,揭示其逆境响应的分子机制具有重要意义。锌指蛋白是一类广泛存在于生物体内的具有重要功能的转录调控因子,大量研究表明,锌指蛋白通过转录激活或抑制逆境相关基因的表达,进而参与应答非生物胁迫过程。C2H2型锌指蛋白大多数参与各种胁迫反应,如拟南芥中的AZF3、STZ参与高盐和冷诱导[10],ZAT12、ZAT7参与氧化胁迫[31];大豆中的SCOF-1、SCTF-1参与低温胁迫[32];水稻中的DST参与干旱和高盐胁迫 等[33]。本研究发现,玉米在干旱、盐、ABA、GA和SA处理下,ZmC2H2-1基因表达水平受到不同程度的影响。在干旱、高盐和ABA处理条件下,ZmC2H2-1表达下调,而转ZmC2H2-1基因拟南芥叶片相较野生型材料表现出明显的不耐旱,对转基因拟南芥进行ABA、高盐和干旱以及PEG渗透胁迫生长实验结果发现,逆境胁迫抑制过表达ZmC2H2-1植株的生长,转基因拟南芥的耐受性显著降低,证明ZmC2H2-1基因参与了植物的抗逆过程,且这种调控属于负调控。部分锌指蛋白是逆境响应的负调节物,当其功能缺失时转基因植株耐逆性增强,过表达时能降低植物的耐逆性,如水稻的DST[33]、棉花的GhDil9-1和GhDil9-2等[34]。本研究结果得到了类似结论,但是ZmC2H2-1基因是直接参与抗逆的信号转导还是通过调控相关基因表达途径来改变生理表型还需要进一步的研究。植物细胞能够感受外界的胁迫信号,通过信号转导调控相关基因的表达并及时作出响应。ZmC2H2-1基因响应玉米逆境胁迫的机制可能包括以下3种:(1)通过ABA的信号通路,当植株受到逆境胁迫时体内ABA水平升高,ZmC2H2-1作为负调控因子受ABA影响表达受到抑制;(2)通过干旱途径,植株感受干旱胁迫时调节叶片上的气孔导度来完成应答;(3)通过渗透调节途径,植株感受盐或渗透胁迫时积累各种有机或无机物质来提高细胞液浓度,降低渗透势适应胁迫的过程。本研究已证实ZmC2H2-1作为负调控因子参与逆境胁迫调控网络,是逆境应答的重要基因,然而具体的调控模式还有待进一步研究。
(责任编委: 严建兵)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLMagsci [本文引用: 1]

锌指蛋白是一类具有指状结构域的转录因子。根据半胱氨酸(C)和组氨酸(H)残基的数目和位置可将锌指蛋白分为C2H2、C2HC、C2C2、C2HCC2C2、C2C2C2C2等亚类。C2H2型锌指蛋白是最多也是研究最为清楚的一类锌指蛋白,在植物中已经克隆了50多个,主要涉及植物的生长发育和对环境胁迫的应答反应。该类锌指蛋白大部分在锌指区具有植物中特有的QALGGH保守结构,可能涉及调控植物特有的生物学功能。文章主要讨论了植物C2H2型锌指转录因子的结构、对靶DNA的识别及在生长发育和环境胁迫反应中可能的调控功能。 Abstract:Zinc finger protein is one of the important transcription factors with zinc finger domain that regulates gene expression in the eukaryotic organisms mainly by specifically interacting with target DNA sequence(cis-acting element). It could be divided into several types of zinc finger proteins, such as C2H2, C2HC, C2C2, C2HCC2C2, C2C2C2C2 etc, based on numbers and positions of Cys and His residues. Of these, C2H2 type zinc finger protein is the most clearly identified zinc finger transcription factor, with the wide existence in human, animals and plants. The characterized plant C2H2 zinc finger proteins are mainly involved in plant growth and development and the responses to environmental stresses. Up to now, more than 50 C2H2 zinc finger proteins have been reported in plants including petunia, Arabidopsis, wheat and rice, and most of them have the plant-specific QALGGH motif in zinc finger domain. This paper briefly introduces the structure, recognition of target-DNA sequence and functions involved in development or environmental stresses of plant C2H2 zinc finger proteins. <br><br><br>
URLMagsci [本文引用: 1]

锌指蛋白是一类具有指状结构域的转录因子。根据半胱氨酸(C)和组氨酸(H)残基的数目和位置可将锌指蛋白分为C2H2、C2HC、C2C2、C2HCC2C2、C2C2C2C2等亚类。C2H2型锌指蛋白是最多也是研究最为清楚的一类锌指蛋白,在植物中已经克隆了50多个,主要涉及植物的生长发育和对环境胁迫的应答反应。该类锌指蛋白大部分在锌指区具有植物中特有的QALGGH保守结构,可能涉及调控植物特有的生物学功能。文章主要讨论了植物C2H2型锌指转录因子的结构、对靶DNA的识别及在生长发育和环境胁迫反应中可能的调控功能。 Abstract:Zinc finger protein is one of the important transcription factors with zinc finger domain that regulates gene expression in the eukaryotic organisms mainly by specifically interacting with target DNA sequence(cis-acting element). It could be divided into several types of zinc finger proteins, such as C2H2, C2HC, C2C2, C2HCC2C2, C2C2C2C2 etc, based on numbers and positions of Cys and His residues. Of these, C2H2 type zinc finger protein is the most clearly identified zinc finger transcription factor, with the wide existence in human, animals and plants. The characterized plant C2H2 zinc finger proteins are mainly involved in plant growth and development and the responses to environmental stresses. Up to now, more than 50 C2H2 zinc finger proteins have been reported in plants including petunia, Arabidopsis, wheat and rice, and most of them have the plant-specific QALGGH motif in zinc finger domain. This paper briefly introduces the structure, recognition of target-DNA sequence and functions involved in development or environmental stresses of plant C2H2 zinc finger proteins. <br><br><br>
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
URL [本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

By sequencing analysis of the clones chosen from the cotton petal cDNA library, a cDNA, homologous to Arabidopsis salt tolerance zinc finger protein, was isolated and designated as CSTZ. This new cDNA (1 012 bp) contains an open reading frame of 819 bp encoding a 272 residue polypeptide that includes a typical plant two fingered Cys2/His2 zinc finger mitif. Nortern blot analysis revealed that the expressed level of CSTZ in cotton seedling increased in response to 220 mmol/L NaCl treatment. CSTZ is highly expressed in leaves, roots, petals, and anthers, but with low expression in stigmas.
Magsci [本文引用: 1]

<P>利用生物信息学和RT-PCR方法从水稻幼苗组织中分离了1个新的C2H2型锌指蛋白基因<EM>RZF71</EM>, 该基因编码一条250个氨基酸残基的多肽, 含有两个典型的C2H2型锌指结构。半定量RT-PCR分析表明: <EM>RZF71</EM>在根、茎、叶和幼穗中呈组成性表达, 在根中的表达丰度略高; 在高盐和PEG6000胁迫的水稻幼苗组织中, <EM>RZF71</EM>的表达显著增强, 但低温和ABA处理对该基因的表达量影响不大。农杆菌介导的洋葱表皮细胞GFP瞬时表达实验表明: RZF71定位于细胞核内。讨论了RZF71可能作为一个转录调控因子在水稻耐高盐和渗透胁迫中的作用。</P>
Magsci [本文引用: 1]

<P>利用生物信息学和RT-PCR方法从水稻幼苗组织中分离了1个新的C2H2型锌指蛋白基因<EM>RZF71</EM>, 该基因编码一条250个氨基酸残基的多肽, 含有两个典型的C2H2型锌指结构。半定量RT-PCR分析表明: <EM>RZF71</EM>在根、茎、叶和幼穗中呈组成性表达, 在根中的表达丰度略高; 在高盐和PEG6000胁迫的水稻幼苗组织中, <EM>RZF71</EM>的表达显著增强, 但低温和ABA处理对该基因的表达量影响不大。农杆菌介导的洋葱表皮细胞GFP瞬时表达实验表明: RZF71定位于细胞核内。讨论了RZF71可能作为一个转录调控因子在水稻耐高盐和渗透胁迫中的作用。</P>
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:14675448 [本文引用: 1]

The petunia gene, ZPT2-3 , encodes a Cys 2 /His 2 -type zinc finger protein. Here, we describe the expression of ZPT2-3 in response to various stresses and the effects of ZPT2-3 overexpression in transgenic petunia. Mechanical wounding induced accumulation of ZPT2-3 transcript, and the activity of ZPT2-3::luciferase was conferred by the 1668-bp ZPT2-3 upstream sequence, both locally and systemically. This induction was mediated by a jasmonic acid (JA)-dependent and ethylene-independent pathway. ZPT2-3 expression was also induced by cold, drought, and heavy metal treatments. The same ZPT2-3 promoter sequence showed similar responsiveness to wounding, cold, drought, and JA treatments in Arabidopsis when investigated in a -glucuronidase (GUS) reporter gene, indicating conservation of similar signaling pathways between the two plant species. ZPT2-3 functioned as an active repressor in a transient assay using Arabidopsis leaves. Constitutive overexpression of ZPT2-3 in transgenic petunia plants increased tolerance to dehydration. These results demonstrate the involvement of ZPT2-3 in plant response to various stresses, and suggest its potential utility to improve drought tolerance.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:24306565 [本文引用: 1]

We have developed a DNA extraction procedure for milligram amounts of plant tissue. Yields ranged from 0.3-200 nanograms of DNA per milligram of tissue. The factors affecting yield are discussed. Fresh tissue, as well as herbarium specimens (22-118 years old) and mummified seeds and embryos (500 to greater than 44 600 years old) were used. All tissues attempted (57 types from 29 species) yielded measurable amounts of DNA. In no case tested was inhibition observed for restriction enzymes BamHI or EcoRI.
URL [本文引用: 1]

Extraction procedures for plant DNA in general must accomplish the following. (1) The cell walls must be broken (or digested away) in order to release the cellular constituents. This is usually done...
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
URL [本文引用: 1]
