 ,2
,2Roles and molecular mechanisms of hypoxia-inducible factors in renal cell carcinoma
Junxia Zou1, Ke Chen ,2
,2通讯作者:
编委: 陈雁
收稿日期:2017-12-11修回日期:2018-01-13网络出版日期:2018-05-20
| 基金资助: |
Editorial board:
Received:2017-12-11Revised:2018-01-13Online:2018-05-20
| Fund supported: |
作者简介 About authors
邹俊遐,本科,护师,研究方向:儿童护理和肿瘤疾病专科护理E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (811KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
邹俊遐, 陈科. 缺氧诱导因子(HIFs)在肾癌发生中的作用及其分子机制. 遗传[J], 2018, 40(5): 341-356 doi:10.16288/j.yczz.17-406
Junxia Zou, Ke Chen.
肾癌是由多种不同类型肾脏肿瘤组成的复杂性疾病。每一种类型的肾脏肿瘤都具有不同的组织学特点和临床预后表现,这主要是由不同的基因突变和治疗手段决定的[1]。在临床上根据肾癌的组织病理学区别,可以将其分为以下3种主要类型:(1)透明细胞癌(clear cell RCC, ccRCC),约占肾癌的70%~ 75%;(2)乳头状肾细胞癌(papillary RCC, pRCC),约占肾癌的10%~16%;(3)嫌色细胞癌(chromophobe RCC, chRCC),约占肾癌的5%[2]。肾细胞癌的发病主要有两种模式,即散发性和遗传性。遗传性肾细胞癌约占全部肾癌的2%~3%,主要包括von Hippel- Lindau(VHL)综合征(由位于3p25-26的VHL基因突变导致)、遗传性乳头状肾细胞癌(由位于7q31-34的c-MET基因突变导致)、Birt-Hogg- Dube(BHD)综合征(由位于17p11的FLCN基因突变导致)、遗传性平滑肌瘤病肾癌(由位于1q42-43的FH基因突变导致)和结节性硬化症相关肾癌(由位于9q34的TSC1基因或位于16p13的TSC2基因突变导致)[3,4]。ccRCC和chRCC分别与VHL综合征和BHD综合征相关联。VHL综合征是一种家族性多发肿瘤综合征,受累的个体由于VHL基因的突变或缺失导致多器官发生肿瘤,包括血管母细胞瘤、肾透明细胞癌、嗜铬细胞瘤等。BHD综合征是一种罕见的常染色体显性遗传病,主要由抑癌基因FLCN突变导致,且 BHD患者中大约有25%~35%会发生肾癌[5]。BHD综合征相关肾癌具有多种不同的组织学表现,其中约90%的表现为chRCC和混合嫌色嗜酸性细胞瘤[5]。遗传性乳头状肾细胞癌也是一种罕见的常染色体显性遗传病,并具有很高的外显率,主要由原癌基因c-MET的突变导致c-MET蛋白持续激活,进而促进细胞增殖和存活以及增加细胞的迁移和侵袭能力等,最终导致肾癌的发生[3,6,7]。
在哺乳动物正常的新陈代谢和生理功能中,氧气是必不可少的。缺氧诱导因子(hypoxia inducible factor, HIF)是细胞感知和适应氧气水平变化的中枢调控因子[8]。目前已发现的HIF家族包括有3个成员:HIF-1、HIF-2和HIF-3,均由对氧敏感的α亚基HIF-α(它们有3个成员:HIF-1α、HIF-2α和HIF-3α)和对氧不敏感的β亚基HIF-1β构成[9]。在富氧条件下,HIF-α与pVHL结合后被降解。在脯氨酰羟化酶(prolyl hydroxylases,PHD)的作用下HIF-α的保守脯氨酸位点会被羟基化修饰,这是其被pVHL识别的前提条件。在缺氧条件下,PHD失活,HIF-α的羟基化受到抑制,使其不能被pVHL识别和降解,进而与HIF-1β形成异源二聚体,它们能结合DNA序列上的缺氧反应元件而激活一系列下游基因的表达,进而引发组织细胞的耐氧适应性反应[3]。
缺氧是实体肿瘤微环境的一个重要的基本特征,因此在许多实体肿瘤中HIF活性增加能激活许多靶基因的转录,从而引起细胞代谢重编程、细胞增殖、侵袭和转移、细胞凋亡以及耐药性等改变,进而发挥其生理调节功能及恶性转化作用。大量研究表明,大多数ccRCC存在VHL基因的失活。与缺氧相似,pVHL的失活也会导致HIF-α的稳定性增加和激活而引起“假缺氧”转录激活应答反应。因此,肾癌中pVHL的失活与HIF的激活密切相关。此外,其他几种主要的肾细胞癌亚型也伴随着HIF的激活[3]。例如,在FH基因突变的肾癌中,由于FH蛋白的失活导致细胞内富马酸盐浓度的上升,从而抑制HIF-α的羟基化而使其避免被VHL识别和降解[3]。因此,HIF-α在肾细胞癌(RCC)的发生发展中发挥着至关重要的作用。
本文主要综述了目前关于缺氧诱导因子的相关研究,特别是HIF-1α和HIF-2α在ccRCC中的作用,并探讨了HIF相关的靶向通路并提高肾癌的疗效的可能性。
1 VHL和HIF-α
1.1 VHL基因失活与肾癌
VHL基因由于可变剪接可以编码两种蛋白pVHL30和pVHL19。目前的研究发现这两种异构体在肾癌中具有相似的功能,基于此,这两种蛋白被统称为pVHL[10]。pVHL具有许多功能,其最经典的功能是作为一个E3泛素连接酶复合物的底物识别亚基识别HIF-α并使其发生泛素化降解[11]。如前所述,大部分肾细胞癌的病理类型是ccRCC,且其中约97%为散发性。通常,大多数ccRCC与3号染色体短臂(3p)的改变有关,其中定位于3p25-26染色体区域的VHL基因的突变或缺失在ccRCC的发生发展中发挥着关键的作用[4]。大量的研究表明,VHL基因在ccRCC中的失活机制主要包括等位基因缺失、杂合性缺失、基因突变和启动子甲基化[12]。癌症基因组图谱(The Cancer Genome Atlas,TCGA)的研究人员分析了417例ccRCC患者的肾癌组织。结果发现,大部分(92%)的ccRCC伴随着染色体3p(包含VHL、PBRM1、BAP1和SETD2基因)的丢失,以及218例(52.28%)ccRCC出现了VHL基因的突变位点[13]。此外,TCGA研究人员还发现大约7%的ccRCC肿瘤中具有VHL基因的启动子区甲基化,值得注意的是,在ccRCC中VHL基因启动子的异常甲基化与其基因突变是相互排斥,不共存的,这进一步说明VHL基因的失活在ccRCC发生发展中起着关键作用[13]。
1.2 VHL基因型与表型的相关性
VHL基因的失活通常可导致内脏囊肿以及一系列的肿瘤,包括神经系统和视网膜的血管母细胞瘤、肾透明细胞癌、嗜铬细胞瘤、胰岛细胞瘤、內淋巴囊肿瘤和附睾囊腺瘤等。在绝大部分遗传性和散发性肾透明细胞癌中可见由基因缺失、突变和甲基化等机制导致的VHL基因失活。根据相对风险的不同,可以将VHL综合征分为两种类型:1型VHL综合征表现为肾癌和血管母细胞瘤,2型VHL综合征则表现有嗜铬细胞瘤[14]。2型VHL综合征可进一步分为2A型(嗜铬细胞瘤和血管母细胞瘤,而ccRCC发生率低),2B型(嗜铬细胞瘤和血管母细胞瘤,且ccRCC发生率高),2C型(仅表现为嗜铬细胞瘤)[3]。这些亚型具有明显不同的VHL基因突变类型(表1)。具体而言,1型VHL综合征(ccRCC高风险)与VHL 基因等位基因的缺失或失活突变(基因组大片段的缺失、移码突变或无义突变导致的pVHL的完全缺失)有关,而大多数2型VHL综合征与VHL 基因错义突变有关[10]。此外,影响elongin C相互作用的VHL 基因错义突变明显增加嗜铬细胞瘤的发展风险,而影响与elongin B和HIF-α相互作用的VHL基因错义突变明显增加ccRCC的发展风险[10, 15]。在体外模拟pVHL突变与VHL综合征亚型之间相关性的研究中发现:HIF通路异常激活程度与ccRCC的发展风险升高似乎密切相关,而那些仅与嗜铬细胞瘤相关的pVHL突变则具有正常调节HIF通路的功 能[16]。因此,在1型和2B型VHL疾病中pVHL突变在HIF-α的调节方面是严重缺陷的,这类肾癌中具有高水平的HIF活性;而2A型相关pVHL突变则具有正常的HIF-α调节功能。尽管这种相关性并不是绝对的,但是这说明了VHL-HIF通路在ccRCC发生发展中起着关键作用。Table 1
表1
表1 VHL综合征中VHL基因型与表型的相关性
Table 1
| VHL综合征亚型 | 临床表型 | VHL基因突变类型 | HIF异常程度 | 参考文献 |
|---|---|---|---|---|
| 1 | ccRCC;血管母细胞瘤 | 基因组大片段的缺失、移码突变或无义突变 | +++ | [3, 10, 16] |
| 2A | 血管母细胞瘤;嗜铬细胞瘤 | 错义突变 | + | [17, 18] |
| 2B | ccRCC;血管母细胞瘤;嗜铬细胞瘤 | 错义突变 | ++ | [17, 18] |
| 2C | 嗜铬细胞瘤 | 错义突变 | 正常 | [16, 19] |
新窗口打开|下载CSV
1.3 HIF-1α与HIF-2α
HIF-α蛋白家族有3个成员:HIF-1α、HIF-2α和HIF-3α,其中研究比较深入的是HIF-1α与HIF-2α[20]。HIF-1α和HIF-2α蛋白具有蛋白质序列和结构的保守性,有48%的蛋白序列相似,具有相同的结构域,包括氨基端的碱性螺旋-环-螺旋(basic-helix-loop-helix,bHLH结构域、两个Per/Amt/ Sim(PAS)结构域、一个氧依赖降解结构域(oxygen- dependent degradation domain,ODDD)、以及位于N末端C末端的反式激活结构域(transactivationdomain, TAD),即TAD-N和TAD-C。HIF-3α由于可变剪接可以产生很多不同的蛋白异构体,它缺少氧依赖降解结构域以及CTAD,尽管它含有NTAD的同源结构域,然而HIF-3α的转录激活活性还没有被证 实[3]。由于HIF-3α缺失ODDD,因此,HIF-3α可能并不直接受到pVHL的调节。但是,HIF-3α是HIF-1α的一个靶基因,因此,HIF-3α也间接受到pVHL的调节。此外,HIF-3α与HIF-1α和HIF-2α一样都与HIF-1β相互作用,因此,HIF-3α通过竞争结合HIF-1β可作为HIF-1α和HIF-2α的显性负效应因子对HIF-1α和HIF-2α起负调控作用[3]。HIF-1α在人体组织中广泛表达,而HIF-2α的表达则主要局限于某些特定组织中[21]。许多HIF的靶基因被认为在肿瘤发生过程中起着重要的作用,这些基因主要涉及血管生成(VEGF、PDGF和CTCF等)、糖酵解(GLUT1、PGK1和PDK等)、染色质重塑(JMJD1A和JMJD1C等)、细胞周期(CCND等)、细胞外基质的形成和更替(MMP1和LOX等)。大规模的染色质免疫共沉淀-测序(ChIP-Seq)和转录组测序显示细胞内有大约500~1000个HIF靶基因[22, 23]。HIF靶基因表现出高度细胞特异性,其中只有一小部分靶基因在所有细胞中是保守的[22]。此外,HIF-1α和HIF-2α既有相同的靶基因(如VEGF 和GLUT1),也有各自特异的下游基因[24]。HIF-1α被证实倾向于诱导醣酵解相关酶基因的表达;而HIF-2α可激活细胞转移相关基因的表达,例如OCT-3/4和MMP等[25,26,27]。最初,研究人员认为可能是HIF-1α和HIF-2α具有不同的DNA结合位点。然而实际上,HIF-1α和HIF-2α具有相同的结合基序。研究证实有许多基因位点能被HIF-1α和HIF-2α结合,但是并不是这两个转录因子都能引起转录激活效应,例如HIF-1α能激活PGK1的表达而HIF-2α则无法激活,HIF-2α能激活CCND1的表达而HIF-1α则不能[3]。此外,最近有研究表明,在VHL缺陷的ccRCC细胞中,HIF-1α倾向结合到靶基因近端启动子区域的缺氧反应元件;而HIF-2α通常结合到位于增强子区(内含子和基因间区域)的缺氧反应元件,随后招募组蛋白乙酰转移酶p300,进而激活下游靶基因的表达[28]。
许多研究表明在ccRCC细胞系中,pVHL调节的基因与缺氧调节基因之间存在明显的重叠[29,30,31]。此外,通过分析HIF的DNA结合位点以及VHL缺陷的ccRCC细胞系的表达谱揭示了许多HIF的靶基因在许多ccRCC细胞系和肿瘤组织中组成型的高表达,进一步说明了VHL-HIF通路的异常在ccRCC中发挥着关键的作用[32]。
2 HIF-1α和HIF-2α与ccRCC的关系
2.1 HIF-1α和HIF-2α在ccRCC中具有相反功能的表型证据
在VHL基因相关肾癌中,HIF-α的激活是正常肾小管上皮细胞中的早期事件,并且伴随着HIF下游基因(如GLUT1和CA9基因)的诱导表达[33]。值得注意的是,在早期癌前病变位点可检测到HIF-1α和HIF-2α的表达,而在正常的肾小管细胞内仅能检测到HIF-1α的表达,这说明HIF-2α的表达才是VHL基因缺陷相关肾癌真正的早期癌前病变事件[33,34]。在小鼠胚胎成纤维细胞或正常肾小管上皮细胞中VHL的缺失将导致细胞衰老,这表明在VHL基因缺陷的肾小管上皮细胞的恶性转化过程中需要一些额外的基因突变或生物学事件来协同作用,最后产生恶性肿瘤[35]。此外,在VHL综合征肾癌患者中可检测到数百个VHL基因缺陷位点,但是仅有一小部分缺陷位点导致发展为肿瘤[3]。总的来说,这些研究表明在肾小管上皮细胞中pVHL的失活主要导致HIF-1α的稳定性增加和HIF-2α的从头表达,以及HIF靶基因的表达上调,此外,还需要一些协同突变或生物学事件来克服细胞衰老最终促进肿瘤的发生发展。
在肾癌的发生发展中,HIF-1α和HIF-2α的功能得到了很好的研究。在人肾癌小鼠异种移植模型中,过表达HIF-2α可增加肿瘤的大小。而过表达DNA结合结构域缺陷的HIF-2α则没有这个表型,这说明HIF-2α的转录激活活性对其促ccRCC发生发展是必不可少的。与此一致,下调HIF-2α的的表达可明显降低肿瘤的大小[36]。与HIF-2α的作用相反,HIF-1α的过表达可降低人肾癌移植瘤的大小,而下调HIF-1α的水平可增加肾癌细胞系的增殖[3]。因此,从中可以得出以下结论:在肾癌发生发展中,HIF-2α是一个促进因子,而HIF-1α是一个抑制因子。但让人意外的是,HIF-1α和HIF-2α在许多其他肿瘤(包括乳腺癌、结直肠癌和前列腺癌等)中却都发挥着相同的促进肿瘤发生发展的作用,并且它们的高表达都与这些肿瘤的不良预后密切相关[37]。
2.2 HIF-1α和HIF-2α在ccRCC中具有相反功能的遗传证据
越来越多的研究表明,HIF-1α和HIF-2α在ccRCC中具有不同的功能。免疫组化分析VHL基因缺陷的肿瘤发现:尽管所有的肿瘤中都高表达HIF-2α,但是很多肿瘤却没有HIF-1α的表达。根据VHL基因突变和HIF-1α与HIF-2α的表达情况可以将ccRCC分为以下3类:(1) pVHL功能正常的肿瘤;(2) pVHL失活且同时表达HIF-1α和HIF-2α的肿瘤;(3) pVHL失活但是仅表达HIF-2α的肿瘤。人肾癌小鼠异种移植瘤实验显示,第三类肿瘤细胞肿瘤生长速度最快[38]。除了染色体3p(包含VHL基因)的缺失,还有许多染色体异常事件与肾癌发生发展密切相关,包括染色体5q的扩增和染色体14q的缺失[39, 40]。许多研究表明染色体14q的缺失是肾癌预后不良的一个重要指标[40,41,42,43]。HIF-1α定位于染色体14q23,且大多数14q染色体的缺失片段中包含HIF-1α基因座位[44]。Shen等[40]通过遗传学和功能学研究证实HIF-1α的部分缺失在14q染色体缺失的肾癌中扮演了十分重要的角色。他们通过一系列的体内体外实验证实HIF-1α可抑制肾癌细胞的增殖。然而,HIF-1α的部分缺失异构体(HIF-1αΔ3-4, HIF-1αΔ2-6, HIF-1αΔ5- 10)以及少量存在于肾癌中的突变体(c.2120delA、c.2180C→A和V116E)抑制肾癌细胞增殖的能力明显减弱。目前的研究数据表明,VHL的缺失或突变最初导致HIF-1α和HIF-2α都被激活,随后由于14q染色体或HIF-1α的的部分缺失将会导致更具侵袭性的VHL基因缺陷型肿瘤[40]。
随着基因组学研究的深入发展,进一步证实了HIF-2α在肾癌的发生中发挥着关键作用。全基因组关联研究(genome-wide association study, GWAS)发现定位于HIF-2α基因第1内含子中的SNP(rs11894252和rs7579899)与肾癌发生发展密切相关[45,46]。此外,GWAS分析也发现了一些其他的基因座位与肾癌的发生发展相关联。其中有一个SNP(rs7105934)定位于11号染色体(11q13.3)的非基因编码区,且其对RCC的发生发展具有保护作用[45]。通过对HIF调节基因的转录组以及大规模的ChIP-Seq数据分析发现rs7105934基因座位正好对应于HIF的结合位点,这一位点作为cyclin D1的增强子序列,在HIF-2α结合后招募p300等转录辅激活因子激活cyclin D1的表达[28, 47]。Cyclin D1是一个功能非常明确的癌基因,它仅在VHL基因缺陷肾癌中作为HIF的一个靶基因[47]。有趣的是,尽管HIF-1α和HIF-2α结合相同的DNA序列且都能与这一增强子序列结合,但是只有HIF-2α的结合能激活cyclin D1的表达。在肾脏中,Cyclin D1在VHL基因缺陷位点也被诱导表达,这暗示VHL-HIF-2α-cyclin D1这一信号通路在肾癌的发生发展中发挥重要作用[34]。
2.3 HIF信号通路与其他突变基因的关系
大规模基因组测序结果显示除VHL基因突变外,ccRCC中还含有其他一些突变频率较高的基因,这些基因主要包括染色质修饰酶类基因(UTX、JARID1C、SETD2和PBRM1等)和去泛素化酶BAP1等。其中,PBRM1的突变频率约为40%,BAP1的突变率大约为14% [48,49,50,51]。此外,ccRCC中存在很大程度的肿瘤内异质性,即同一患者来源肿瘤不同部位肿瘤细胞间从基因型到表型上存在着明显的差 异[52, 53]。目前主流的观点认为,在ccRCC的发生中VHL基因的失活作为肿瘤起始发生的关键步骤,随后,其他基因包括前面提到的一些基因突变将进一步促进肿瘤的发生发展[3]。到目前为止,染色质修饰酶类基因突变是如何与pVHL失活产生联系的还不是很清楚。DNA和组蛋白的修饰是通过表观遗传调节基因转录的重要机制。研究表明许多组蛋白赖氨酸甲基转移酶(histone lysine demethylases,KDM)是HIF的靶基因,缺氧可诱导它们的表达,例如,KDM5C (JARID1C)、KDM3A、KDM2B、KDM4B、KDM5B、KDM6B和KDM4C等[8]。KDMs在功能上既可以促进也可以抑制基因的表达。例如,KDMs可以介导组蛋白H3K27和H3K9的去甲基化从而促进其下游基因的表达,以及介导组蛋白H3K4和H3K36的去甲基化从而抑制基因的表达[3]。JARID1C基因编码一个H3K4me3去甲基化酶。Niu 等[54]证实在VHL基因缺陷的ccRCC细胞中H3K4me3的整体水平要低于VHL正常的对应细胞系,且HIF-2α可激活JARID1C基因的表达。虽然HIF-2α在肾癌中作为一个癌基因发挥功能,但是其激活的下游基因也有一些起抑制肿瘤发生的功能。例如,在VHL基因缺陷的786-O细胞中下调JARID1C的表达明显增加肿瘤细胞的生长,说明JARID1C在肾癌中是一个抑癌基因。在786-O细胞中抑制JARID1C的活性可上调HIF靶基因启动子区的H3K4me3水平,同时上调这些HIF靶基因的表达,这说明了HIF活性和染色质构象之间存在着密切联系[54]。SETD2基因编码一个甲基转移酶蛋白,负责H3K36三甲基化(H3K36me3)修饰。近几年的研究表明,SETD2是一个关键的抑癌基因,其突变与肾癌、白血病和胶质瘤等多种癌症相关[55]。研究发现SETD2基因突变将导致组蛋白缺失H3K36三甲基化修饰,从而增加了开放的染色质结构,因此,SETD2功能的缺失进一步放大了缺氧/HIF信号通路,进一步促进肿瘤的生长[8]。HIF反应依赖于开放的染色质构象,且HIF结合于染色质对胞嘧啶甲基化敏感。因此,我们可以推测在pVHL失活的细胞内,染色质修饰酶类基因突变可导致染色质构象异常进而影响HIF调节下游基因表达。与此推测一致,目前的研究表明JARID1C和SETD2基因对许多HIF靶基因具有明显的调节作用,且染色质构象是影响ccRCC发生发展的一个重要因素[54, 56]。此外,大规模的Chip-Seq研究揭示出肿瘤细胞特异的开放染色质构象是HIF结合的前提条件。PBRM1基因编码的蛋白是SWI/SNF复合物中的一个亚基,SWI/SNF复合物是一种染色质重塑复合物,在肿瘤中起抑癌基因作用,其多个亚基失活与肾癌的发生、发展关系密切,且常常提示肿瘤预后不良。在786-O细胞系(VHL基因缺失但含有功能正常的PBRM1基因)中敲除PBRM1基因将促进细胞的增殖[48]。此外,在VHL基因缺陷的ccRCC细胞中,PBRM1基因的缺失能够放大HIF信号通路[57]。最近,Espana-Agusti等[58]证实在小鼠肾脏中同时缺失VHL和PBRM1基因可导致全浸润、多病灶肿瘤的发展,与人类ccRCC非常相似。其机制可能是PBRM1功能的缺失可以抵消由pVHL失活诱导的复制胁迫,从而促进肾癌发展。由于缺氧也可以诱导DNA复制胁迫,因此pVHL失活导致DNA复制胁迫和DNA损伤积累很可能依赖于HIF的活性[59, 60]。BAP1基因编码一个去泛素化酶,定位于细胞核,该蛋白羧基末端含有泛素水解酶(UCH)结构域。Wang等[61]证实在小鼠中同时缺失VHL和BAP基因将促进肾癌的发展。然而,在肾小管内单独的VHL或BAP基因敲除仅仅会导致肾囊肿和肾功能衰竭。在BAP和VHL基因缺失的小鼠模型中,HIF的下游基因被明显激活,然而,在这个模型中HIF是否发挥重要功能,以及BAP和VHL基因的缺失是否与HIF通路一起协同作用促进肿瘤发生,以及它们之间的联系还不是很清楚,还需要进一步深入研究。
总的来说,这些研究证实了表观遗传修饰与缺氧/HIF信号通路之间具有很大的关联,既这些表观遗传因子能够调节HIF的转录激活活性,HIF反过来也可以调节部分表观遗传因子的表达。但是,它们之间的具体关系和这些基因突变的联合效应还不是很清楚。HIF与表观遗传、以及肾癌中关键基因之间的关系将会是肾癌研究中的一个热点。此外,这些泛基因组的研究结果进一步说明了HIF-α在ccRCC发展中具有关键的作用。
3 HIF-1α和HIF-2α在ccRCC中具有相反功能的分子作用机制
大多数实体肿瘤都显示一定程度的缺氧,且缺氧程度与肿瘤患者的不良预后明显相关[8]。在常氧下,HIF-α可与pVHL蛋白复合物相互作用,最终通过泛素蛋白酶体酶降解HIF-α。HIF-α的脯氨酸残基被脯氨酰羟化酶羟基化是其与VHL相互作用所必须的。在缺氧时,脯氨酰羟化酶失活,HIF-α的羟基化被抑制而使其稳定性增加,进而与HIF-1α形成异源二聚体激活一系列下游基因的表达[3]。HIF-α除了受到氧气依赖的PHD/VHL通路的调节外,它们还受到许多其他信号通路的调控[62]。此外,HSP90以及钙信号通路可以增加HIF-1α的蛋白稳定性,从而能够在常氧下激活HIF信号通路[63]。HIF-1α和HIF-2α亚基虽然结构相似,但在ccRCC中二者的功能相反,以下主要探讨在ccRCC中这二者发挥相反功能的分子作用机制(图1)。图1
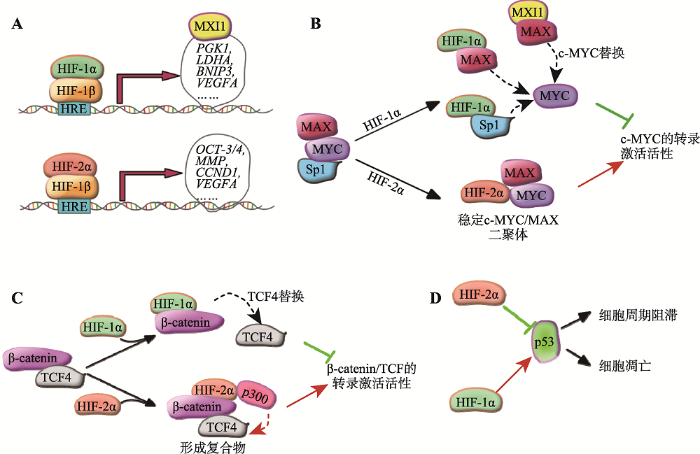 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1HIF-1α和HIF-2α在ccRCC中具有相反功能的分子作用机制
A:HIF-1α和HIF-2α具有各自特异的靶基因; B:HIF-1α与MYC竞争结合Sp1和MAX,以及HIF-1α的下游基因MXI1与MYC竞争结合MAX,从而抑制MYC/MAX二聚体作用于目的基因启动子。而HIF-2α与MAX和MYC相互作用后可促进MAX/MYC二聚体的形成,从而促进MYC的转录激活活性;C:HIF-1α与TCF-4竞争结合β-catenin,抑制β-catenin/TCF-4复合物的形成,从而抑制β-catenin/TCF-4的功能。HIF-2α与β-catenin/TCF-4复合物相互作用后,招募辅助激活因子p300进而促进β-catenin的转录激活活性; D:HIF-1α激活而HIF-2α抑制p53的活性,进而调节细胞周期的阻滞和细胞凋亡。
Fig. 1Molecular mechanisms that HIF-1α and HIF-2α have opposing effects in ccRCC biology
3.1 HIF-1α和HIF-2α对靶基因表达调控的差异
如前所述,尽管HIF-1α和HIF-2α具有相同的结合基序,但是它们具有各自特异的靶基因。这些差异的下游基因很可能是HIF-1α和HIF-2α具有相反功能的重要分子机制(图1A)。此外,HIF-1α和HIF-2α具有不同的转录激活能力,其部分归因于它们的C端转录激活结构域具有对FIH1的不同敏感性。HIF-1α和HIF-2α转录激活结构域内保守的天冬氨酰残基(Asn)被FIH1羟基化,形成空间位阻,抑制辅助激活因子p300和CBP的募集,从而抑制HIF的转录活性。研究证实HIF-1α较HIF-2α对FIH1更加敏感[3]。此外,在RCC细胞系中下调FIH1的表达可增加HIF-1α的转录激活活性并促进细胞凋亡。这说明在肾癌发生发展中,FIH1具有降低HIF-1α的活性和促进细胞成活的作用,是一个促癌基因。然而,FIH1在细胞核中的低表达与ccRCC患者的不良预后相关[64]。这与在RCC中FIH1起促癌基因的功能是矛盾的,因此,FIH1很可能还具有其他的重要功能。3.2 HIF-1α和HIF-2α在信号通路中的不同作用
除了通过转录激活来直接控制肿瘤代谢和细胞存活等相关通路的基因表达外,HIF-1α和HIF-2α也可以直接与一些重要癌基因相互作用并可调节这些癌基因的活性,例如MYC和β-catenin。MYC基因定位于染色体8q24,与多种肿瘤发生发展密切有关,它可通过调节许多细胞周期和细胞代谢相关基因的表达,从而促进细胞的增殖和生长。ccRCC通常伴有MYC基因的异常扩增[13, 65]。虽然HIF-1α和HIF-2α是非常相似,但是现在有证据表明它们在一些情况下是互相拮抗的。例如,HIF-1α抑制而HIF-2α激活MYC的活性[38, 66, 67](图1B)。目前的研究证实HIF-1α可在许多不同的层次上抑制MYC的功能。首先,HIF-1α与MYC竞争结合Sp1,从而抑制MYC作用于目的基因启动子[68]。其次,HIF-1α可与MAX相互作用而阻止MAX-MYC复合物的形成,MAX/ MYC的二聚化是转录因子Myc的活性所必须的。再者,HIF-1α可促进MYC蛋白的蛋白酶体降解,但确切机制远未阐明[67]。最后,HIF-1α可直接结合MXI1基因的启动子从而激活其表达,MXI1与MYC竞争结合MAX,从而破坏MAX/MYC的二聚化而抑制MYC的活性[67]。此外,MXI1/MAX二聚体可结合一些MYC的靶基因的启动子从而抑制这些基因的表达[69]。因此,HIF-1α可拮抗MYC介导的生物学过程。与HIF-1α相反,HIF-2α与MAX和MYC相互作用后可促进MAX/MYC二聚体的形成,从而促进MYC的转录激活活性[66]。鉴于HIF-1α和HIF-2α对MYC活性明显相反的影响,在同时表达HIF-1α和HIF-2α的细胞中,MYC活性是如何被调节的仍然有待进一步的研究。越来越多的研究表明β-catenin信号通路的异常与人类肿瘤密切相关。目前的研究证实HIF-1α抑制β-catenin信号通路,而HIF-2α激活β-catenin的活性(图1C)。HIF-1α与TCF-4竞争结合β-catenin,抑制β-catenin/TCF-4复合物的形成,从而抑制β-catenin/ TCF-4的功能。有趣的是β-catenin /HIF-1α复合物可结合到HIF-1α靶基因启动子处,促进HIF-1α的转录激活活性[70]。HIF-2α也可与β-catenin/TCF-4复合物相互作用,并招募辅助激活因子p300进而促进β-catenin的转录激活活性[71]。此外,在肾癌细胞系中,β-catenin的蛋白水平与VHL负相关,其机制可能是pVHL通过与Jade-1相互作用后增加了Jade-1的蛋白稳定性,而Jade-1可促进β-catenin的降解[72]。因此,在ccRCC中,pVHL的失活可在不同层次上增加β-catenin的功能。
p53是一个抑癌基因,它在许多人类肿瘤中突变或失活。在ccRCC患者中也发现了p53基因的突变,然而,与其他的大多数肿瘤类型相比,在肾癌中,p53基因的突变频率较低[13]。这说明在ccRCC中很可能存在其他的基因突变或机制抑制p53信号通路。HIF-1α能够增强辐射诱导的p53活化,导致增强p53的磷酸化和p53介导的细胞凋亡[73]。此外,HIF-1α可与p53相互作用,并增强p53的蛋白稳定性[74, 75]。相反,HIF-2α可抑制p53的磷酸化,从而抑制其活性。在ccRCC细胞系中下调HIF-2α的表达可促进p53蛋白水平和活性,从而促进细胞周期的阻滞和增加细胞凋亡[76]。因此,HIF-α通过调节p53信号通路很可能在肾癌发生发展中发挥着重要作用(图1D)。
缺氧相关因子(hypoxia-associated factor, HAF)在许多肿瘤组织中高表达,它可以与HIF-1α相互作用并促进HIF-1α的泛素化降解。HAF介导的HIF-1α降解不依赖于细胞内O2/PHD/VHL途径。此外,在RCC4和RCC10细胞系中由于VHL基因突变,pVHL的E3 连接酶活性缺失,pVHL突变体可以与HAF竞争结合HIF-1α。因此,在常氧下,pVHL突变体可以抑制HAF促进的HIF-1α降解,而在缺氧条件下,pVHL突变体不能和HIF-1α相互作用,从而不能保护HIF-1α免于被HAF的泛素化降解[77]。HAF也可以与HIF-2α相互作用,但是HAF并不导致HIF-2α的泛素化降解,相反的,而是促进HIF-2α的转录激活活性[78]。
此外,在VHL基因失活的RCC细胞系中,HIF-1α和HIF-2α可相互调节彼此的蛋白质水平, 例如, HIF-1α的缺失会上调HIF-2α的蛋白水平,反之亦然[34]。因此,遗传学和功能上的数据表明在ccRCC的发生发展中HIF-1α和HIF-2α具有相反的作用。
4 HIF在肾癌临床疗效和靶向治疗上的作用
虽然目前的实验指出HIF-1α和HIF-2α是ccRCC发生发展的重要预后因子,但是仍缺乏在大型病例队列中研究关于HIF-1α和HIF-2α的表达与临床预后之间的关系。Biswas等[79]发现仅有HIF-1α或HIF-2α高表达与患者预后不良相关。然而,当HIF-1α和HIF-2α同时高表达却并没有提示患者预后不良。与以前的研究结果一致,HIF-2α的表达水平与肿瘤大小正相关[38,79]。有趣的是,HIF-2α的下游基因CCND1和GLUT1的表达水平却与患者预后没有关联。同样的,在一个大型研究队列中,Klatte等[80]对357名患者进行分析显示HIF-1α的高表达提示着转移性肾癌患者的不良预后。与这些研究结论相反,在一个含有92名肾癌患者的队列研究中,研究者通过Western blotting手段确定蛋白表达显示HIF-1α的高表达提示预后良好[81]。然而,这两项研究中都没有对HIF-2α的表达水平进行分析。因此,关于HIF-1α和HIF-2α在肾癌中的研究还远远没有结束。自2005年以来,肾癌的靶向治疗药物主要有针对VEGF途径起作用的TKI药物,以及mTOR通路抑制剂类药物。目前有9种靶向VHL-HIF通路的药物被美国食品药品监督管理局(Food and Drug Administration,FDA)批准用于高级别肾癌患者的治疗,其中7种(sorafenib、sunitinib、bevacizumab、pazopanib、axitinib、levantinib和cabozantinib)是靶向VEGF和PDGFR等HIF下游基因的药物。剩下的2种,即temsirolimus和everolimus是靶向mTOR通路的药物。由于VEGFA是HIF的靶基因,以及mTOR具有调节HIF翻译的功能,因此,这2类药物都可以干扰HIF信号通路[3]。虽然这些药物的使用取得了良好的疗效,例如它延长了患者的无疾病进展生存期(progression free survival,PFS)和生存时间,但是最终患者都会对这些药物产生耐药性从而复发。由于HIF在ccRCC中的重要地位,因此提出这样一个问题,肿瘤组织中HIF的水平是否可以预测肿瘤细胞对靶向药物的反应?帕唑帕尼(pazopanib)是一个多靶点的VEGF、PDGFR、FGFR和c-Kit抑制剂,它可明显延长晚期转移性肾癌患者的无疾病进展生存期[82]。然而,Choueiri 等[83]的研究结果显示VHL/ HIF-1α/HIF-2α信号通路与晚期转移性肾癌患者对pazopanib的药物敏感性没有关联。此外,另外一项通过对123例转移性肾癌患者的研究显示患者对VEGF靶向药物的敏感性与VHL和HIF的表达都没有直接关联[84]。与以前的研究结果不一致的是,最近的基于1538例肾癌患者的研究结果显示pazopanib并没有明显改善患者的无疾病进展生存期[85]。因此,仍需研发更有效的新药和更准确的药效评估标志物以提高疗效。
目前,尽管缺乏VHL/HIF信号通路的异常与临床预后之间的确切关联,但是实验数据明确显示HIF通路在ccRCC的发生发展和转移上发挥着关键作 用[3]。Vanharanta等[86]发现在ccRCC的发生发展过程中,肿瘤细胞会利用多种表观遗传机制放大和增强VHL-HIF通路的功能。例如PRC2介导的组蛋白H3第27位赖氨酸上三甲基化(H3K27me3)的缺失将进一步促进HIF激活CXCR4的表达,以及DNA甲基化的缺失有助于HIF激活CYTIP的表达,这些下游基因的表达使得肿瘤细胞具有更强的侵袭和转移能力。这些研究与遗传学研究结论一致,即表观遗传的改变在肿瘤发生发展过程中发挥着至关重要的作用,并且许多负责表观遗传修饰的酶是HIF信号通路的下游基因。这些信号通路是否可以被用来设计新型靶向药物用于治疗ccRCC还需要更加进一步的研究。Chen等[87]通过系统筛选VHL基因缺陷的协同致死基因,发现GLUT1基因是VHL缺陷的ccRCC细胞生存的必需组分。在小鼠异种移植模型中用药物抑制GLUT1的活性能够降低ccRCC细胞的生长速度。此外,有研究证实HIF通路还可以通过AXL-MET信号通路来促进ccRCC的转移[88]。这些研究进一步的说明HIF信号通路在肾癌发生发展中具有至关重要的作用,它能够做为靶点用于开发更有效的新型靶向治疗药物。
最近有研究表明HIF-2α的拮抗剂PT2399可以下调HIF-2下游基因的转录表达以及抑制VHL基因缺陷的ccRCC细胞的生长[89]。其作用机制是:小分子抑制剂PT2399能直接结合到HIF-2α的PAS B结构域上,从而抑制HIF-2α/HIF-1β二聚体的形成。Chen等[89]证实PT2399能抑制56%(10/18)的肿瘤患者来源的移植瘤(patient-derived xenografts, PDXs)的生长,且其抑制效果比当前的一线药物sunitinib更好。他们发现有44%(8/18)的移植瘤对PT2399具有耐药性,尽管在这些细胞中PT2399仍然具有解离HIF-2α/HIF-1β二聚体的作用。大多数对PT2399敏感的移植瘤表达高水平的HIF-2α蛋白。然而,这些耐药的ccRCC移植瘤具有较低表达水平的HIF-2α以及较高表达水平的HIF-1α。其可能的原因是,HIF-1α在不同的pVHL失活的ccRCC中具有不同的作用,它很可能在这些PT2399耐药的ccRCC移植瘤中作为一个促癌基因发挥功能。同样的,Cho等[90]通过一系列体内和体外实验证实PT2399具有抑制大多数VHL缺陷的ccRCC细胞系的生长的作用。与Chen等[89]结果不一样,他们发现ccRCC细胞对PT2399的敏感性与HIF-1α和HIF-2α表达没有相关性,即对PT2399具有耐药性的细胞也表达高水平的HIF-2α。有趣的是,有一个细胞系对HIF-2α的敲除敏感却对PT2399具有耐药性,这说明细胞具有其他的内在耐药机制以及HIF-2α具有不依赖于HIF-2α/ HIF-1β二聚体的功能。此外,Chen等[90] 还报道了利用PT2385(HIF-2α的拮抗剂,与PT2399机制一样)治疗一个高级别肾癌患者的一期临床试验结果。尽管这名患者在接受PT2385治疗之前,已经受到了高剂量的IL12、bevacizumab、sorafenib、everolimus、sunitinib、pazopanib和axitinib的治疗,但是这名患者在长达近一年内没有复发。最近,关于新药PT2385的一期临床试验已经结束,证实了它具有良好的安全性,以及对已经接受过治疗的ccRCC具有良好的抗肿瘤活性,这为其进一步临床应用提供了坚实基础[91]。总的来说,这些研究进一步说明VHL/HIF通路在ccRCC发生发展中发挥着关键作用,并且HIF-2α的抑制剂很可能作为ccRCC治疗的高效靶向药物。
5 结语与展望
在过去的数十年里,遗传学上和功能基因组学上的研究证据都显示缺氧信号通路与ccRCC的发生发展紧密相关。在功能上,HIF-1α和HIF-2α这两个调节氧稳态的核心转录因子在ccRCC的发生发展中具有不同的功能,即HIF-1α作为一个抑癌基因,而HIF-2α作为一个促癌基因发挥作用。目前普遍认为肿瘤的发生发展是多个遗传学改变积累的结果,即肿瘤发生是一个多基因、多因素的过程,涉及不同通路上不同分子事件的相互作用。随着测序技术和分析工具日新月异的发展,产生了一些类型肿瘤变化的综合全景图,使人们对不同肿瘤阶段的基因型和表型有了一个更完整的描述,同时也开辟了一个全新的研究领域。目前,泛基因组学分析显示与ccRCC风险相关的SNP位点与HIF结合位点重叠,因此,这些位点可能通过影响HIF信号通路而调节ccRCC的发生发展。HIF-2α的聚集是肾癌的早期癌前病变事件之一,基因组层面的研究发现在肾癌细胞的不断进化过程中伴随着许多次级遗传事件,这些事件包括HIF-1α的丢失、其他抑癌基因或促癌基因的突变等。此外,这些事件的发生能够进一步影响HIF-2α的活性。这些突变基因中有很大一部分具有染色质修饰的功能,这说明表观遗传学的改变在肾癌发生发展中发挥着至关重要的作用。由于HIF主要作为转录因子发挥功能,需要结合到开放的活性染色质区域,因此染色质的结构改变很可能影响HIF信号通路的活性。但是,到目前为止,具体有哪些遗传事件以及这些事件在癌前病变灶发展为肿瘤过程中的贡献程度大小还不是很清楚,这些事件的具体作用仍需进一步探索。因此,更进一步阐明表观遗传学在HIF通路中的作用机制,将促进人们对低氧胁迫下细胞调控以及VHL-HIF通路在肾癌的发生发展中作用机制的理解,这对将来新药物的开发具有重要的指导意义。目前,对高级别ccRCC的治疗药物主要是靶向HIF信号通路的药物,包括抑制HIF翻译的药物(mTOR抑制剂)和抑制HIF下游基因功能的药物(VEGF抑制剂)。由于这些药物并不是靶向某一个特定的HIF-α异构体,因此HIF-α异构体在这些药物治疗过程中的贡献程度大小还不清楚。此外,这些药物的使用仅仅是延长部分RCC患者无进展生存期,几乎所有的患者都会出现获得性耐药,以及这些药物具有明显的细胞毒性。因此,有必要研发更安全有效的药物用于ccRCC的治疗。最近,有研究表明HIF-2α的拮抗剂PT2399和PT2385可能是用于治疗肾癌很有希望的靶向药物,并且临床1期试验结果显示PT2385安全性良好且在接受过治疗的ccRCC患者中具有抗肿瘤活性,表明HIF-2α拮抗剂在ccRCC患者中具有直接的治疗作用[91]。与TKI药物一样,PT2385和PT2399的耐药问题仍然存 在[89, 90]。大量的研究表明,肿瘤异质性是产生耐药性的主要原因。高级别的ccRCC中确实存在高度异质性,包括在单个肿瘤中染色质重塑相关基因突变的不一致,这说明肿瘤内不同细胞间的表观遗传状态可能存在极大的差异。因此,将HIF-2α的拮抗剂结合靶向表观遗传改变的药物可能会显著提高ccRCC的总体治疗效率。因此,寻找有效的能预测靶向治疗疗效及不良反应的生物标志物、阐明肿瘤的耐药性机制以及寻找克服耐药性的方法,已经成为了当前分子靶向药物研究的热点。可以预测在将来,随着肿瘤生物学及相关学科的飞速发展,将鉴定出更多的药物新靶点,并且会有更多、更有效的药物用于肾癌的治疗。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:20448661 [本文引用: 1]

Abstract Kidney cancer is not a single disease but comprises a number of different types of cancer that occur in the kidney, each caused by a different gene with a different histology and clinical course that responds differently to therapy. Each of the seven known kidney cancer genes, VHL, MET, FLCN, TSC1, TSC2, FH and SDH, is involved in pathways that respond to metabolic stress or nutrient stimulation. The VHL protein is a component of the oxygen and iron sensing pathway that regulates hypoxia-inducible factor (HIF) levels in the cell. HGF-MET signaling affects the LKB1-AMPK energy sensing cascade. The FLCN-FNIP1-FNIP2 complex binds AMPK and, therefore, might interact with the cellular energy and nutrient sensing pathways AMPK-TSC1/2-mTOR and PI3K-Akt-mTOR. TSC1-TSC2 is downstream of AMPK and negatively regulates mTOR in response to cellular energy deficit. FH and SDH have a central role in the mitochondrial tricarboxylic acid cycle, which is coupled to energy production through oxidative phosphorylation. Mutations in each of these kidney cancer genes result in dysregulation of metabolic pathways involved in oxygen, iron, energy or nutrient sensing, suggesting that kidney cancer is a disease of cell metabolism. Targeting the fundamental metabolic abnormalities in kidney cancer provides a unique opportunity for the development of more-effective forms of therapy for this disease.
URLPMID:24857407 [本文引用: 1]

Kidney cancer can be subdivided into related but different cancers that arise from the kidney's tubules. In this article we review current classifications for kidney cancer, discuss their characteristics, and provide an overview of each subtype's clinical behavior and treatment. We stress that each subtype harbors unique biology and thus responds differently to available treatment strategies.
URLPMID:5012644 [本文引用: 17]

In contrast to many tumor types, HIF-1α and HIF-2α have opposing effects on clear cell renal cell carcinoma biology, with HIF-1α acting as a tumor suppressor and HIF-2α as an oncogene. The overall effect of VHL inactivation will depend on fine-tuning of the hypoxia-inducible transcription factor response.
URL [本文引用: 2]
URLPMID:20817385 [本文引用: 2]

Hereditary renal cancers (HRCs) comprise approximately 3-5% of renal cell carcinomas (RCCs).Our aim was to provide an overview of the currently known HRC syndromes in adults.Data on HRC syndromes were analysed using PubMed and Online Mendelian Inheritance in Man with an emphasis on kidney cancer, clinical criteria, management, treatment, and genetic counselling and screening.Ten HRC syndromes have been described that are inherited with an autosomal dominant trait. Eight genes have already been identified (VHL, MET, FH, FLCN, TSC1, TSC2, CDC73, and SDHB). These HRC syndromes involve one or more RCC histologic subtypes and are generally bilateral and multiple. Computed tomography and magnetic resonance imaging are the best imaging techniques for surveillance and assessment of renal lesions, but there are no established guidelines for follow-up after imaging. Except for hereditary leiomyomatosis RCC tumours, conservative treatments favour both an oncologically effective therapeutic procedure and a better preservation of renal function.HRC involves multiple clinical manifestations, histologic subtypes, genetic alterations, and molecular pathways. Urologists should know about HRC syndromes in the interest of their patients and families.
URLPMID:28237281 [本文引用: 1]

Hereditary renal cancers account for approximately 5%-8% of all renal tumors. Over the past 2 decades, a number of syndromes have been identified that predispose patients to early renal cancer development, representing all the major histologic types of tumor pathology. In this article, we describe the current knowledge concerning the cell type, known mechanism of tumor development, other manifestations of the syndrome, imaging findings, genetic screening, and imaging surveillance recommendations for each of the major syndromes associated with hereditary renal cancers.
URLPMID:4775252 [本文引用: 1]

Background Papillary renal-cell carcinoma, which accounts for 15 to 20% of renal-cell carcinomas, is a heterogeneous disease that consists of various types of renal cancer, including tumors with indolent, multifocal presentation and solitary tumors with an aggressive, highly lethal phenotype. Little is known about the genetic basis of sporadic papillary renal-cell carcinoma, and no effective forms of therapy for advanced disease exist. Methods We performed comprehensive molecular characterization of 161 primary papillary renal-cell carcinomas, using whole-exome sequencing, copy-number analysis, messenger RNA and microRNA sequencing, DNA-methylation analysis, and proteomic analysis. Results Type 1 and type 2 papillary renal-cell carcinomas were shown to be different types of renal cancer characterized by specific genetic alterations, with type 2 further classified into three individual subgroups on the basis of molecular differences associated with patient survival. Type 1 tumors were associated with MET alterations, whereas type 2 tumors were characterized by CDKN2A silencing, SETD2 mutations, TFE3 fusions, and increased expression of the NRF2-antioxidant response element (ARE) pathway. A CpG island methylator phenotype (CIMP) was observed in a distinct subgroup of type 2 papillary renal-cell carcinomas that was characterized by poor survival and mutation of the gene encoding fumarate hydratase (FH). Conclusions Type 1 and type 2 papillary renal-cell carcinomas were shown to be clinically and biologically distinct. Alterations in the MET pathway were associated with type 1, and activation of the NRF2-ARE pathway was associated with type 2; CDKN2A loss and CIMP in type 2 conveyed a poor prognosis. Furthermore, type 2 papillary renal-cell carcinoma consisted of at least three subtypes based on molecular and phenotypic features. (Funded by the National Institutes of Health.).
URLPMID:29129785 [本文引用: 4]

Abstract Hypoxia-inducible factor (HIF), a central regulator for detecting and adapting to cellular oxygen levels, transcriptionally activates genes modulating oxygen homeostasis and metabolic activation. Beyond this, HIF influences many other processes. Hypoxia, in part through HIF-dependent mechanisms, influences epigenetic factors, including DNA methylation and histone acetylation, which modulate hypoxia-responsive gene expression in cells. Hypoxia profoundly affects expression of many noncoding RNAs classes that have clinicopathological implications in cancer. HIF can regulate noncoding RNAs production, while, conversely, noncoding RNAs can modulate HIF expression. There is recent evidence for crosstalk between circadian rhythms and hypoxia-induced signaling, suggesting involvement of molecular clocks in adaptation to fluxes in nutrient and oxygen sensing. HIF induces increased production of cellular vesicles facilitating intercellular communication at a distance-for example, promoting angiogenesis in hypoxic tumors. Understanding the complex networks underlying cellular and genomic regulation in response to hypoxia via HIF may identify novel and specific therapeutic targets. Copyright 2017 Elsevier Inc. All rights reserved.
[本文引用: 1]
[本文引用: 1]
URLPMID:25533676 [本文引用: 3]

Abstract Since the Von Hippel-Lindau (VHL) disease tumour suppressor gene VHL was identified in 1993 as the genetic basis for a rare disorder, it has proved to be of wide medical and scientific interest. VHL tumour suppressor protein (pVHL) plays a key part in cellular oxygen sensing by targeting hypoxia-inducible factors for ubiquitylation and proteasomal degradation. Early inactivation of VHL is commonly seen in clear-cell renal cell carcinoma (ccRCC), and insights gained from the functional analysis of pVHL have provided the foundation for the routine treatment of advanced-stage ccRCC with novel targeted therapies. However, recent sequencing studies have identified additional driver genes that are involved in the pathogenesis of ccRCC. As our understanding of the importance of VHL matures, it is timely to review progress from its initial description to current knowledge of VHL biology, as well as future prospects for novel medical treatments for VHL disease and ccRCC.
URL [本文引用: 1]

抑癌基因VHL(von Hippel-Lindau)的突变可导致VHL综合征。其所编码的蛋白产物——肿瘤抑制蛋白VHL(von Hippel-Lindau tumor suppressor protein,p VHL)可与多种底物特别是低氧诱导因子(hipoxia inducible factor,HIF)相互作用。p VHL在有氧状态下主要通过参与组成泛素连接酶复合体作用于HIF,从而介导其泛素化降解。VHL突变所致的HIF活化,导致肿瘤血管生成、肿瘤细胞增多,与肾细胞癌的发生、发展密切相关,可作为肾细胞癌潜在治疗靶点之一。
URL [本文引用: 1]

抑癌基因VHL(von Hippel-Lindau)的突变可导致VHL综合征。其所编码的蛋白产物——肿瘤抑制蛋白VHL(von Hippel-Lindau tumor suppressor protein,p VHL)可与多种底物特别是低氧诱导因子(hipoxia inducible factor,HIF)相互作用。p VHL在有氧状态下主要通过参与组成泛素连接酶复合体作用于HIF,从而介导其泛素化降解。VHL突变所致的HIF活化,导致肿瘤血管生成、肿瘤细胞增多,与肾细胞癌的发生、发展密切相关,可作为肾细胞癌潜在治疗靶点之一。
URLPMID:23797736 [本文引用: 1]

Clear-cell renal cell carcinoma (ccRCC) is the most prevalent kidney cancer and its molecular pathogenesis is incompletely understood. Here we report an integrated molecular study of ccRCC in which 鈮100 ccRCC cases were fully analyzed by whole-genome and/or whole-exome and RNA sequencing as well as by array-based gene expression, copy number and/or methylation analyses. We identified a full spectrum of genetic lesions and analyzed gene expression and DNA methylation signatures and determined their impact on tumor behavior. Defective VHL-mediated proteolysis was a common feature of ccRCC, which was caused not only by VHL inactivation but also by new hotspot TCEB1 mutations, which abolished Elongin C-VHL binding, leading to HIF accumulation. Other newly identified pathways and components recurrently mutated in ccRCC included PI3K-AKT-mTOR signaling, the KEAP1-NRF2-CUL3 apparatus, DNA methylation, p53-related pathways and mRNA processing. This integrated molecular analysis unmasked new correlations between DNA methylation, gene mutation and/or gene expression and copy number profiles, enabling the stratification of clinical risks for patients with ccRCC.
URLPMID:23792563 [本文引用: 4]

Abstract Genetic changes underlying clear cell renal cell carcinoma (ccRCC) include alterations in genes controlling cellular oxygen sensing (for example, VHL) and the maintenance of chromatin states (for example, PBRM1). We surveyed more than 400 tumours using different genomic platforms and identified 19 significantly mutated genes. The PI(3)K/AKT pathway was recurrently mutated, suggesting this pathway as a potential therapeutic target. Widespread DNA hypomethylation was associated with mutation of the H3K36 methyltransferase SETD2, and integrative analysis suggested that mutations involving the SWI/SNF chromatin remodelling complex (PBRM1, ARID1A, SMARCA4) could have far-reaching effects on other pathways. Aggressive cancers demonstrated evidence of a metabolic shift, involving downregulation of genes involved in the TCA cycle, decreased AMPK and PTEN protein levels, upregulation of the pentose phosphate pathway and the glutamine transporter genes, increased acetyl-CoA carboxylase protein, and altered promoter methylation of miR-21 (also known as MIR21) and GRB10. Remodelling cellular metabolism thus constitutes a recurrent pattern in ccRCC that correlates with tumour stage and severity and offers new views on the opportunities for disease treatment.
URL [本文引用: 1]

Von Hippel-Lindau(VHL)综合征是一种较为罕见的常染色体显性遗传疾病,可引起包括中枢神经系统在内的多系统肿瘤。VHL基因是一种抑癌基因,VHL综合征由VHL基因突变引起,VHL基因通过促进缺氧诱导因子1α(HIF-1α)降解导致疾病发生,它通过编码VHL蛋白来调控其mRNA,在缺氧条件下,导致血管内皮生长因子过表达,影响肿瘤的生长浸润。随着基因检测技术日益成熟,VHL综合征的筛查与治疗逐渐出现了多种手段。因为VHL综合征具有遗传性,深入研究患者致病基因类型特点并结合临床表现,有助于深一步挖掘VHL综合征的发病机制,更好地完善基因靶向药物的研究,从而为患者及其家属提供更好的医疗指导。
.
URL [本文引用: 1]

Von Hippel-Lindau(VHL)综合征是一种较为罕见的常染色体显性遗传疾病,可引起包括中枢神经系统在内的多系统肿瘤。VHL基因是一种抑癌基因,VHL综合征由VHL基因突变引起,VHL基因通过促进缺氧诱导因子1α(HIF-1α)降解导致疾病发生,它通过编码VHL蛋白来调控其mRNA,在缺氧条件下,导致血管内皮生长因子过表达,影响肿瘤的生长浸润。随着基因检测技术日益成熟,VHL综合征的筛查与治疗逐渐出现了多种手段。因为VHL综合征具有遗传性,深入研究患者致病基因类型特点并结合临床表现,有助于深一步挖掘VHL综合征的发病机制,更好地完善基因靶向药物的研究,从而为患者及其家属提供更好的医疗指导。
URLPMID:21715564 [本文引用: 1]

Abstract Mutations of the von Hippel-Lindau (VHL) gene are frequent in clear cell renal cell carcinomas (ccRCC). Nonsense and frameshift mutations abrogate the function of the VHL protein (pVHL), whereas missense mutations can have different effects. To identify those missense mutations with functional consequences, we sequenced VHL in 256 sporadic ccRCC and identified 187 different VHL mutations of which 65 were missense mutations. Location and destabilizing effects of VHL missense mutations were determined in silico. The majority of the thermodynamically destabilizing missense mutations were located in exon 1 in the core of pVHL, whereas protein surface mutations in exon 3 affected the interaction domains of elongin B and C. Their impact on pVHL's functionality was further investigated in vitro by stably reintroducing VHL missense mutations into a VHL null cell line and by monitoring the green fluorescent protein (GFP) signals after the transfection of a hypoxia inducible factor (HIF) -GFP expression vector. pVHL's functionality ranged from no effect to complete HIF stabilization. Interestingly, Asn78Ser, Asp121Tyr, and Val130Phe selectively influenced HIF1 and HIF2 degradation. In summary, we obtained three different groups of missense mutations: one with severe destabilization of pVHL; a second without destabilizing effects on pVHL but relevance for the interaction with HIF , elongin B, and elongin C; and a third with pVHL functions comparable with wild type. We therefore conclude that the specific impact of missense mutations may help to distinguish between driver and passenger mutations and may explain responses of ccRCC patients to HIF-targeted therapies.
URLPMID:20368728 [本文引用: 1]

Germ line mutations in the VHL tumor-suppressor gene cause von Hippel-Lindau (VHL) disease, a hereditary neoplastic disease associated with clear-cell renal-cell carcinomas (ccRCCs), central nervous system hemangioblastomas and pheochromocytomas. Disruption of VHL, by somatic mutation, hypermethylation of its promoter or chromosomal loss, is also seen in the majority of cases of sporadic ccRCC. The protein product of VHL, pVHL, has multiple functions, the best-documented of which relates to its ability to target hypoxia-inducible factors (HIFs) for polyubiquitination and proteasomal degradation through its role in substrate recognition as part of a ubiquitin ligase complex. Consequently, pVHL-defective ccRCCs overexpress mRNAs that are under the transcriptional control of HIF. Drugs that modulate the downstream targets of the pVHL/HIF pathway, including sunitinib, sorafenib, temsirolimus and bevacizumab, have proven benefit in treating ccRCC. In VHL disease, clear evidence supports strong genotype-phenotype correlations, but the situation in sporadic ccRCC is less clear. Data indicate that VHL alterations have a potential role as prognostic and predictive markers in ccRCC. Future clinical trials should prospectively define the VHL alteration status of study participants so that the true utility of such markers can be determined.
URLPMID:17526729

Abstract Clear cell carcinoma of the kidney is a major cause of mortality in patients with von Hippel-Lindau (VHL) disease, which is caused by germ line mutations that inactivate the VHL tumor suppressor gene. Biallelic VHL inactivation, due to mutations or hypermethylation, is also common in sporadic clear cell renal carcinomas. The VHL gene product, pVHL, is part of a ubiquitin ligase complex that targets the alpha subunits of the heterodimeric transcription factor hypoxia-inducible factor (HIF) for destruction under well-oxygenated conditions. All VHL mutations linked to classical VHL disease compromise this pVHL function although some missense mutations result in a low risk of kidney cancer (type 2A VHL disease) while others result in a high risk (type 2B VHL disease). We found that type 2A mutants were less defective than type 2B mutants when reintroduced into VHL-/- renal carcinoma cells with respect to HIF regulation. A stabilized version of HIF2alpha promoted tumor growth by VHL-/- cells engineered to produce type 2A mutants, while knock-down of HIF2alpha in cells producing type 2B mutants had the opposite effect. Therefore, quantitative differences with respect to HIF deregulation are sufficient to account for the differential risks of kidney cancer linked to VHL mutations.
URLPMID:16261165

Abstract The von Hippel-Lindau (VHL) tumor suppressor protein is the substrate binding subunit of the CBC(VHL) E3 ubiquitin ligase complex. Mutations in the VHL gene cause a variety of tumors with complex genotype/phenotype correlations. Type 2A and type 2B VHL disease are characterized by a low or high risk of renal cell carcinoma, respectively. To investigate the molecular basis underlying the difference between disease types 2A and 2B, we performed a detailed biochemical analysis of the two most frequent type 2A mutations, Y98 H and Y112 H, in comparison to type 2B mutations in the same residues, Y98N and Y112N. While none of these mutations affected the assembly of CBC(VHL) complexes, the type 2A mutant proteins exhibited higher stabilities at physiological temperature. Moreover, the type 2A mutant proteins possessed higher binding affinities for the key cellular substrate, hypoxia-inducible transcription factor 1 (HIF-1alpha). Consistent with these results, type 2A but not type 2B mutant VHL proteins retained significant ubiquitin ligase activity towards HIF-1alpha in vitro. We propose that this residual ubiquitin ligase activity is sufficient to suppress renal cell carcinogenesis in vivo.
URLPMID:11331612

Abstract von Hippel-Lindau (VHL) disease is a hereditary cancer syndrome caused by germ line mutation of the von Hippel-Lindau tumor suppressor gene (VHL). Tumors observed in this disorder include retinal and central nervous system hemangioblastomas, clear cell renal carcinomas and pheochromocytomas. The VHL gene product, pVHL, is a component of a ubiquitin ligase which targets the transcription factor known as hypoxia-inducible factor (HIF) for degradation in the presence of oxygen. pVHL also plays roles in the control of extracellular matrix formation and cell-cycle exit. Different VHL mutations confer different site-specific risks of cancer. Type 2C VHL mutations confer an increased risk of pheochromocytoma without the other stigmata of VHL disease. Here we report that the products of such type 2C VHL alleles retain the ability to down regulate HIF but are defective for promotion of fibronectin matrix assembly. Furthermore, pVHL L188V, a well studied type 2C mutant, retained the ability to suppress renal carcinoma growth in vivo. These studies strengthen the notion that HIF deregulation plays a causal role in hemangioblastoma and renal carcinoma, and raises the possibility that abnormal fibronectin matrix assembly contributes to pheochromocytoma pathogenesis in the setting of VHL disease.
URLMagsci [本文引用: 1]

细胞内所有的蛋白质和大多数的细胞外蛋白都在不断的进行更新, 即它们在不断地被降解, 并被新合成的蛋白质取代。细胞内蛋白的降解主要通过两个途径, 即自噬和泛素蛋白酶体系统。自噬是一种由溶酶体介导的细胞内过多或异常蛋白质的降解机制。在细胞内主要有3种类型的自噬, 即分子伴侣介导的自噬、微自噬和巨自噬。泛素蛋白酶体系统是由泛素介导的一种高度复杂的蛋白降解机制, 它参与降解细胞内许多蛋白质并且这个过程具有高度特异性。细胞内蛋白质的降解参与调节许多细胞过程, 包括细胞周期、DNA修复、细胞生长和分化、细胞质量的控制、病原生物的感染反应和细胞凋亡等。许多严重的人类疾病被认为是由于蛋白质降解系统的紊乱而引起的。文章综述了自噬和泛素化途径及其分子机制, 以及蛋白质降解系统紊乱的病理学意义。
URLMagsci [本文引用: 1]

细胞内所有的蛋白质和大多数的细胞外蛋白都在不断的进行更新, 即它们在不断地被降解, 并被新合成的蛋白质取代。细胞内蛋白的降解主要通过两个途径, 即自噬和泛素蛋白酶体系统。自噬是一种由溶酶体介导的细胞内过多或异常蛋白质的降解机制。在细胞内主要有3种类型的自噬, 即分子伴侣介导的自噬、微自噬和巨自噬。泛素蛋白酶体系统是由泛素介导的一种高度复杂的蛋白降解机制, 它参与降解细胞内许多蛋白质并且这个过程具有高度特异性。细胞内蛋白质的降解参与调节许多细胞过程, 包括细胞周期、DNA修复、细胞生长和分化、细胞质量的控制、病原生物的感染反应和细胞凋亡等。许多严重的人类疾病被认为是由于蛋白质降解系统的紊乱而引起的。文章综述了自噬和泛素化途径及其分子机制, 以及蛋白质降解系统紊乱的病理学意义。
URLPMID:12490539 [本文引用: 1]

Cellular responses to oxygen are increasingly recognized as critical in normal development and physiology, and are implicated in pathological processes. Many of these responses are mediated by the transcription factors HIF-1 and HIF-2. Their regulation occurs through oxygen-dependent proteolysis of the alpha subunits HIF-1alpha and HIF-2alpha, respectively. Both are stabilized in cell lines exposed to hypoxia, and recently HIF-1alpha was reported to be widely expressed in vivo. In contrast, regulation and sites of HIF-2alpha expression in vivo are unknown, although a specific role in endothelium was suggested. We therefore analyzed HIF-2alpha expression in control and hypoxic rats. Although HIF-2alpha was not detectable under baseline conditions, marked hypoxic induction occurred in all organs investigated, including brain, heart, lung, kidney, liver, pancreas, and intestine. Time course and amplitude of induction varied between organs. Immunohistochemistry revealed nuclear accumulation in distinct cell populations of each tissue, which were exclusively non-parenchymal in some organs (kidney, pancreas, and brain), predominantly parenchymal in others (liver and intestine) or equally distributed (myocardium). These data indicate that HIF-2 plays an important role in the transcriptional response to hypoxia in vivo, which is not confined to the vasculature and is complementary to rather than redundant with HIF-1.
[本文引用: 2]
URLPMID:5100585 [本文引用: 1]

Abstract A wide range of diseases course with an unbalance between the consumption of oxygen by tissues and its supply. This situation triggers a transcriptional response, mediated by the hypoxia inducible factors (HIFs), that aims to restore oxygen homeostasis. Little is known about the inter-individual variation in this response and its role in the progression of disease. Herein, we sought to identify common genetic variants mapping to hypoxia response elements (HREs) and characterize their effect on transcription. To this end, we constructed a list of genome-wide HIF-binding regions from publicly available experimental datasets and studied the genetic variability in these regions by targeted re-sequencing of genomic samples from 96 chronic obstructive pulmonary disease and 144 obstructive sleep apnea patients. This study identified 14 frequent variants disrupting potential HREs. The analysis of the genomic regions containing these variants by means of reporter assays revealed that variants rs1009329, rs6593210 and rs150921338 impaired the transcriptional response to hypoxia. Finally, using genome editing we confirmed the functional role of rs6593210 in the transcriptional regulation of EGFR. In summary, we found that inter-individual variability in non-coding regions affect the response to hypoxia and could potentially impact on the progression of pulmonary diseases. The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
URLPMID:26431912 [本文引用: 1]

Sarcomatoid renal cell carcinomas, highly aggressive variants of renal cell carcinoma subtypes, often present with or develop metastases soon after the primary diagnosis. Most metastatic cases do not respond to immunotherapy or aggressive chemotherapy. Recently targeted therapies, particularly those targeting hypoxia inducible pathway molecules, have been tested clinically on metastatic clear cell renal cell carcinoma with promising initial results. No such studies are available on sarcomatoid renal cell carcinoma. We investigated the hypoxia inducible pathway marker immunohistochemical expression profile, and any potential therapeutic implications that such expression may have, in these tumors.Immunohistochemical staining for hypoxia inducible factor-1alpha, glucose transporter 1, carbonic anhydrase IX and vascular endothelial growth factor was performed in 22 clear cell and 12 nonclear cell sarcomatoid renal cell carcinomas. The immunoreactivity in the tumors was graded from 0 to 3+ (0-no staining, 1+-1% to 25% cells positive, 2+-26% to 50% cells positive and 3+-greater than 50% cells positive). The results were then compared with various clinical parameters to assess for associations.Most clear cell renal cell carcinomas over expressed (2+ or 3+) hypoxia inducible factor-1alpha (in 59%), carbonic anhydrase IX (95%), glucose transporter 1 (91%) and vascular endothelial growth factor (95%). None of the nonclear cell sarcomatoid renal cell carcinomas showed 2+ or 3+ expression of hypoxia inducible factor-1alpha, carbonic anhydrase IX or glucose transporter 1, but 92% showed diffuse positivity for vascular endothelial growth factor. Over expression of carbonic anhydrase IX showed no association with survival, unlike that reported in (nonsarcomatoid) clear cell renal cell carcinoma. There was significant discordance in the staining grades among hypoxia inducible factor-1alpha, carbonic anhydrase IX and glucose transporter 1 in clear cell renal cell carcinoma, suggesting that mechanisms other than hypoxia inducible pathway may be involved in some sarcomatoid clear cell renal cell carcinoma.Hypoxia inducible pathway markers continue to be over expressed in sarcomatoid clear cell renal cell carcinoma, and can be of diagnostic usefulness in such high grade tumors. Over expression of vascular endothelial growth factor in the clear and nonclear cell groups raises the possibility that vascular endothelial growth factor targeted therapies may have a role in the management of sarcomatoid renal cell carcinoma, and deserve further investigation.
URLPMID:1472400 [本文引用: 1]

Metastatic renal cell carcinoma (RCC) resulting from the hereditary loss of the von Hippel-Lindau (VHL) tumor suppressor gene is the leading cause of death in VHL patients due to the deleterious effects of the metastatic tumor(s). VHL functions in the destruction of the alpha subunits of the heterodimeric transcription factor, hypoxia-inducible factor (HIF-1 alpha and HIF-2 alpha), in normoxic conditions. When VHL function is lost, HIF-alpha protein is stabilized, and target hypoxia-inducible genes are transcribed. The process of tumor invasion and metastasis involves the destruction of the extracellular matrix, which is accomplished primarily by the matrix metalloproteinase (MMP) family of enzymes. Here, we describe a connection between the loss of VHL tumor suppressor function and the upregulation of membrane type-1 MMP (MT1-MMP) gene expression and protein. Specifically, MT1-MMP is upregulated in VHL-/- RCC cells through an increase in gene transcription, which is mediated by the cooperative effectsof the transcription factors, HIF-2 and Sp1. Further, we identify a functional HIF-binding site in the proximal promoter of MT1-MMP. To our knowledge, this is the first report to show direct regulation of MT1-MMP by HIF-2 and to provide a direct link between the loss of VHL tumor suppressor function and an increase in MMP gene and protein expression.
URLPMID:16510872 [本文引用: 1]

The division, differentiation, and function of stem cells and multipotent progenitors are influenced by complex signals in the microenvironment, including oxygen availability. Using a genetic "knock-in" strategy, we demonstrate that targeted replacement of the oxygen-regulated transcription factor HIF-1alpha with HIF-2alpha results in expanded expression of HIF-2alpha-specific target genes including Oct-4, a transcription factor essential for maintaining stem cell pluripotency. We show that HIF-2alpha, but not HIF-1alpha, binds to the Oct-4 promoter and induces Oct-4 expression and transcriptional activity, thereby contributing to impaired development in homozygous Hif-2alpha KI/KI embryos, defective hematopoietic stem cell differentiation in embryoid bodies, and large embryonic stem cell (ES)-derived tumors characterized by altered cellular differentiation. Furthermore, loss of HIF-2alpha severely reduces the number of embryonic primordial germ cells, which require Oct-4 expression for survival and/or maintenance. These results identify Oct-4 as a HIF-2alpha-specific target gene and indicate that HIF-2alpha can regulate stem cell function and/or differentiation through activation of Oct-4, which in turn contributes to HIF-2alpha's tumor promoting activity.
URLPMID:15833863 [本文引用: 1]

Abstract Cells exposed to hypoxia respond by increasing the level of hypoxia-inducible factor-1 (HIF-1). This factor then activates a number of genes by binding to hypoxia response elements in their promoter regions. A second hypoxia-responsive factor, HIF-2, can activate many of the same genes as HIF-1. Overexpression of HIFs accompanies the pathogenesis of many tumors. It is unclear, however, as to the respective role of these factors in responsiveness to hypoxia and other stresses. To address this issue, we used microarray technology to study the genes activated in HEK293T cells by hypoxia or transfection with the alpha chain of HIF-1 (or mutant HIF-1 resistant to degradation) or HIF-2. Fifty-six genes were found to be up-regulated at least 3-fold by either hypoxia or transfection. Of these, 21 were elevated both by transfection with HIF-1alpha and with HIF-2alpha, and 14 were preferentially activated by HIF-1alpha including several involved in glycolysis. Ten genes were preferentially activated by HIF-2alpha, including two (CACNA1A and PTPRZ1) implicated in neurologic diseases. Interestingly, most HIF-2alpha-responsive genes were not substantially activated by hypoxia. An additional 10 genes were up-regulated by hypoxia but minimally activated by HIF-1alpha or HIF-2alpha transfection. Ten of the genes were studied by quantitative real-time PCR and/or by Northern blot and the results paralleled those found with microarray technology. Although confirmation in other systems will be necessary, these results indicate that whereas some genes are robustly activated by both HIF-1 and HIF-2, others can be preferentially activated by one or the other factor.
URLPMID:28893800 [本文引用: 2]

Abstract Protein-coding mutations in clear cell renal cell carcinoma (ccRCC) have been extensively characterized, frequently involving inactivation of the von Hippel Lindau (VHL) tumor suppressor. Roles for non-coding cis-regulatory aberrations in ccRCC tumorigenesis, however, remain unclear. Analyzing 10 primary tumor/normal pairs and 9 cell lines across 79 chromatin profiles, we observed pervasive enhancer malfunction in ccRCC, with cognate enhancer-target genes associated with tissue-specific aspects of malignancy. Super-enhancer profiling identified ZNF395 as a ccRCC-specific and VHL-regulated master regulator, whose depletion causes near-complete tumor elimination in vitro and in vivo. VHL loss predominantly drives enhancer/super-enhancer deregulation more so than promoters, with acquisition of active enhancer marks (H3K27ac, H3K4me1) near ccRCC hallmark genes. Mechanistically, VHL loss stabilizes HIF2 -HIF1 heterodimer binding at enhancers, subsequently recruiting histone acetyltransferase P300 without overtly affecting pre-existing promoter-enhancer interactions. Subtype-specific driver mutations such as VHL may thus propagate unique pathogenic dependencies in ccRCC by modulating epigenomic landscapes and cancer gene expression. Copyright 2017, American Association for Cancer Research.
URLPMID:11175344 [本文引用: 1]

The von Hippel-Lindau tumour suppressor gene (VHL) targets hypoxia inducible factor (HIF)-02± subunits for ubiquitin dependent proteolysis. To better understand the role of this and other putative pathways of gene regulation in VHL function we subjected mRNA from VHL defective renal carcinoma cells and transfectants re-expressing a wild type VHL allele to differential expression profiling, and analysed VHL target genes for oxygen regulated expression. Among a group of newly identified VHL target genes the majority but not all were regulated by oxygen, indicating that whilst dysregulation of the HIF system makes a dominant contribution to alterations in transcription, VHL has other influences on patterns of gene expression. Genes newly defined as targets of the VHL/hypoxia pathway (conditionally downregulated by VHL in normoxic cells) include aminopeptidase A, collagen type V, alpha 1, cyclin G2, DEC1/Stra13, endothelin 1, low density lipoprotein receptor-related protein 1, MIC2/CD99, and transglutaminase 2. These genes have a variety of functions relevant to tumour biology. However, not all are connected with the promotion of tumour growth, some being pro-apoptotic or growth inhibitory. We postulate that co-ordinate regulation as part of the HIF pathway may explain this paradox, and that evolution of anti-apoptotic pathways may be required for tumour growth under VHL-dysregulation. Our results indicate that it will be necessary to consider the effects of abnormal activity in integral regulatory pathways, as well as the effects of individual genes to understand the role of abnormal patterns of gene expression in cancer. Oncogene (2000) 19, 629709“6305.
URLPMID:15026807 [本文引用: 1]

Gene expression analysis was performed on a human renal cancer cell line (786-0) with mutated VHL gene and a transfectant with wild-type VHL to analyse genes regulated by VHL and to compare with the gene programme regulated by hypoxia. There was a highly significant concordance of the global gene response to hypoxia and genes suppressed by VHL. Cyclin D1 was the most highly inducible transcript and 14-3-3 epsilon was downregulated. There were some genes regulated by VHL but not hypoxia in the renal cell line, suggesting a VHL role independent of hypoxia. However in nonrenal cell lines they were hypoxia regulated. These included several new pathways regulated by hypoxia, including RNase 6PL, collagen type I alpha 1, integrin alpha 5, ferritin light polypeptide, JM4 protein, transgelin and L1 cell adhesion molecule. These were not found in a recent SAGE analysis of the same cell line. Hypoxia induced downregulation of Cyclin D1 in nonrenal cells via an HIF independent pathway. The selective regulation of Cyclin D I by hypoxia in renal cells may therefore contribute to the tissue selectivity of VHL mutation.
[本文引用: 1]
URLPMID:23324384 [本文引用: 1]

Hypoxia-inducible transcription factors (HIFs) mediate the cellular response to hypoxia. HIF-DNA binding triggers a transcriptional program that acts to both restore oxygen homeostasis and adapt cells to low oxygen availability. In this context, HIF is centrally involved in many physiologic and pathophysiological processes such as development, high altitude adaptation, ischemic disease, inflammation, and cancer. The recent development of chromatin immunoprecipitation coupled to genome-wide DNA sequence analysis allows the position and extent of HIF binding to DNA to be characterized across the entire genome and correlated with genetic, epigenetic, and transcriptional analyses. This review summarizes recent pan-genomic analyses of HIF binding and HIF-dependent transcriptional regulation.
URL [本文引用: 2]
URLPMID:15964822 [本文引用: 3]

Defective function of the von Hippel-Lindau (VHL) tumor suppressor ablates proteolytic regulation of hypoxia-inducible factor alpha subunits (HIF-1alpha and HIF-2alpha), leading to constitutive activation of hypoxia pathways in renal cell carcinoma (RCC). Here we report a comparative analysis of the functions of HIF-1alpha and HIF-2alpha in RCC and non-RCC cells. We demonstrate common patterns of HIF-alpha isoform transcriptional selectivity in VHL-defective RCC that show consistent and striking differences from patterns in other cell types. We also show that HIF-alpha isoforms display unexpected suppressive interactions in RCC cells, with enhanced expression of HIF-2alpha suppressing HIF-1alpha and vice-versa. In VHL-defective RCC cells, we demonstrate that the protumorigenic genes encoding cyclin D1, transforming growth factor alpha, and vascular endothelial growth factor respond specifically to HIF-2alpha and that the proapoptotic gene encoding BNip3 responds positively to HIF-1alpha and negatively to HIF-2alpha, indicating that HIF-1alpha and HIF-2alpha have contrasting properties in the biology of RCC. In keeping with this, HIF-alpha isoform-specific transcriptional selectivity was matched by differential effects on the growth of RCC as tumor xenografts, with HIF-1alpha retarding and HIF-2alpha enhancing tumor growth. These findings indicate that therapeutic approaches to targeting of the HIF system, at least in this setting, will need to take account of HIF isoform-specific functions.
URLPMID:18583945 [本文引用: 1]

Abstract not yet available.
URL [本文引用: 1]
URLPMID:22169972 [本文引用: 1]

Hypoxia-inducible factors (HIFs) are broadly expressed in human cancers, and HIF1α and HIF2α were previously suspected to promote tumour progression through largely overlapping functions. However, this relatively simple model has now been challenged in light of recent data from various approaches that reveal unique and sometimes opposing activities of these HIFα isoforms in both normal physiology and disease. These effects are mediated in part through the regulation of unique target genes, as well as through direct and indirect interactions with important oncoproteins and tumour suppressors, including MYC and p53. As HIF inhibitors are currently undergoing clinical evaluation as cancer therapeutics, a more thorough understanding of the unique roles performed by HIF1α and HIF2α in human neoplasia is warranted.
[本文引用: 3]
URLPMID:4760374 [本文引用: 1]

The signaling adaptor sequestosome 1 (SQSTM1)/p62 is frequently overexpressed in tumors and plays an important role in the regulation of tumorigenesis. Although great progress has been made, biological roles of p62 and relevant molecular mechanisms responsible for its pro-tumor activity remain largely unknown. Here, we show that p62 knockdown reduces cell growth and the expression of glycolytic genes in a manner that depends on HIF1α activity in renal cancer cells. Knockdown of p62 decreases HIF1α levels and transcriptional activity by regulating mTORC1 activity and NF-κB nuclear translocation. Furthermore, p62 interacts directly with the von Hippel-Lindau (VHL) E3 ligase complex to modulate the stability of HIF1α. Mechanistically, p62 binds to the VHL complex and competes with HIF1α. Expression of p62 inhibits the interaction of DCNL1 (also known as DCUN1D1) with CUL2 and attenuates the neddylation of CUL2, and thus downregulates the VHL E3 ligase complex activity. Functionally, HIF1α expression is required for p62-induced glucose uptake, lactate production and soft agar colony growth. Taken together, our findings demonstrate that p62 is a crucial positive regulator of HIF1α, which is a facilitating factor in p62-enhanced tumorigenesis. Highlighted Article:p62 is a crucial positive regulator of HIF1α, which is a facilitating factor in p62-enhanced tumorigenesis.
URLPMID:3202343 [本文引用: 4]

Abstract Kidney cancers often delete chromosome 3p, spanning the VHL tumor suppressor gene, and chromosome 14q, which presumably harbors ≥ 1 tumor suppressor genes. pVHL inhibits the hypoxia-inducible transcription factor (HIF), and HIF2α is a kidney cancer oncoprotein. In this article, we identify focal, homozygous deletions of the HIF1α locus on 14q in clear cell renal carcinoma cell lines. Wild-type HIF1α suppresses renal carcinoma growth, but the products of these altered loci do not. Conversely, downregulation of HIF1α in HIF1α-proficient lines promotes tumor growth. HIF1α activity is diminished in 14q-deleted kidney cancers, and all somatic HIF1α mutations identified in kidney cancers tested to date are loss of function. Therefore, HIF1α has the credentials of a kidney cancer suppressor gene. SIGNIFICANCE: Deletion of 14q is a frequent event in clear cell renal carcinoma and portends a poor prognosis. In this study, we provide genetic and functional evidence that HIF1α is a target of 14q loss in kidney cancer.
URLPMID:12210070 [本文引用: 1]

Abstract The progression of a malignant tumour is understood to be the result of the accumulation of multiple genetic aberrations. As up to 14% of organ-confined renal cell carcinomas will recur after surgery, tumour clones with metastatic potential must already be present in some of these localized tumours. The association of 14q LOH with high-grade tumours and advanced tumour stage suggests an important role for the gene in tumour progression. Chromosome 14q LOH has been analysed in microdissected specimens from 130 organ-confined (UICC TNM stage 1 and 2) clear cell renal cell carcinomas using three microsatellite markers (D14S588, D14S617, GATA136B01). Tumours were classified as 14q LOH or not on the basis of LOH at one or more of the markers. The allelic imbalance ratio was used to determine both LOH and LOH proportion and the association between LOH and mortality, tumour size, histological grade and growth kinetics, measured by quantification of nucleolar organizer regions, was analysed. 14q LOH was present in 35.4% of informative cases at marker D14S588, 24.4% at D14S617, 36.4% at GATA136B01 and 39.5% for any one of the three markers. The mean 14q LOH proportion was 0.24 (range 0.009-0.80). LOH proportion correlated significantly with tumour size, AgNOR score and histological grade. It was also significantly associated with disease-specific mortality; (hazard ratio 1.22; 95% CI 1.02-1.45; p = 0.039). LOH proportion did not remain significant after adjusting for tumour size (hazard ratio 0.98; 95% CI 0.76-1.27; p = 0.90). These results indicate that the proportion of cells with 14q LOH in the tumour is associated with tumour aggressiveness; while this is not an independent predictor of survival, it may have some utility as a marker of latent metastatic potential. Copyright 2002 John Wiley & Sons, Ltd.
URLPMID:15245966 [本文引用: 1]

Our inter- Alu long PCR genomic scan method is a powerful method for the screening of DNA alterations, and our data suggest that the chromosome 14q24-31 region contains likely tumor suppressor genes associated with the progression of RCC.
URLPMID:15202004 [本文引用: 1]

The present study was undertaken to further approach the importance of 14q deletions in renal cell carcinoma (RCC) development. The initial screening using 2 RFLP markers from distal 14q identified loss of heterozygosity (LOH) in 17 of 45 informative cases (38%). In addition, in 37 patients with primary RCCs, it was shown that cases with LOH at D14S1 had significantly shorter survival as compared to cases with-out LOH (p<0.005). Subsequently, 19 primary tumors and 6 metastases were genotyped for 20 polymorphic markers and the findings were evaluated in relation to the clinical characteristics of the primary tumor and the survival during follow-up. Overall LOH was identified in 11 of the primary tumors (58%) and 4 of the metastases (66%). In metastases as well as in primary tumors the highest frequency of LOH was detected with markers from the distal part of the chromosome i.e., 14q32. Five minimal regions of overlapping deletions were identified, three of which (II, IV and V) were defined from the primary RCCs. From centromere to telomere these include region I proximal of D14S259, region II between D14S255 and D14S588, region III in the D14S61-D14S617 interval, region IV between D14S617 and D14S260, and region V telomeric of D14S1007. For the primary tumors, losses in regions IV and V were each significantly associated with high tumor grade (i.e., grade 3; p<0.05). Furthermore, LOH within region IV was also associated with a significantly shorter survival (p=0.02). In conclusion, the high frequency of distal 14q LOH supports the relevance of this alteration for the development of RCC.
URLPMID:2745239 [本文引用: 1]

Recent insights into the role of the von-Hippel Lindau (VHL) tumor suppressor gene in hereditary and sporadic clear-cell renal cell carcinoma (ccRCC) have led to new treatments for patients with metastatic ccRCC, although virtually all patients eventually succumb to the disease. We performed an integrated, genome-wide analysis of copy-number changes and gene expression profiles in 90 tumors, including both sporadic and VHL disease-associated tumors, in hopes of identifying new therapeutic targets in ccRCC. We identified 14 regions of nonrandom copy-number change, including 7 regions of amplification (1q, 2q, 5q, 7q, 8q, 12p, and 20q) and 7 regions of deletion (1p, 3p, 4q, 6q, 8p, 9p, and 14q). An analysis aimed at identifying the relevant genes revealed VHL as one of three genes in the 3p deletion peak, CDKN2A and CDKN2B as the only genes in the 9p deletion peak, and MYC as the only gene in the 8q amplification peak. An integrated analysis to identify genes in amplification peaks that are consistently overexpressed among amplified samples confirmed MYC as a potential target of 8q amplification and identified candidate oncogenes in the other regions. A comparison of genomic profiles revealed that VHL disease-associated tumors are similar to a subgroup of sporadic tumors and thus more homogeneous overall. Sporadic tumors without evidence of biallelic VHL inactivation fell into two groups: one group with genomic profiles highly dissimilar to the majority of ccRCC and a second group with genomic profiles that are much more similar to tumors with biallelic inactivation of VHL.
URL [本文引用: 2]
URLPMID:3277315 [本文引用: 1]

In follow-up of a recent genome-wide association study (GWAS) that identified a locus in chromosome 2p21 associated with risk for renal cell carcinoma (RCC), we conducted a fine mapping analysis of a 120 kb region that includes EPAS1. We genotyped 59 tagged common single-nucleotide polymorphisms (SNPs) in 2278 RCC and 3719 controls of European background and observed a novel signal for rs9679290 [P = 5.75 × 10(-8), per-allele odds ratio (OR) = 1.27, 95% confidence interval (CI): 1.17-1.39]. Imputation of common SNPs surrounding rs9679290 using HapMap 3 and 1000 Genomes data yielded two additional signals, rs4953346 (P = 4.09 × 10(-14)) and rs12617313 (P = 7.48 × 10(-12)), both highly correlated with rs9679290 (r(2) > 0.95), but interestingly not correlated with the two SNPs reported in the GWAS: rs11894252 and rs7579899 (r(2) < 0.1 with rs9679290). Genotype analysis of rs12617313 confirmed an association with RCC risk (P = 1.72 × 10(-9), per-allele OR = 1.28, 95% CI: 1.18-1.39) In conclusion, we report that chromosome 2p21 harbors a complex genetic architecture for common RCC risk variants.
URLPMID:22406644 [本文引用: 2]

Although genome-wide association studies (GWAS) have identified the existence of numerous population-based cancer susceptibility loci, mechanistic insights remain limited, particularly for intergenic polymorphisms. Here, we show that polymorphism at a remote intergenic region on chromosome 11q13.3, recently identified as a susceptibility locus for renal cell carcinoma, modulates the binding and function of hypoxia-inducible factor (HIF) at a previously unrecognized transcriptional enhancer of CCND1 (encoding cyclin D1) that is specific for renal cancers characterized by inactivation of the von Hippel-Lindau tumor suppressor (pVHL). The protective haplotype impairs binding of HIF-2, resulting in an allelic imbalance in cyclin D1 expression, thus affecting a link between hypoxia pathways and cell cycle control.
URLPMID:21248752 [本文引用: 2]

The genetics of renal cancer is dominated by inactivation of the VHL tumour suppressor gene in clear cell carcinoma (ccRCC), the commonest histological subtype. A recent large-scale screen of 3,300 genes by PCR-based exon re-sequencing identified several new cancer genes in ccRCC including UTX (also known as KDM6A), JARID1C (also known as KDM5C) and SETD2 (ref. 2). These genes encode enzymes that demethylate (UTX, JARID1C) or methylate (SETD2) key lysine residues of histone H3. Modification of the methylation state of these lysine residues of histone H3 regulates chromatin structure and is implicated in transcriptional control. However, together these mutations are present in fewer than 15% of ccRCC, suggesting the existence of additional, currently unidentified cancer genes. Here, we have sequenced the protein coding exome in a series of primary ccRCC and report the identification of the SWI/SNF chromatin remodelling complex gene PBRM1 (ref. 4) as a second major ccRCC cancer gene, with truncating mutations in 41% (92/227) of cases. These data further elucidate the somatic genetic architecture of ccRCC and emphasize the marked contribution of aberrant chromatin biology. 2011 Macmillan Publishers Limited. All rights reserved.
URLPMID:2820242 [本文引用: 1]

Abstract Clear cell renal cell carcinoma (ccRCC) is the most common form of adult kidney cancer, characterized by the presence of inactivating mutations in the VHL gene in most cases, and by infrequent somatic mutations in known cancer genes. To determine further the genetics of ccRCC, we have sequenced 101 cases through 3,544 protein-coding genes. Here we report the identification of inactivating mutations in two genes encoding enzymes involved in histone modification-SETD2, a histone H3 lysine 36 methyltransferase, and JARID1C (also known as KDM5C), a histone H3 lysine 4 demethylase-as well as mutations in the histone H3 lysine 27 demethylase, UTX (KMD6A), that we recently reported. The results highlight the role of mutations in components of the chromatin modification machinery in human cancer. Furthermore, NF2 mutations were found in non-VHL mutated ccRCC, and several other probable cancer genes were identified. These results indicate that substantial genetic heterogeneity exists in a cancer type dominated by mutations in a single gene, and that systematic screens will be key to fully determining the somatic genetic architecture of cancer.
URLPMID:22683710 [本文引用: 1]

The molecular pathogenesis of renal cell carcinoma (RCC) is poorly understood. Whole-genome and exome sequencing followed by innovative tumorgraft analyses (to accurately determine mutant allele ratios) identified several putative two-hit tumor suppressor genes, including BAP1. The BAP1 protein, a nuclear deubiquitinase, is inactivated in 15% of clear cell RCCs. BAP1 cofractionates with and binds to HCF-1 in tumorgrafts. Mutations disrupting the HCF-1 binding motif impair BAP1-mediated suppression of cell proliferation but not deubiquitination of monoubiquitinated histone 2A lysine 119 (H2AK119ub1). BAP1 loss sensitizes RCC cells in vitro to genotoxic stress. Notably, mutations in BAP1 and PBRM1 anticorrelate in tumors (P = 3 脳 10(-5)), [corrected] and combined loss of BAP1 and PBRM1 in a few RCCs was associated with rhabdoid features (q = 0.0007). BAP1 and PBRM1 regulate seemingly different gene expression programs, and BAP1 loss was associated with high tumor grade (q = 0.0005). Our results establish the foundation for an integrated pathological and molecular genetic classification of RCC, paving the way for subtype-specific treatments exploiting genetic vulnerabilities.
URLPMID:19330029 [本文引用: 1]

Somatically acquired epigenetic changes are present in many cancers. Epigenetic regulation is maintained via post-translational modifications of core histones. Here, we describe inactivating somatic mutations in the histone lysine demethylase gene UTX, pointing to histone H3 lysine methylation deregulation in multiple tumor types. UTX reintroduction into cancer cells with inactivating UTX mutations resulted in slowing of proliferation and marked transcriptional changes. These data identify UTX as a new human cancer gene. 漏 2009 Nature America, Inc. All rights reserved.
URL [本文引用: 1]
URLPMID:4636053 [本文引用: 1]

Clear cell renal carcinomas (ccRCCs) can display intratumor heterogeneity (ITH). We applied multiregion exome sequencing (M-seq) to resolve the genetic architecture and evolutionary histories of ten ccRCCs. Ultra-deep sequencing identified ITH in all cases. We found that 73-75% of identified ccRCC driver aberrations were subclonal, confounding estimates of driver mutation prevalence. ITH increased with the number of biopsies analyzed, without evidence of saturation in most tumors. Chromosome 3p loss and VHL aberrations were the only ubiquitous events. The proportion of C>T transitions at CpG sites increased during tumor progression. M-seq permits the temporal resolution of ccRCC evolution and refines mutational signatures occurring during tumor development.
URLPMID:21725364 [本文引用: 3]

Abstract In clear-cell renal cell carcinoma (ccRCC), inactivation of the tumor suppressor von Hippel-Lindau (VHL) occurs in the majority of the tumors and is causal for the pathogenesis of ccRCC. Recently, a large-scale genomic sequencing study of ccRCC tumors revealed that enzymes that regulate histone H3 lysine 4 trimethylation (H3K4Me3), such as JARID1C/KDM5C/SMCX and MLL2, were mutated in ccRCC tumors, suggesting that H3K4Me3 might have an important role in regulating gene expression and tumorigenesis. In this study we report that in VHL-deficient ccRCC cells, the overall H3K4Me3 levels were significantly lower than that of VHL+/+ counterparts. Furthermore, this was hypoxia-inducible factor (HIF) dependent, as depletion of HIF subunits by small hairpin RNA in VHL-deficient ccRCC cells restored H3K4Me3 levels. In addition, we demonstrated that only loss of JARID1C, not JARID1A or JARID1B, abolished the difference of H3K4Me3 levels between VHL-/- and VHL+/+ cells, and JARID1C displayed HIF-dependent expression pattern. JARID1C in VHL-/- cells was responsible for the suppression of HIF-responsive genes insulin-like growth factor-binding protein 3 (IGFBP3), DNAJC12, COL6A1, growth and differentiation factor 15 (GDF15) and density-enhanced phosphatase 1. Consistent with these findings, the H3K4Me3 levels at the promoters of IGFBP3, DNAJC12, COL6A1 and GDF15 were lower in VHL-/- cells than in VHL+/+ cells, and the differences disappeared after JARID1C depletion. Although HIF2 is an oncogene in ccRCC, some of its targets might have tumor suppressive activity. Consistent with this, knockdown of JARID1C in 786-O VHL-/- ccRCC cells significantly enhanced tumor growth in a xenograft model, suggesting that JARID1C is tumor suppressive and its mutations are tumor promoting in ccRCC. Thus, VHL inactivation decreases H3K4Me3 levels through JARID1C, which alters gene expression and suppresses tumor growth.
[本文引用: 1]
URLPMID:24158655 [本文引用: 1]

Comprehensive sequencing of human cancers has identified recurrent mutations in genes encoding chromatin regulatory proteins. For clear cell renal cell carcinoma (ccRCC), three of the five commonly mutated genes encode the chromatin regulators PBRM1, SETD2, and BAP1. How these mutations alter the chromatin landscape and transcriptional program in ccRCC or other cancers is not understood. Here, we identified alterations in chromatin organization and transcript profiles associated with mutations in chromatin regulators in a large cohort of primary human kidney tumors. By associating variation in chromatin organization with mutations in SETD2, which encodes the enzyme responsible for H3K36 trimethylation, we found that changes in chromatin accessibility occurred primarily within actively transcribed genes. This increase in chromatin accessibility was linked with widespread alterations in RNA processing, including intron retention and aberrant splicing, affecting 25% of all expressed genes. Furthermore, decreased nucleosome occupancy proximal to misspliced exons was observed in tumors lacking H3K36me3. These results directly link mutations in SETD2 to chromatin accessibility changes and RNA processing defects in cancer. Detecting the functional consequences of specific mutations in chromatin regulatory proteins in primary human samples could ultimately inform the therapeutic application of an emerging class of chromatin-targeted compounds.
URLPMID:28082722 [本文引用: 1]

react-text: 154 In most patients with renal cell carcinoma (RCC) of clear cell subtype, there is inactivation of the von Hippel-Lindau (VHL) tumor-suppressor gene, which leads to a proangiogenic state with overexpression of vascular endothelial growth factor (VEGF). This molecular level knowledge has led to the development of multiple antiangiogenic therapies directed against the VEGF protein or the VEGF... /react-text react-text: 155 /react-text [Show full abstract]
URLPMID:5725450 [本文引用: 1]

Abstract Inactivation of the VHL (Von Hippel Lindau) tumour suppressor has long been recognised as necessary for the pathogenesis of clear cell renal cancer (ccRCC); however, the molecular mechanisms underlying transformation and the requirement for additional genetic hits remain unclear. Here, we show that loss of VHL alone results in DNA replication stress and damage accumulation, effects that constrain cellular growth and transformation. By contrast, concomitant loss of the chromatin remodelling factor PBRM1 (mutated in 40% of ccRCC) rescues VHL-induced replication stress, maintaining cellular fitness and allowing proliferation. In line with these data we demonstrate that combined deletion of Vhl and Pbrm1 in the mouse kidney is sufficient for the development of fully-penetrant, multifocal carcinomas, closely mimicking human ccRCC. Our results illustrate how VHL and PBRM1 co-operate to drive renal transformation and uncover replication stress as an underlying vulnerability of all VHL mutated renal cancers that could be therapeutically exploited.
URLPMID:25956861 [本文引用: 1]

Hypoxia, as a pervasive feature in the microenvironment of solid tumors, plays a significant role in cancer progression, metastasis, and ultimately clinical outcome. One key cellular consequence of hypoxic stress is the regulation of DNA repair pathways, which contributes to the genomic instability and mutator phenotype observed in human cancers. Tumor hypoxia can vary in severity and duration, ranging from acute fluctuating hypoxia arising from temporary blockages in the immature microvasculature, to chronic moderate hypoxia due to sparse vasculature, to complete anoxia at distances more than 150 M from the nearest blood vessel. Paralleling the intra-tumor heterogeneity of hypoxia, the effects of hypoxia on DNA repair occur through diverse mechanisms. Acutely, hypoxia activates DNA damage signaling pathways, primarily via post-translational modifications. On a longer timescale, hypoxia leads to transcriptional and/or translational downregulation of most DNA repair pathways including DNA double-strand break repair, mismatch repair, and nucleotide excision repair. Furthermore, extended hypoxia can lead to long-term persistent silencing of certain DNA repair genes, including BRCA1 and MLH1 , revealing a mechanism by which tumor suppressor genes can be inactivated. The discoveries of the hypoxic modulation of DNA repair pathways have highlighted many potential ways to target susceptibilities of hypoxic cancer cells. In this review, we will discuss the multifaceted hypoxic control of DNA repair at the transcriptional, post-transcriptional, and epigenetic levels, and we will offer perspective on the future of its clinical implications.
URLPMID:3898930 [本文引用: 1]

ATM-mediated signaling in response to DNA damage is a barrier to tumorigenesis. Here we asked whether replication stress could also contribute to ATM signaling. We demonstrate that, in the absence of DNA damage, ATM responds to replication stress in a hypoxia-induced heterochromatin-like context. In certain hypoxic conditions, replication stress occurs in the absence of detectable DNA damage. Hypoxia also induces H3K9me3, a histone modification associated with gene repression and heterochromatin. Hypoxia-induced replication stress together with increased H3K9me3 leads to ATM activation. Importantly, ATM prevents the accumulation of DNA damage in hypoxia. Most significantly, we describe a stress-specific role for ATM in maintaining DNA replication rates in a background of increased H3K9me3. Furthermore, the ATM-mediated response to oncogene-induced replication stress is enhanced in hypoxic conditions. Together, these data indicate that hypoxia plays a critical role in the activation of the DNA damage response, therefore contributing to this barrier to tumorigenesis.
URLPMID:25359211 [本文引用: 1]

Abstract Why different species are predisposed to different tumor spectra is not well understood. In particular, whether the physical location of tumor suppressor genes relative to one another influences tumor predisposition is unknown. Renal cancer presents a unique opportunity to explore this question. Renal cell carcinoma (RCC) of clear-cell type (ccRCC), the most common type, begins with an intragenic mutation in the von Hippel-Lindau (VHL) gene and loss of 3p (where VHL is located). Chromosome 3p harbors several additional tumor suppressor genes, including BRCA1-associated protein-1 (BAP1). In the mouse, Vhl is on a different chromosome than Bap1. Thus, whereas loss of 3p in humans simultaneously deletes one copy of BAP1, loss of heterozygosity in the corresponding Vhl region in the mouse would not affect Bap1. To test the role of BAP1 in ccRCC development, we generated mice deficient for either Vhl or Vhl together with one allele of Bap1 in nephron progenitor cells. Six2-Cre;Vhl F/F ;Bap1 F /+ mice developed ccRCC, but Six2-Cre;Vhl F/F mice did not. Kidneys from Six2-Cre;Vhl F/F ;Bap1 F/+ mice resembled kidneys from humans with VHL syndrome, containing multiple lesions spanning from benign cysts to cystic and solid RCC. Although the tumors were small, they showed nuclear atypia and exhibited features of human ccRCC. These results provide an explanation for why VHL heterozygous humans, but not mice, develop ccRCC. They also explain why a mouse model of ccRCC has been lacking. More broadly, our data suggest that differences in tumor predisposition across species may be explained, at least in part, by differences in the location of two-hit tumor suppressor genes across the genome.
[本文引用: 1]
URL [本文引用: 1]
URLPMID:20709525 [本文引用: 1]

These results show that low nuclear expression of FIH is a strong independent prognostic factor for a poor overall survival in ccRCC.
URLPMID:22605478 [本文引用: 1]

Abstract BACKGROUND: The aim of this study was to evaluate the prevalence of chromosome 8q gain in clear cell renal cell carcinoma (CCRCC) and to correlate the findings with tumor phenotype and disease-specific survival (DSS). METHODS: The tumor karyotypes of 336 consecutive patients with CCRCC were prospectively evaluated with classical cytogenetic analysis. Chromosome 8q status was correlated with clinicopathological variables, and its impact on DSS was evaluated. RESULTS: Gain of 8q occurred in 28 tumors (8.3%). Gain of 8q was associated with a higher risk of regional lymph node (21.4% vs 6.2%, P = .011) and distant metastases (50.0% vs 24.4%, P = .006), and greater tumor sizes (P = .030). Patients with gain of 8q had a 3.22-fold increased risk of death from CCRCC (P < .001). In multivariable analysis, gain of 8q was identified as an independent prognostic factor (hazard ratio, 2.37; P = .006). The concordance index of a multivariable base model increased significantly following inclusion of 8q gain (P = .0015). CONCLUSIONS: Gain of chromosome 8q occurs in a subset of CCRCCs and is associated with an increased risk of metastases and death from CCRCC. Because the proto-oncogene c-MYC is among the list of candidate genes located on 8q, our data suggest that these tumors may have unique pathways activated, which are associated with an aggressive tumor phenotype. If confirmed, defining tumors with gain of 8q may assist in identifying patients who would benefit for specific c-MYC inhibitors or agents that target the MAPK/ERK (mitogen-activated protein kinase) pathway. Copyright 脗漏 2012 American Cancer Society.
URLPMID:3145406 [本文引用: 2]

Abstract HIF-2alpha promotes von Hippel-Lindau (VHL)-deficient renal clear cell carcinoma (RCC) tumorigenesis, while HIF-1alpha inhibits RCC growth. As HIF-1alpha antagonizes c-Myc function, we hypothesized that HIF-2alpha might enhance c-Myc activity. We demonstrate here that HIF-2alpha promotes cell-cycle progression in hypoxic RCCs and multiple other cell lines. This correlates with enhanced c-Myc promoter binding, transcriptional effects on both activated and repressed target genes, and interactions with Sp1, Miz1, and Max. Finally, HIF-2alpha augments c-Myc transformation of primary mouse embryo fibroblasts (MEFs). Enhanced c-Myc activity likely contributes to HIF-2alpha-mediated neoplastic progression following loss of the VHL tumor suppressor and influences the behavior of hypoxic tumor cells.
URLPMID:17482131 [本文引用: 3]

Many cancer cells are characterized by increased glycolysis and decreased respiration, even under aerobic conditions. The molecular mechanisms underlying this metabolic reprogramming are unclear. Here we show that hypoxia-inducible factor 1 (HIF-1) negatively regulates mitochondrial biogenesis and O consumption in renal carcinoma cells lacking the von Hippel-Lindau tumor suppressor (VHL). HIF-1 mediates these effects by inhibiting C-MYC activity via two mechanisms. First, HIF-1 binds to and activates transcription of the gene, which encodes a repressor of C-MYC transcriptional activity. Second, HIF-1 promotes MXI-1-independent, proteasome-dependent degradation of C-MYC. We demonstrate that transcription of the gene encoding the coactivator PGC-1尾 is C-MYC dependent and that loss of PGC-1尾 expression is a major factor contributing to reduced respiration in VHL-deficient renal carcinoma cells.
URL [本文引用: 1]

Hypoxia promotes genetic instability by undefined mechanisms. The transcription factor HIF-1α is crucial for the cellular response to hypoxia and is frequently overexpressed in human cancers, resulting in the activation of genes essential for cell survival. Here, we demonstrate that HIF-1α is responsible for genetic instability at the nucleotide level by inhibiting MSH2 and MSH6 , thereby decreasing levels of the MSH2-MSH6 complex, MutSα, which recognizes base mismatches. HIF-1α displaces the transcriptional activator Myc from Sp1 binding to repress MutSα expression in a p53-dependent manner; Sp1 serves as a molecular switch by recruiting HIF-1α to the gene promoter under hypoxia. Furthermore, in human sporadic colon cancers, HIF-1α overexpression is statistically associated with the loss of MSH2 expression, especially when p53 is immunochemically undetectable. These findings indicate that the regulation of DNA repair is an integral part of the hypoxic response, providing molecular insights into the mechanisms underlying hypoxia-induced genetic instability.
URLPMID:10655054 [本文引用: 1]

Myc and Mad family proteins regulate multiple biological processes through their capacity to influence gene expression directly. Here we show that the basic regions of Myc and Mad proteins are not functionally equivalent in oncogenesis, have separable E-box-binding activities and engage both common and distinct gene targets. Our data support the view that the opposing biological actions of Myc and Mxi1 extend beyond reciprocal regulation of common gene targets. Identification of differentially regulated gene targets provides a framework for understanding the mechanism through which the Myc superfamily governs the growth, proliferation and survival of normal and neoplastic cells.
URLPMID:17220880 [本文引用: 1]

Aberrant activation of beta-catenin promotes cell proliferation and initiates colorectal tumorigenesis. However, the expansion of tumours and the inadequacy of their local vasculature results in areas of hypoxia where cell growth is typically constrained. Here, we report a novel diversion in beta-catenin signalling triggered by hypoxia. We show that hypoxia inhibits beta-catenin-T-cell factor-4 (TCF-4) complex formation and transcriptional activity, resulting in a G1 arrest that involves the c-Myc-p21 axis. Additionally, we find that hypoxia inducible factor-1alpha (HIF-1alpha) competes with TCF-4 for direct binding to beta-catenin. DNA-protein interaction studies reveal that beta-catenin-HIF-1alpha interaction occurs at the promoter region of HIF-1 target genes. Furthermore, rigorous analyses indicate that beta-catenin can enhance HIF-1-mediated transcription, thereby promoting cell survival and adaptation to hypoxia. These findings demonstrate a dynamic role for beta-catenin in colorectal tumorigenesis, where a functional switch is instigated to meet the ever-changing needs of the tumour. This study highlights the importance of the microenvironment in transcriptional regulation.
URL [本文引用: 1]
URLPMID:18806787 [本文引用: 1]

The von Hippel-Lindau protein pVHL suppresses renal tumorigenesis in part by promoting the degradation of hypoxia-inducible HIF-alpha transcription factors; additional mechanisms have been proposed. pVHL also stabilizes the plant homeodomain protein Jade-1, which is a candidate renal tumour suppressor that may correlate with renal cancer risk. Here we show that Jade-1 binds the oncoprotein beta-catenin in Wnt-responsive fashion. Moreover, Jade-1 destabilizes wild-type beta-catenin but not a cancer-causing form of beta-catenin. Whereas the well-established beta-catenin E3 ubiquitin ligase component beta-TrCP ubiquitylates only phosphorylated beta-catenin, Jade-1 ubiquitylates both phosphorylated and non-phosphorylated beta-catenin and therefore regulates canonical Wnt signalling in both Wnt-off and Wnt-on phases. Thus, the different characteristics of beta-TrCP and Jade-1 may ensure optimal Wnt pathway regulation. Furthermore, pVHL downregulates beta-catenin in a Jade-1-dependent manner and inhibits Wnt signalling, supporting a role for Jade-1 and Wnt signalling in renal tumorigenesis. The pVHL tumour suppressor and the Wnt tumorigenesis pathway are therefore directly linked through Jade-1.
URLPMID:16098463 [本文引用: 1]

We have previously shown that radiation increases HIF-1 activity in tumors, causing significant radioprotection of the tumor vasculature. The impact that HIF-1 activation has on overall tumor radiosensitivity, however, is unknown. We reveal here that HIF-1 plays an important role in determining tumor radioresponsiveness through regulating four distinct processes. By promoting ATP metabolism, proliferation, and p53 activation, HIF-1 has a radiosensitizing effect on tumors. Through stimulating endothelial cell survival, HIF-1 promotes tumor radioresistance. As a result, the net effect of HIF-1 blockade on tumor radioresponsiveness is highly dependent on treatment sequencing, with “radiation first” strategies being significantly more effective than the alternative. These data provide a strong rationale for pursuing sequence-specific combinations of HIF-1 blockade and conventional therapeutics.
URL [本文引用: 1]
URL [本文引用: 1]

Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric transcription factor that plays a crucial role in mediating oxygen response in the cell. Using biophysical techniques, we characterized two fragments of the HIF-1alpha subunit, one the full-length ODD domain (residues 403-603) and the second comprising the N-TAD (N-transactivation domain) and inhibitory domain (residues 530-698). Both were unstructured in solution under physiological conditions and so belong to the family of natively unfolded proteins. The HIF-1alpha ODD domain binds weakly to the isolated p53 core domain but tightly to full-length p53 to give a complex of one HIF-1alpha ODD domain with a p53 dimer. By being unstructured, the HIF-1alpha ODD domain can thread both its binding sites through the p53 multimer and bind tightly by the "chelate effect." These results support the idea that hypoxic p53-mediated apoptosis does involve the direct binding of HIF-1alpha to p53.
URLPMID:19706526 [本文引用: 1]

Abstract Approximately 50% of cancer patients receive radiation treatment, either alone or in combination with other therapies. Tumor hypoxia has long been associated with resistance to radiation therapy. Moreover, the expression of hypoxia inducible factors HIF1alpha and/or HIF2alpha correlates with poor prognosis in many tumors. Recent evidence indicates that HIF1alpha expression can enhance radiation-induced apoptosis in cancer cells. We demonstrate here that HIF2alpha inhibition promotes tumor cell death and, in contrast to HIF1alpha, enhances the response to radiation treatment. Specifically, inhibiting HIF2alpha expression augments p53 activity, increases apoptosis, and reduces clonogenic survival of irradiated and non-irradiated cells. Moreover, HIF2alpha inhibition promotes p53-mediated responses by disrupting cellular redox homeostasis, thereby permitting reactive oxygen species (ROS) accumulation and DNA damage. These results correlate with altered p53 phosphorylation and target gene expression in untreated human tumor samples and show that HIF2alpha likely contributes to tumor cell survival including during radiation therapy.
URLPMID:25915846 [本文引用: 1]

Inactivation of the von Hippel-Lindau (VHL) tumor suppressor drives the development of clear-cell renal cell carcinoma (ccRCC) through hypoxia-inducible factors (HIFs). Although ccRCC cells exhibit constitutive normoxic HIF signaling, the potential role of hypoxia in this setting is not fully understood. We show here that the ccRCC cell lines RCC4 and RCC10, which express mutant versions of VHL, have reduced HIF1α expression in hypoxia, whereas HIF2α expression is increased or not affected. Similar findings were observed in normoxia after abrogation of prolyl hydroxylase activity by siRNA or pharmacological inhibition, and by siRNA inhibition of mutant VHL. This reduction of HIF1α protein is due to proteasome-dependent degradation and is mediated by the E3 ubiquitin ligase SART1. HIF1α degradation favors ccRCC proliferation, in line with the previously recognized tumor suppressor capability of HIF1α. Our data indicate that mutant VHL can protect HIF1α from SART1-dependent degradation in normoxic conditions, but this protection is lost in hypoxic settings, favoring hypoxia-dependent ccRCC proliferation. This mechanism of HIF1α degradation might operate in some VHL-related clear-cell renal carcinomas in which the deletion of HIF1α locus does not occur.
URLPMID:3268651 [本文引用: 1]

Abstract Most solid tumors and their metastases experience periods of low oxygen or hypoxia, which is of major clinical significance as it promotes both tumor progression and resistance to therapy. Critical mediators of the hypoxic response are the hypoxia-inducible factors HIF-1α and HIF-2α. The HIFs are nonredundant and regulate both overlapping and unique downstream target genes. Here, we describe a novel mechanism for the switch between HIF-1α- and HIF-2α-dependent transcription during tumor hypoxia caused by the hypoxia associated factor (HAF). HAF is overexpressed in a variety of tumors and its levels are decreased during acute hypoxia, but increased following prolonged hypoxia. We have previously identified HAF as an E3 ubiquitin ligase that binds and ubiquitinates HIF-1α by an oxygen and pVHL-independent mechanism, thus targeting HIF-1α for proteasomal degradation. Here, we show that HAF also binds to HIF-2α, but at a different site than HIF-1α, and increases HIF-2α transactivation without causing its degradation. HAF, thus, switches the hypoxic response of the cancer cell from HIF-1α-dependent to HIF-2α-dependent transcription and activates genes involved in invasion such as MMP9, PAI-1, and the stem cell factor OCT-3/4. The switch to HIF-2α-dependent gene expression caused by HAF also promotes an enriched tumor stem cell population, resulting in highly aggressive tumors in vivo. Thus, HAF, by causing a switch from a HIF-1α- to HIF-2α-dependent response to hypoxia, provides a mechanism for more aggressive growth of tumors under prolonged hypoxia.
URL [本文引用: 2]
URL [本文引用: 1]

ABSTRACT Hypoxia-inducible factor-1 alpha (HIF-1 alpha) plays an important role in tumoral adaptation to hypoxic conditions by serving as a transcription factor for several crucial proteins, including vascular endothelial growth factor and carbonic anhydrase IX (CAIX). Here, we evaluated the significance of HIF-1 alpha in renal cell carcinoma (RCC). Immunohistochemical analysis was done on a tissue microarray constructed from paraffin-embedded primary tumor specimens from 357 patients treated by nephrectomy for RCC. Nuclear expression was evaluated by a single pathologist who was blinded to outcome. The expression levels were associated with pathologic variables and survival. HIF-1 alpha expression was greater in RCC than in benign tissue. Clear cell RCC showed the highest expression levels. In clear cell RCC, HIF-1 alpha was significantly correlated with markers of apoptosis (p21, p53), the mammalian target of rapamycin pathway (pAkt, p27), CXCR3, and proteins of the vascular endothelial growth factor family. HIF-1 alpha was correlated with CAIX and CAXII in localized, but not in metastatic RCC. HIF-1 alpha expression predicted outcome in metastatic patients: patients with high HIF-1 alpha expression (>35%) had significantly worse survival than patients with low expression (< or =35%); median survival, 13.5 versus 24.4 months, respectively (P = 0.005). Multivariate analysis retained HIF-1 alpha and CAIX expression as the strongest independent prognostic factors for patients with metastatic clear cell RCC. HIF-1 alpha is an important independent prognostic factor for patients with metastatic clear cell RCC. Because HIF-1 alpha and CAIX are independently and differentially regulated in metastatic clear cell RCC, both tumor markers can be complementary in predicting prognosis.
URLPMID:15709180 [本文引用: 1]

Abstract PURPOSE: Renal cell carcinoma (RCC) is the most common malignancy of the kidney composed of specific tumor types. The sporadic conventional RCCs are, in contrast to the other RCC types, characterized by a high rate of von Hippel-Lindau (VHL) mutations and hypermethylation. The majority of these tumors lack functional VHL protein (pVHL) that leads to increased hypoxia-inducible factor 1alpha (HIF-1alpha) expression. The pVHL is the physiologic regulator of the activity of HIF-1alpha by targeting it to the proteasome for degradation under normoxia. Both pVHL and HIF-1alpha target other genes that are important for cancer survival and proliferation. Expression of HIF-1alpha has been linked to poor prognosis in different malignancies, although few studies have been done on the relation between HIF-1alpha and clinical variables in RCC. EXPERIMENTAL DESIGN: HIF-1alpha protein expression was analyzed in tumor tissue from 92 patients with RCC. HIF-1alpha was quantified by Western blot relative to a positive control. RESULTS: The HIF-1alpha protein was expressed as two bands which strongly correlated (r = 0.906, P < 0.001); therefore, they were added and the sum evaluated against clinicopathologic variables. There was no association between HIF-1alpha and gender, stage, grade, tumor size, or vein invasion. Conventional RCCs had significantly higher HIF-1alpha expression compared with papillary and chromophobe RCCs and kidney cortex. In conventional RCC, HIF-1alpha was an independent prognostic factor. CONCLUSION: HIF-1alpha levels varied significantly between the different RCC types. In conventional RCC, HIF-1alpha was an independent prognostic factor. These data indicate that HIF-1alpha is involved in tumorogenesis and progression of RCC. Evaluation of other HIF target gene products and correlation to angiogenesis seems warranted.
URLPMID:20100962 [本文引用: 1]

Abstract PURPOSE Pazopanib is an oral angiogenesis inhibitor targeting vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and c-Kit. This randomized, double-blind, placebo-controlled phase III study evaluated efficacy and safety of pazopanib monotherapy in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). PATIENTS AND METHODS Adult patients with measurable, locally advanced, and/or metastatic RCC were randomly assigned 2:1 to receive oral pazopanib or placebo. The primary end point was progression-free survival (PFS). Secondary end points included overall survival, tumor response rate (Response Evaluation Criteria in Solid Tumors), and safety. Radiographic assessments of tumors were independently reviewed. Results Of 435 patients enrolled, 233 were treatment naive (54%) and 202 were cytokine pretreated (46%). PFS was significantly prolonged with pazopanib compared with placebo in the overall study population (median, PFS 9.2 v 4.2 months; hazard ratio [HR], 0.46; 95% CI, 0.34 to 0.62; P < .0001), the treatment-naive subpopulation (median PFS 11.1 v 2.8 months; HR, 0.40; 95% CI, 0.27 to 0.60; P < .0001), and the cytokine-pretreated subpopulation (median PFS, 7.4 v 4.2 months; HR, 0.54; 95% CI, 0.35 to 0.84; P < .001). The objective response rate was 30% with pazopanib compared with 3% with placebo (P < .001). The median duration of response was longer than 1 year. The most common adverse events were diarrhea, hypertension, hair color changes, nausea, anorexia, and vomiting. There was no evidence of clinically important differences in quality of life for pazopanib versus placebo. CONCLUSION Pazopanib demonstrated significant improvement in PFS and tumor response compared with placebo in treatment-naive and cytokine-pretreated patients with advanced and/or metastatic RCC.
URLPMID:4522695 [本文引用: 1]

Abstract PURPOSE: Inactivation of von Hippel-Lindau (VHL) gene in clear-cell renal cell carcinoma (RCC) leads to increased levels of hypoxia-inducible factors (HIF) and overexpression of HIF target genes, such as VEGF and others. VEGF-targeted agents are standard in advanced clear-cell RCC but biomarkers of activity are lacking. EXPERIMENTAL DESIGN: We analyzed tumor tissue samples from metastatic clear-cell RCC patients who received pazopanib as part of clinical trial VEG102616. We evaluated several components of the VHL/HIF pathway: VHL gene inactivation (mutation and/or methylation), HIF-102± and HIF-202± immunohistochemistry staining, and HIF-102± transcriptional signature. We evaluated the association of these biomarkers with best overall response rate (ORR) and progression-free survival (PFS) to pazopanib, a standard first-line VEGF-targeted agent. RESULTS: The VEG102616 trial enrolled 225 patients, from whom 78 samples were available for tumor DNA extraction. Of these, 70 patients had VHL mutation or methylation. VHL gene status did not correlate with ORR or PFS. Similarly, HIF-102± (65 samples) and HIF-202± (66 samples) protein levels (high vs. low) did not correlate with ORR or PFS to pazopanib. The HIF-102± transcriptional signature (46 samples) was enriched in tumors expressing high HIF-102± levels. However, the HIF-102± gene expression signature was not associated with clinical outcome to pazopanib. CONCLUSIONS: In patients with advanced clear-cell RCC, several potential biomarkers along the VHL/HIF-102±/HIF-202± axis were not found to be predictive for pazopanib activity. Additional efforts must continue to identify biomarkers associated with clinical outcome to VEGF-targeted agents in metastatic RCC. 00082013 AACR.
URL [本文引用: 1]
URLPMID:28902533 [本文引用: 1]

Abstract Purpose This phase III trial evaluated the efficacy and safety of pazopanib versus placebo in patients with locally advanced renal cell carcinoma (RCC) at high risk for relapse after nephrectomy. Patients and Methods A total of 1,538 patients with resected pT2 (high grade) or 鈮 pT3, including N1, clear cell RCC were randomly assigned to pazopanib or placebo for 1 year; 403 patients received a starting dose of 800 mg or placebo. To address toxicity attrition, the 800-mg starting dose was lowered to 600 mg, and the primary end point analysis was changed to disease-free survival (DFS) for pazopanib 600 mg versus placebo (n = 1,135). Primary analysis was performed after 350 DFS events in the intent-to-treat (ITT) pazopanib 600 mg group (ITT 600mg ), and DFS follow-up analysis was performed 12 months later. Secondary end point analyses included DFS with ITT pazopanib 800 mg (ITT 800mg ) and safety. Results The primary analysis results of DFS ITT 600mg favored pazopanib but did not show a significant improvement over placebo (hazard ratio [HR], 0.86; 95% CI, 0.70 to 1.06; P = .165). The secondary analysis of DFS in ITT 800mg (n = 403) yielded an HR of 0.69 (95% CI, 0.51 to 0.94). Follow-up analysis in ITT 600mg yielded an HR of 0.94 (95% CI, 0.77 to 1.14). Increased ALT and AST were common adverse events leading to treatment discontinuation in the pazopanib 600 mg (ALT, 16%; AST, 5%) and 800 mg (ALT, 18%; AST, 7%) groups. Conclusion The results of the primary DFS analysis of pazopanib 600 mg showed no benefit over placebo in the adjuvant setting.
URLPMID:3540187 [本文引用: 1]

Abstract Inactivation of the von Hippel-Lindau tumor suppressor gene, VHL, is an archetypical tumor-initiating event in clear cell renal carcinoma (ccRCC) that leads to the activation of hypoxia-inducible transcription factors (HIFs). However, VHL mutation status in ccRCC is not correlated with clinical outcome. Here we show that during ccRCC progression, cancer cells exploit diverse epigenetic alterations to empower a branch of the VHL-HIF pathway for metastasis, and the strength of this activation is associated with poor clinical outcome. By analyzing metastatic subpopulations of VHL-deficient ccRCC cells, we discovered an epigenetically altered VHL-HIF response that is specific to metastatic ccRCC. Focusing on the two most prominent pro-metastatic VHL-HIF target genes, we show that loss of Polycomb repressive complex 2 (PRC2)-dependent histone H3 Lys27 trimethylation (H3K27me3) activates HIF-driven chemokine (C-X-C motif) receptor 4 (CXCR4) expression in support of chemotactic cell invasion, whereas loss of DNA methylation enables HIF-driven cytohesin 1 interacting protein (CYTIP) expression to protect cancer cells from death cytokine signals. Thus, metastasis in ccRCC is based on an epigenetically expanded output of the tumor-initiating pathway.
[本文引用: 1]
URLPMID:25187556 [本文引用: 1]

Abstract Dysregulation of the von Hippel-Lindau/hypoxia-inducible transcription factor (HIF) signaling pathway promotes clear cell renal cell carcinoma (ccRCC) progression and metastasis. The protein kinase GAS6/AXL signaling pathway has recently been implicated as an essential mediator of metastasis and receptor tyrosine kinase crosstalk in cancer. Here we establish a molecular link between HIF stabilization and induction of AXL receptor expression in metastatic ccRCC. We found that HIF-1 and HIF-2 directly activate the expression of AXL by binding to the hypoxia-response element in the AXL proximal promoter. Importantly, genetic and therapeutic inactivation of AXL signaling in metastatic ccRCC cells reversed the invasive and metastatic phenotype in vivo. Furthermore, we define a pathway by which GAS6/AXL signaling uses lateral activation of the met proto-oncogene (MET) through SRC proto-oncogene nonreceptor tyrosine kinase to maximize cellular invasion. Clinically, AXL expression in primary tumors of ccRCC patients correlates with aggressive tumor behavior and patient lethality. These findings provide an alternative model for SRC and MET activation by growth arrest-specific 6 in ccRCC and identify AXL as a therapeutic target driving the aggressive phenotype in renal clear cell carcinoma.
URLPMID:27595394 [本文引用: 4]

Clear cell renal cell carcinoma (ccRCC) is characterized by inactivation of the von Hippel-Lindau tumour suppressor gene (VHL). Because no other gene is mutated as frequently in ccRCC and VHL mutations are truncal, VHL inactivation is regarded as the governing event. VHL loss activates the HIF-2 transcription factor, and constitutive HIF-2 activity restores tumorigenesis in VHL-reconstituted ccRCC cells. HIF-2 has been implicated in angiogenesis and multiple other processes, but angiogenesis is the main target of drugs such as the tyrosine kinase inhibitor sunitinib. HIF-2 has been regarded as undruggable. Here we use a tumourgraft/patient-derived xenograft platform to evaluate PT2399, a selective HIF-2 antagonist that was identified using a structure-based design approach. PT2399 dissociated HIF-2 (an obligatory heterodimer of HIF-2 -HIF-1 ) in human ccRCC cells and suppressed tumorigenesis in 56% (10 out of 18) of such lines. PT2399 had greater activity than sunitinib, was active in sunitinib-progressing tumours, and was better tolerated. Unexpectedly, some VHL-mutant ccRCCs were resistant to PT2399. Resistance occurred despite HIF-2 dissociation in tumours and evidence of Hif-2 inhibition in the mouse, as determined by suppression of circulating erythropoietin, a HIF-2 target and possible pharmacodynamic marker. We identified a HIF-2-dependent gene signature in sensitive tumours. Gene expression was largely unaffected by PT2399 in resistant tumours, illustrating the specificity of the drug. Sensitive tumours exhibited a distinguishing gene expression signature and generally higher levels of HIF-2伪. Prolonged PT2399 treatment led to resistance. We identified binding site and second site suppressor mutations in HIF-2 and HIF-1 , respectively. Both mutations preserved HIF-2 dimers despite treatment with PT2399. Finally, an extensively pretreated patient whose tumour had given rise to a sensitive tumourgraft showed disease control for more than 11 months when treated with a close analogue of PT2399, PT2385. We validate HIF-2 as a target in ccRCC, show that some ccRCCs are HIF-2 independent, and set the stage for biomarker-driven clinical trials.
URLPMID:27595393 [本文引用: 3]

Clear cell renal cell carcinoma, the most common form of kidney cancer, is usually linked to inactivation of the pVHL tumour suppressor protein and consequent accumulation of the HIF-2 transcription factor (also known as EPAS1). Here we show that a small molecule (PT2399) that directly inhibits HIF-2伪 causes tumour regression in preclinical mouse models of primary and metastatic pVHL-defective clear cell renal cell carcinoma in an on-target fashion. pVHL-defective clear cell renal cell carcinoma cell lines display unexpectedly variable sensitivity to PT2399, however, suggesting the need for predictive biomarkers to be developed to use this approach optimally in the clinic.
URLPMID:29257710 [本文引用: 2]

Abstract Purpose The von Hippel-Lindau tumor suppressor is inactivated in the majority of clear cell renal cell carcinomas (ccRCCs), leading to inappropriate stabilization of hypoxia-inducible factor-2 (HIF-2 ). PT2385 is a first-in-class HIF-2 antagonist. Objectives of this first-in-human study were to characterize the safety, pharmacokinetics, pharmacodynamics, and efficacy, and to identify the recommended phase II dose (RP2D) of PT2385. Patients and Methods Eligible patients had locally advanced or metastatic ccRCC that had progressed during one or more prior regimens that included a vascular endothelial growth factor inhibitor. PT2385 was administered orally at twice-per-day doses of 100 to 1,800 mg, according to a 3 + 3 dose-escalation design, followed by an expansion phase at the RP2D. Results The dose-escalation and expansion phases enrolled 26 and 25 patients, respectively. Patients were heavily pretreated, with a median of four (range, one to seven) prior therapies. No dose-limiting toxicity was observed at any dose. On the basis of safety, pharmacokinetic, and pharmacodynamic profiling, the RP2D was defined as 800 mg twice per day. PT2385 was well tolerated, with anemia (grade 1 to 2, 35%; grade 3, 10%), peripheral edema (grade 1 to 2, 37%; grade 3, 2%), and fatigue (grade 1 to 2, 37%; no grade 3 or 4) being the most common treatment-emergent adverse events. No patients discontinued treatment because of adverse events. Complete response, partial response, and stable disease as best response were achieved by 2%, 12%, and 52% of patients, respectively. At data cutoff, eight patients remained in the study, with 13 patients in the study for 1 year. Conclusion PT2385 has a favorable safety profile and is active in patients with heavily pretreated ccRCC, validating direct HIF-2伪 antagonism for the treatment of patients with ccRCC.
