 ,陕西省干细胞工程技术研究中心,西北农林科技大学动物医学院,杨凌 712100
,陕西省干细胞工程技术研究中心,西北农林科技大学动物医学院,杨凌 712100Research progress on the asymmetric division in mammalian oocytes
Junyu Zhang, Shan Lv, Huimin Niu, Anmin Lei ,College of Veterinary Medicine ,Shaanxi Center of Stem Cell Engineering &Technology , Northwest Sci-Tech University of Agriculture and Forestry , Yangling , Shaanxi 712100, China
,College of Veterinary Medicine ,Shaanxi Center of Stem Cell Engineering &Technology , Northwest Sci-Tech University of Agriculture and Forestry , Yangling , Shaanxi 712100, China通讯作者:
编委: 史庆华
收稿日期:2017-11-28修回日期:2018-03-4网络出版日期:2018-04-20
| 基金资助: |
Editorial board:
Received:2017-11-28Revised:2018-03-4Online:2018-04-20
| Fund supported: |
作者简介 About authors
张俊玉,硕士研究生,专业方向:动物胚胎工程E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (609KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张俊玉, 吕珊, 牛慧敏, 雷安民. 哺乳动物卵母细胞不对称分裂的研究进展. 遗传[J], 2018, 40(4): 279-291 doi:10.16288/j.yczz.17-195
Junyu Zhang, Shan Lv, Huimin Niu, Anmin Lei.
哺乳动物卵母细胞的不对称分裂可以大致分为两个方面:一个是形态学意义的细胞质不对称分裂;另外一个是细胞核的不对称分裂。以小鼠卵母细胞为例(图1),第一次减数分裂中细胞质不对称分裂过程包括纺锤体在卵母细胞近中心或中心形成,纺锤体以肌动蛋白依赖的方式沿着长轴,向离它最近的皮质区迁移,并形成一个富含纤维状肌动蛋白(F-actin)的区域[1, 2]。同时,这个富含F-actin的区域(即肌动蛋白帽)的皮质颗粒密度降低,形成一个无皮质颗粒区域(cortical granule-free domain,CGFD),这是极体的排出位点[3],皮质极性也伴随着这一过程建立。在减数分裂中后期转换时,收缩环在富含F-actin的皮质区装配形成,并环绕着纺锤体中部区域。在减数分裂的后期,富含肌动蛋白的区域向外突出,同时收缩环开始收缩,随后排出第一极体[1]。卵母细胞排出第一极体后便阻滞在第二次减数分裂中期(metaphase Ⅱ, MⅡ),此时的纺锤体定位在极化的皮质区下并与皮质表面平行。卵母细胞被激活后,卵母细胞从MⅡ阻滞中释放,纺锤体旋转后排出第二极体[2],减数分裂结束。
图1
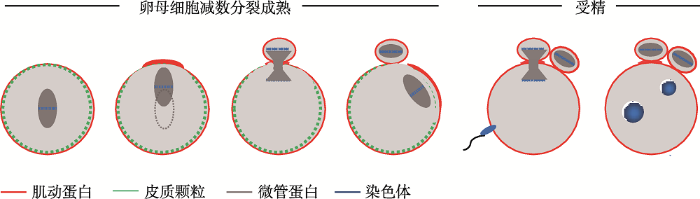 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1小鼠卵母细胞减数分裂模型
Fig. 1Meiotic model for meiosis in mouse oocytes
卵母细胞细胞核的不对称分裂表现在染色体的非随机分离。生发泡破裂(germinal vesicle breakdown, GVBD)后形成的纺锤体是对称的,在纺锤体向皮质区迁移的过程中,皮质区的CDC42诱导纺锤体微管发生不对称的酪氨酸修饰。而自私元件利用这种不对称性操控小鼠卵母细胞的染色体分离过程,使染色体发生非随机分离。
本文从细胞质和细胞核两个方面对卵母细胞的不对称分裂现象、形态学过程以及与调控机制进行阐述,并对染色体的非随机分离机制的研究前景和未来应用进行探讨。
1 卵母细胞细胞质不对称分裂的分子机制
哺乳动物卵母细胞的不对称分裂是一个复杂且精细的过程[3],需要染色体、细胞骨架网络及多种蛋白分子协同有序发挥作用以确保对不对称分裂的精准调控[3,4,5],任何一个环节出错都可能影响减数分裂的正常进行和卵母细胞的不对称分裂过程。表1汇总了涉及的有关哺乳动物卵母细胞不对称分裂有关的因子。Table 1
表1
表1 哺乳动物卵母细胞不对称分裂的调控因子
Table 1
| 类别 | 蛋白名称 | 处理方法 | 表型/作用 | 参考文献 | |
|---|---|---|---|---|---|
| 英文全称 | 简称 | ||||
| 肌动蛋 白成核 因子 | Actin-related protein-2/3 | Arp2/3复合物 | RNA干扰、抑制剂 | 纺锤体迁移失败、胞质分裂失败、破坏肌动蛋白帽和CGFD;控制皮质下肌动蛋白厚度 | [6~9] |
| Formin2 | FMN2 | mRNA、 注射基因敲除鼠 | 纺锤体迁移失败、胞质分裂失败、发生对等分裂;纺锤体形成 | [10~12] | |
| Spire | Spire | mRNA注射、RNAi | 纺锤体迁移失败、胞质分裂失败、发生对等分裂 | [13] | |
| Mammalian diaphanous1 | mDia1 | mRNA注射 | mDia定位在纺锤体极点 | [11,14] | |
| Formin-like 1 | FMN1 | MO注射 | 破坏皮质极性、破坏肌动蛋白帽和CGFD的形成、破坏纺锤体迁移、极体排出率降低、排出大极体、异常的肌动蛋白表达水平 | [14,15] | |
| 成核促 进因子 | Neural-Wiskott-Aldrich syndrome protein | N-WASP | MO注射、 基因敲除鼠 | Arp2表达降低;破坏第二次减数分裂、小鼠生育能力低下 | [16,17] |
| Wasp-family verprolin homologous protein2 | WAVE2 | RNAi、抗体注射 | 纺锤体迁移失败、胞质分裂失败、破坏肌动蛋白帽和CGFD | [18] | |
| Junction mediating and regulatory protein | JMY | RNAi、抗体注射 | 纺锤体迁移失败、胞质分裂失败、发生对称分裂、破坏肌动蛋白帽和CGFD,减低降低激动蛋白水平 | [19,20] | |
| WAS protein homolog associated with actin,golgi membranes and microtubules | WHAMM | SiRNA | 纺锤体迁移失败、胞质分裂失败、发生对称分裂、破坏肌动蛋白帽 | [21] | |
| Wiskott-Aldrich Syndrome homologue | WASH | MO注射、抗体注射 | 纺锤体形成异常、纺锤体迁移失败、极体排除率降低、发生对称分裂 | [22] | |
| 小GTP 酶 | Ras homolog family member A | RhoA | mRNA注射、 siRNA、抑制剂 | 纺锤体形成异常、纺锤体迁移失败、胞质分裂失败 | [23,24] |
| Cell division cycle 42 | Cdc42 | 突变体、抑制剂 | 纺锤体迁移失败、抑制肌动蛋白装配、膜突出失败、第一极体排除率降低 | [25,26] | |
| Ras-related nuclear protein | Ran | 突变体 | 破坏皮质区极性、对肌动蛋白帽的形成和微绒毛去装配有作用 | [6,27] | |
| Ras-related C3 botulinum toxin substrate | Rac | 突变体 | 纺锤体延伸、胞质分裂失败 | [28,29] | |
| ADP-ribosylation factor 1 | Arf1 | 突变体 | 对等分裂纺锤体迁移失败、纺锤体延伸 | [30] | |
| 其他调 控因子 | Myosin Ⅱ | Myosin Ⅱ | 抗体注射 | 胞质分裂失败 | [31] |
| Ezrin-radixin-moesin | ERM家族 | 突变体 | 破坏皮质区极性、胞质分裂失败 | [32] | |
| Dynamin2 | Dynamin2 | 抑制剂 | 破坏肌动蛋白帽和CGFD、极体排出受阻、纺锤体定位异常 | [33] | |
| LIM kinase1/2 | LIMK1/2 | 抑制剂 | 纺锤体定位异常、破坏肌动蛋白分布、破坏胞质分裂 | [34] | |
| myosin light chain kinase | MLCK | 抑制剂 | 破坏皮质区重组和皮质区极性、破坏胞质分裂、影响纺锤体迁移、减少皮质张力 | [35~37] | |
| 类别 | 蛋白名称 | 处理方法 | 表型/作用 | 参考文献 | |
| 英文全称 | 简称 | ||||
| 其他调 控因子 | Golgi matrix protein 130 kDa | GM130 | MO注射、RNAi | 纺锤体延伸、纺锤体迁移失败、发生对称分裂、肌动蛋白帽丢失 | [21,38,39] |
| Tyrosine-protein kinase | Fyn | 基因敲鼠、RNAi、 抑制剂 | 胞质分裂失败、无微绒毛区域丢失、CGFD丢失、与F-actin共定位 | [40~42] | |
| Sad-1 UNC-84 homology1 | SUN1 | MO注射 | 降低极体排出率、排出大极体、影响纺锤体形态、影响纺锤体迁移、降低皮质区肌动蛋白密度 | [43] | |
| Klarsicht ANC-1 Syne-homology5 | KASH5 | siRNA | 降低极体排出率、排出大极体、影响纺锤体形态、影响纺锤体迁移、降低皮质区肌动蛋白密度 | [43] | |
| Cytoskeleton- associated protein 5 | CKAP5 | MO注射 | 第一极体排出失败、严重破坏纺锤体组装、破坏染色体排列 | [44] | |
| Mos/Mitogen-activated protein kinase | Mos/MAPK | 基因敲出鼠、 抑制剂 | 纺锤体延伸、纺锤体迁移失败、发生对称分裂 | [31] | |
新窗口打开|下载CSV
1.1 减数分裂染色体信号与细胞皮质区的极化
皮质肌动蛋白帽是卵母细胞皮质区极性形成的典型物理结构,也是保证极体排出的一个重要的结构。在第一次减数分裂中期(metaphase I, M I)纺锤体向皮质区迁移的过程中,皮质肌动蛋白帽逐渐形成。当卵母细胞阻滞在MⅡ期时,纺锤体在皮质帽下维持自身的不对称定位[5,45]。早期研究结果证实,皮质结构的建立依赖于从染色体发出的信号,即染色体介导的一种“距离效应”。传递染色体信号的是RanGTP的浓度梯度[1,46,47],这种浓度梯度在MⅠ、MⅡ期的卵母细胞中都存在[48]。它的形成依赖于定位在染色体上的Ran鸟苷酸交换因子,或染色体浓缩调控蛋白与高活性的RanGTPase激活蛋白之间的空间互作[49]。如图2所示,RanGTP浓度梯度在胞质中的靶标是GVBD后,减数分裂纺锤体在胞质中心形成,肌动蛋白、皮质颗粒在皮质区均匀的分布。随后纺锤体以肌动蛋白依赖的方式迁移到皮质区,伴随着这个过程的是肌动蛋白帽的和CGFD的形成,这些特征标志着皮质极化的完成。随后,第一极体排出并产生一个高度极化的卵母细胞。受精激活后,卵母细胞排出第二极体完成减数分裂。参考文献[4]修改绘制。图2
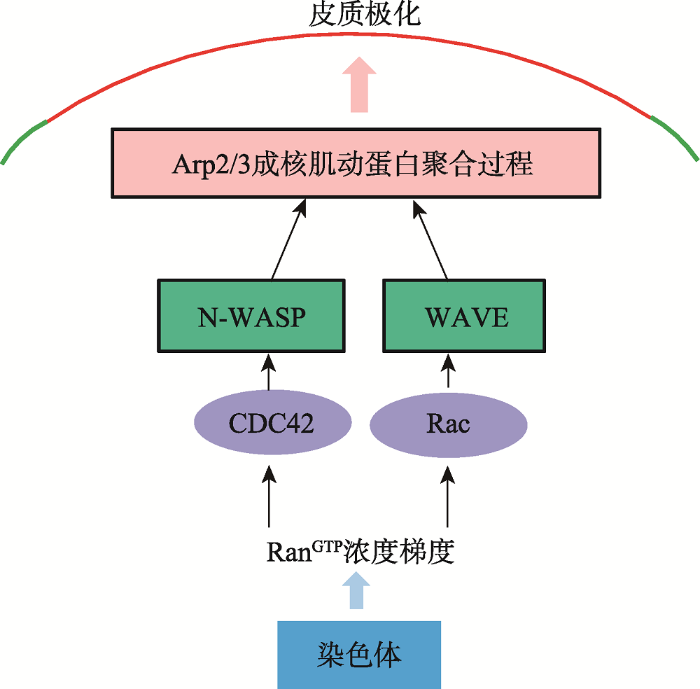 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2介导皮质极化的分子信号通路
Fig. 2Molecular pathway leading to cortical polarization
Arp2/3复合物[6],Arp2/3复合物是Cdc42下游的肌动蛋白成核因子。Cdc42是小GTP酶家族的成员,在多种细胞类型中通过N-WASP来调控Arp2/3的活性[25]。因此,Cdc42-N-WASP-Arp2/3信号通路操纵着Ran信号的下游来调控皮质肌动蛋白帽形成。另一个小GTP酶家族的成员,Rac1在RanGTP浓度梯当纺锤体临近皮质区,染色质介导的RanGTP浓度梯度通过Cdc42,来激活皮质区的N-WASP和Arp2/3复合体,从而介导肌动蛋白成核并聚合,这也是皮质极化的过程。另一方面,Rac1-WAVE2可能作为一个平行的通路来激活Arp2/3复合体,这也是受RanGTP浓度梯度的控制。
度的控制下也定位在皮质帽区域,Rac1通过它的特殊的效应因子WAVE2来调控皮质帽的形成[18,28],Rac1-WAVE2可能作为一个平行的通路来激活Arp2/3复合体,这个通路同样受RanGTP浓度梯度的调控[1]。
1.2 细胞骨架在卵母细胞不对称分裂过程中的作用
在卵母细胞成熟过程中,细胞骨架的结构发生了一系列变化,在减数分裂器形成和动态迁移以及极体排出等关键事件中均发挥重要的作用。1.2.1 微管在卵母细胞不对称分裂中的作用
纺锤体对卵裂沟的正确定位至关重要,并且纺锤体形态的异常往往会影响甚至破坏卵母细胞不对等分裂过程[4]。生发泡(germinal vesicle, GV)期小鼠卵母细胞中,微管呈现相对均匀的分布, GVBD后微管在染色体周围聚拢,并且开始向皮质区迁移。在MⅡ期成熟的小鼠卵母细胞中,微管主要聚集在皮质区胞质中,几乎全部用来组装纺锤体[50]。
在哺乳动物卵母细胞中,中心体在减数分裂之前被清除[51]。对于这种无典型中心体细胞,减数分裂纺锤体的形成主要包括3个关键过程,即不依赖于中心体的微管成核、纺锤体两极化以及纺锤体极的形成[52]。在卵母细胞中,有3条信号通路来取代有丝分裂细胞中的依赖于中心体的成核过程,分别是RanGTP通路、Augmin通路以及染色体乘客复合体(chromosomal passenger complex, CPC)通路[52]。在小鼠卵母细胞中,除了这些微管成核途径,非中心粒微管组织中心(aMTOCs)也能够组织微管成核。aMTOCs的成核能力在第一次减数分裂进程中不断增加。TPX2是RanGTPase的效应因子,它通过加强aMTOCs蛋白的转化相关酸性卷曲蛋白(transforming acidic coiled coil protein, TACC)的磷酸化水平,来增加aMTOCs的微管成核活性[53,54]。一旦微管形成,纺锤体以双极的形式组装,从而将染色体精确的一分为二。在卵母细胞中,纺锤体两极化是一个有序且缓慢的过程[55],需要aMTOCs有序地组织来确保正确的纺锤体两极分化。这个过程需要Kinesin-5先来打破aMTOCs的凝集状态,使它被分成更小的片段。随后在微管结合蛋白(microtubule- associated protein, MAP)和肝瘤上调蛋白(hepatoma up-regulated protein, HURP)蛋白的作用下[56],aMTOCs沿着纺锤体形成明确的两极[57]。双极确定之后,aMTOCs逐渐聚集在一起使纺锤体极更加完整,随后双极纺锤体形成[58]。
1.2.2 微丝在卵母细胞不对称分裂中的作用
微丝系统在卵母细胞减数分裂中,参与纺锤体的迁移、定位,同时还参与卵裂沟的形成和收缩。作为控制哺乳动物卵母细胞不对称分裂的关键调控因子,已经发现肌动蛋白成核剂如formin-2、spire和ARP2 / 3复合物是卵母细胞成熟中肌动蛋白重塑的重要调节因子[59,60]。另外一类肌动蛋白结合蛋白包括cofilin,原肌球蛋白,肌球蛋白,加帽蛋白,和ERM家族蛋白质在卵母细胞成熟的各个步骤中控制肌动蛋白细胞骨架动力学。成核促进因子如N-WASP、WAVE2、JMY、WASH和WHAMM等也参与哺乳动物卵母细胞不对称分裂过程[60]。小GTP酶家族(ARF1、Rac1、Ran、RhoA、Cdc42)是肌动蛋白成核因子的上游调控因子,研究表明它们通过Dynamin-WAVE2/ JMY/WASH-Arp2/3[33]及RhoA主导的ROCK-LIMK- Cofilin和FMNL1-mDia1-Profilin[14,24,34,61]等信号通路来调控微丝骨架动力学变化,参与卵母细胞的不对称分裂(图3)[3]。以下从纺锤体迁移和定位、收缩环的形成两个方面来阐述微丝在不对称分裂过程中的作用。
图3
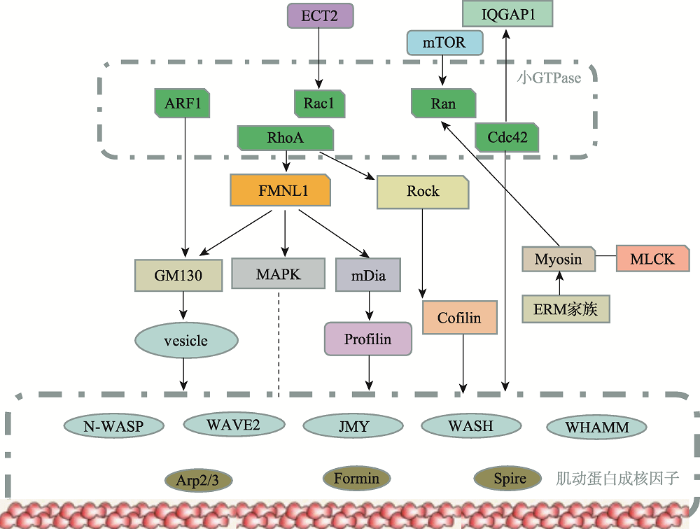 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3调控哺乳动物卵母细胞不对称分裂的主要信号通路
Fig. 3The core signaling pathways which regulate mammalian oocyte asymmetric division
1.2.2.1 纺锤体的迁移和定位
极体的成功排出依赖于不对称纺锤体位置的建立和皮质极性的建立。纺锤体的不对称定位直接决定了卵母细胞胞质的不对称分裂。
Yi等[1]提出第一次减数分裂纺锤体迁移可以分为两个时期。纺锤体迁移的起始是随机的“walk”,这个时期的驱动力来自与纺锤体外围内质网相结合的Fmn2成核肌动蛋白聚合过程所产生的推力。第二个时期则是快速的且有方向的,Arp2/3主导的胞质环流以一个快速的、有方向的方式将纺锤体推向皮质。这个环流一直持续到第一极体排出后,并且对维持MⅡ期纺锤体在皮质帽下的定位有作用。在这个模型中,对称性破坏的发生依赖于Arp2/3介导的皮质肌动蛋白聚合和染色体自身运动之间的正反馈环的结果。
当纺锤体临近皮质区,染色质介导的RanGTP浓度梯度通过皮质区的Cdc42来激活N-WASP和Arp2/3复合体从而完成核肌动蛋白聚合过程,这同时抑制了myosinⅡ环的提前收缩。用F-actin的活体探针GFP-UtrCH标记卵母细胞,发现皮质帽区Arp2/3成核的肌动纤维是流动的并且朝向细胞内部[6]。当Arp2/3活性被抑制,myosinⅡ环的收缩导致纺锤体离开皮质(图4)[59]。因此染色体和纺锤体定位与皮质极化之间存在正反馈环,纺锤体的不对称定位和皮质极化是相互依赖的。这个反馈环既将MⅠ期纺锤体朝向皮质推动,又维持了MⅡ期纺锤体的皮质帽下定位。
图4
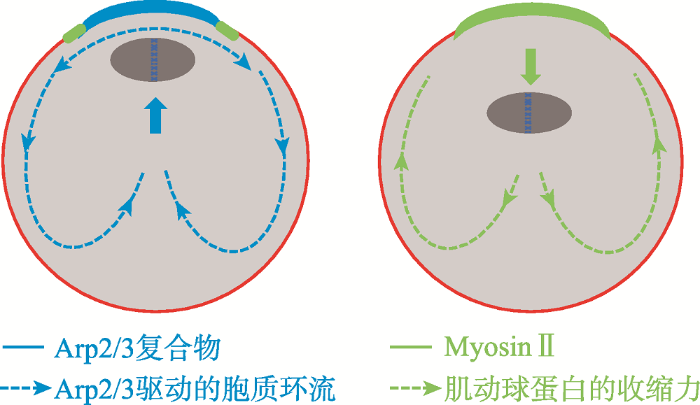 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4Arp2/3驱动的胞质环流维持纺锤体的定位
Arp2/3驱动的肌动蛋白流(左图)从皮质帽(蓝色)开始,向前发动胞质环流将纺锤体推向皮质区的,当Arp2/3活性被抑制(右图),myosinⅡ环(绿色)的收缩产生驱动纺锤体离开皮质的反转流。参考文献[59]修改绘制。
Fig. 4Meiotic spindle position is maintained through the Arp2/3-complex-driven cytoplasmic streaming
1.2.2.2 收缩环的形成
胞质分裂发生是在严格的时空控制下发生的,可分为4个时期,即分裂环定位、招募相关蛋白构建收缩环、分裂环收缩、环解聚同时细胞分离[62]。卵母细胞减数分裂中收缩环的收缩与有丝分裂及生精细胞的胞质分裂过程类似,收缩环中富含肌动蛋白和肌球蛋白Ⅱ,环肌动蛋白和肌球蛋白Ⅱ是Rho的下游效应因子,在活化的Rho的作用下激活并聚合[63]。
在多细胞生物中,分裂位点的选择依赖于活性的RhoA在赤道膜上的定位[64],这个过程的信号来
肌动蛋白成核因子(N-WASP, JMY, WAVE2, cortactin, CYA, Arp2/3 complex, formin, mDia, Spire)均与肌动蛋白装配过程直接相关。小GTPases(ARF1、Rac1、Ran、RhoA、Cdc42)是肌动蛋白成核因子的上游。小GTP酶的效应物和上游因子间接地促进肌动蛋白装配过程。
源于后期纺锤体和星体微管。活化的RhoA招募formin和anillin到分裂位点,随后激酶激活肌球蛋白Ⅱ[65]。后期纺锤体信号起源于纺锤体中部,这个区域招募中心纺锤体信号复合体centralspindlin和染色体乘客复合体 (chromosomal passenger complex, CPC)[65]。中部纺锤体蛋白是由平行的二聚体MKLP1和MgcRacGAP组成的复合体,其定位依赖于aurora B激酶[61]。然后中部纺锤体蛋白激活ECT2,ECT2随后招募并活化RhoA,RhoA通过一些肌动蛋白成核蛋白如formins或者Arp2/3[66,67]来促进卵裂沟处收缩环的形成[68]。
SFKs(Src family kinases)是一组重要的激酶,在控制肌动蛋白的多种功能方面有重要作用[69]。它调控fomins的功能,正常情况下formin-1定位在核里,Src能使它重新定位到质膜上[70]。Rho GTP酶与活化的Src在卵裂沟上共定位并结合formins[71]。另一方面,Src活化mDia相关蛋白来调控肌动蛋白的聚合过程[72],皮质上的SFKs也通过结合并激活Rho蛋白来调控肌动蛋白聚合[73,74]。
1.3 卵母细胞成熟过程中细胞器的重组分配
卵母细胞非对称分裂过程中,其内的细胞器等亚细胞结构也表现为非对称地向卵内和极体分离。其中较为明显的细胞器包含线粒体、高尔基体、内质网以及皮质颗粒等。1.3.1 线粒体
减数分裂期间的蛋白质合成和细胞质RNA的转录都需要线粒体提供能量[50]。线粒体向高能量消耗区的运动对卵母细胞成熟有重要作用。在未成熟的鼠卵中,线粒体呈簇状聚集在生发泡周围,GVBD后线粒体远离核周区并占据MⅡ期卵的大部分体 积[75,76]。在第一次减数分裂中,线粒体在动力蛋白的作用下聚集在纺锤体上,并随着纺锤体一起迁移到细胞皮质区。在细胞分裂时,线粒体进行不均等分离,它们朝向卵母细胞端的纺锤体方向移动并且被第一极体排除在外。但第二极体却随机地继承了MⅡ纺锤体周围的小线粒体簇[77]。这可能是由于第一极体和第二极体的挤压模式存在固有的差异,或者是由于与这两个过程相关的胞质动力和肌动蛋白不同造成的[6,78]。
1.3.2 高尔基体
高尔基体在蛋白质修饰和转运中起核心作用[50]。在GV期小鼠卵母细胞中,高尔基体以连续的膜系统形式分散在卵子的胞浆中。但是相比皮质区,高尔基体更多的集中在胞质内[79]。GVBD后高尔基体进行分裂,在卵母细胞中央呈点状分布[79]。随后高尔基体进一步分裂,分散在整个MⅠ期卵母细胞中,这种分布在排出极体后仍然维持。高尔基体在哺乳动物卵母细胞不对称分裂过程中扮演着不可缺少的角色。BFA(蛋白转运抑制剂)处理GV期小鼠卵母细胞使成熟进程阻滞在GVBD与纺锤体装配之间[79]。GM130(顺式高尔基体的基质蛋白)敲低的卵母细胞不能形成肌动蛋白帽,纺锤体迁移失败[38]。这可能是通过GM130-Cdc42-WAVE2-Arp2/3和GM130- MAPK-WAVE2-Arp2/3 通路在卵母细胞不对称分裂中发挥作用[38]。
1.3.3 内质网
内质网是钙离子的主要内贮体,在蛋白质折叠、降解和脂质代谢中发挥着重要作用[50]。卵母细胞成熟过程中,内质网进行再分配和结构重组。GV期的卵母细胞胞质中均匀分布着内质网。然而当卵母细胞发育到MⅡ期时,内质网定位在皮质区并在胞质中聚集成群。这样的聚集集中在成熟卵的植物极,而在动物极却明显很少[80]。
1.3.4 皮质颗粒
皮质颗粒是卵中特有的细胞器,皮质颗粒来源于高尔基体[81],其组分包含不同数量的蛋白质、结构分子、酶以及多糖[50]。皮质颗粒的迁移及分布与卵母细胞的成熟度有直接关系,并且在保证正常受精及阻止多精受精方面有重要作用[47,81]。在GV期卵母细胞中,皮质颗粒分布在胞质中。GVBD后,胞质中的颗粒细胞向皮质区迁移。随着纺锤体的迁移,卵母细胞皮质帽区域的皮质颗粒密度降低,形成CGFD[4,38]。这个区域是极体的排出位点,它的形成对卵母细胞的不对称分裂而言至关重要[4]。在小鼠卵母细胞中,这个过程是由染色体介导[47]。
2 卵母细胞细胞核不对称分裂:染色体的非随机分离
卵母细胞细胞的不对称分裂不单单表现为细胞质的不对称分裂,另一个表现就是细胞核的不对称分裂,也即卵母细胞在选择遗传物质的过程中有独特的偏好性[82]。基因随机分离定律是孟德尔第一定律,任何染色体都有50%的概率被分配到卵子或极体中。然而在很多物种中,有些基因拷贝得到遗传的概率远远大于50%[83]。几十年前,Sandler等[84,85]提出“减数分裂驱动”的概念,用来解释实验中“一些遗传性状通过性细胞向后代遗传的过程中,不同性状的遗传机会并不均等”的现象。2015年,基于芯片技术对于人类卵子测序结果的大数据分析表明,重组交换率较高的染色体倾向于留在卵母细胞中[86,87]。这说明当代的信息学手段获得结论与60年前的结果是一致的。即一致表明“性状遗传机会的不均等性”,也称之为“染色体非随机分离”。染色体非随机分离在果蝇的精原干细胞上的研究中得以确认[88,89,90,91]。果蝇精原干细胞非对等分裂时,细胞核的分离首先表现为中心体极性的确认,即旧的中心体留在未来仍为干细胞的子代细胞中,而新合成的中心体则进入未来分化的子代细胞中;然后命运已定的两个中心体分别与标记染色体与非标记染色体结合,最终实现对于姐妹染色单体的选择性分离[92]。参与中心体极性分配的成分包含中心体周围材料的核心组件,如SUN域蛋白KOI和KASH域蛋白KASH,这些复合体参与了姐妹染色单体的差异性分离[93]。这依托于中心体对染色体的选择依赖于组蛋白上的特定位点的修饰机制。Histone H3的第三位上的特定修饰是姐妹染色单体进行非对等分裂的识别位点[88]。在此过程中,LINC复合物(包含SUN-和KASH-结构域蛋白,负责核骨架和细胞骨架的连接)介导了两个中心体与各姐妹染色单体的连接。哺乳动物中至少包含5个不同的SUN域蛋白编码基因和5个KASH蛋白质编码基因[94]。在小鼠卵母细胞中,SUN结构域与kash5结合形成一个减数分裂特有的“核质桥”[94]。这不仅将端粒连接到核膜上,而且还介导了减数分裂染色体端粒与微管骨架的连接。目前还没有直接的证据证明哺乳动物卵母细胞中LINC复合物介导对于姐妹染色单体的选择性分离。
近期,研究者从另一个方面揭示和确认了在卵母细胞中染色体非随机分离的机制。研究发现着丝粒作为一种自私基因元件,可以利用纺锤体微管酪氨酸化(tyrosination)的不对称性来使自身优先遗传给后代[83,95]。研究人员观察小鼠卵母细胞组成纺锤体的微管,发现与靠近皮质部微管相比,靠近卵子一边的微管酪氨酸修饰比较少,这种现象是由CDC42蛋白所决定。纺锤体微管酪氨酸修饰的不对称仅仅在纺锤体向皮质区移动时出现。正如上文所描述,当纺锤体临近皮质区,染色质介导的RanGTP浓度激活皮质区的Cdc42,它使靠近皮质区的纺锤体微管被大量的酪氨酸化(图5)。突变Cdc42则纺锤体微管酪氨酸修饰的不对称性消失。这种由CDC42负责的纺锤体微管不对称修饰,是自私基因操纵减数分裂染色体分离的关键。着丝点是纺锤体微管与染色体附着的点,有些着丝粒有更多的着丝粒重复拷贝和更多的动力蛋白(强着丝粒),而有些着丝粒则重复拷贝和动力蛋白相对较少(弱着丝粒)。强着丝粒与被大量酪氨酸化的微管之间的附着比弱着丝粒更不稳定。结果导致近膜区的强着丝点上与酪氨酸化微管之间的附着往往被随机的破坏。强着丝点滑落,建立新的、更稳定的、反方向的(卵子端)附着。就这样,强着丝粒就更大可能地留在卵母细胞里,并且被胚胎继承。但是在这个过程中CDC42-GTP是如何局部增加微管酪氨酸化或者抑制去酪氨酸的?另外,着丝点是如何从大量酪氨酸化的微管上解脱下来的?这些问题还有待继续研究[83]。
图5
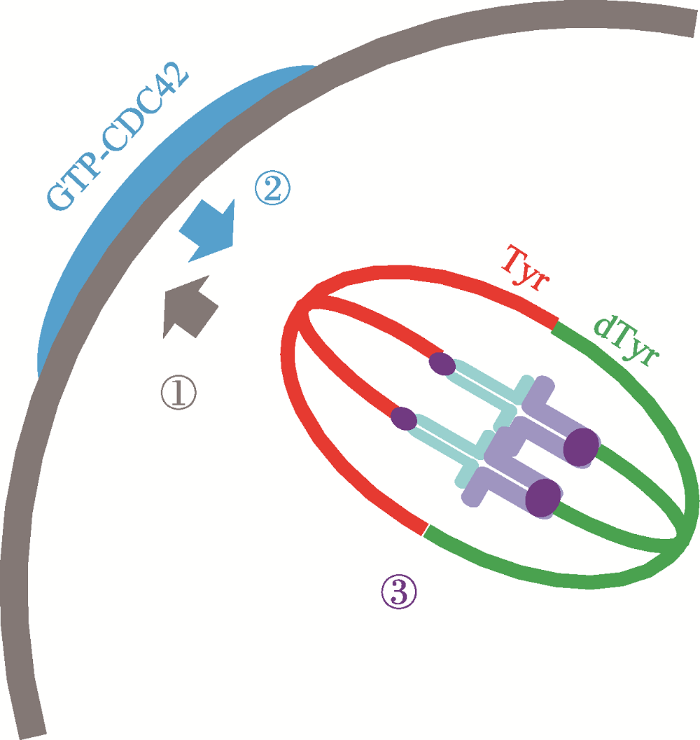 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5非随机分离的模型模型
①染色体介导的RANGTP浓度梯度诱导皮质极化。②极化的皮质区的CDC42GTP诱导纺锤体酪氨酸修饰不对称。③纺锤体不对称诱导自私基因的偏见附着。参考文献[95]修改绘制。
Fig. 5A model for nonrandom segregation
2014年,Wang等[96]提出利用极体遗传物质移植技术治疗线粒体遗传疾病的设想,但根据近几年的研究留在卵子中的染色体和极体中被丢弃的染色体是不同的,卵母细胞减数分裂过程中对于遗传物质是有选择性的,这就提示我们不能轻易地用极体中的遗传物质代替卵子中的遗传物质进行后续的胚胎发育。除了染色体非随机分离外,极体和卵细胞中的DNA突变和损伤的发生率是否相同?表观修饰是否相同[97]?这些问题仍不清楚。未来要致力于研究哺乳动物卵母细胞细胞核不对称分裂的研究,一方面这有利于我们深入了解卵母细胞对遗传物质的选择规律,另一方面可以帮助我们更好地解决人类临床医学中的不育不孕问题,这对染色体错误配对导致的疾病以及线粒体遗传病的解决至关重要。
极性和不对称分裂是许多细胞类型的重要特征。它们协调作用以确保负责细胞多样性的分子能够正确地向子代传递[98]。对于干细胞的不对称分裂而言,细胞极性在有丝分裂之前就已经预先建立。这种预先存在的极性不仅将命运决定子分离而且还决定了有丝分裂纺锤体的方向和位置[99],最终决定了细胞的不对称分裂。但对于小鼠卵母细胞而言,不对称分裂决定了卵母细胞的极性。第一次减数分裂在卵母细胞中形成动物/植物轴(animal/vegetalaxis, A/V axis),建立了卵母细胞极性。而早期胚胎发育的极性根源于卵子极性[100]因为研究表明,受精卵第一次卵裂通常是沿着AV轴[101],到第二次卵裂的时候才进行赤道板分裂,将动物极和植物极分离[102]。研究表明,受精卵是沿着AV轴还是与AV轴垂直进行分裂,这影响小鼠胚胎的发育[103]。
3 结语与展望
哺乳动物卵母细胞减数分裂是典型的不对称分裂过程,这种不对称分裂表现在细胞质和细胞核两个方面。卵母细胞细胞质的不对称分裂过程中,最关键的事件是纺锤体打破对称性向皮质区迁移。纺锤体迁移先后在FMN2和Arp2/3的主导下经历“慢”和“快”两个阶段。当纺锤体临近皮质区,染色质介导的RanGTP浓度通过Cdc42,来激活N-WASP和Arp2/3复合体在皮质区成核肌动蛋白聚合过程,从而介导皮质区极化(图2)。皮质极化完成的标志为肌动蛋白帽和CGFD的形成。在经历分裂位点的选择及收缩环的形成后,排出第一极体,完成第一次减数分裂。这时的卵母细胞是一个高度极化的细胞,纺锤体在收缩力和肌动蛋白纤维流的共同作用下维持皮质区的定位。受精过程后,纺锤体旋转并排出第二极体,完成减数分裂过程。与此同时,卵母细胞细胞核在减数分裂驱动的操控下发生了不对称分裂:染色体非随机分离。CDC42诱导了纺锤体微管上不对称的酪氨酸修饰,重复序列数目多且大的着丝粒利用这种纺锤体不对称性使自己更大概率的附着到卵子边的微管上,从而使他们所携带的遗传信息得到优先遗传。尽管影响着丝粒强弱的特征还未研究明确,但是对于哺乳动物卵母细胞中的染色体非随机分离已经得以确认,取得很大的进展。未来的研究应该着重在现有资料的基础上继续深入研究染色体的分离机制,相信一定能找到染色体疾病的根本原因并找到解决办法。除此之外,有一个问题也不容忽视:目前哺乳动物卵母细胞不对称分裂的研究大多数都是来源于小鼠卵母细胞。虽然之前Neslon等[104]提出过极性形成的核心机制适用于一切细胞的极性形成。但相比于其他哺乳动物的卵母细胞,小鼠卵母细胞可能是非常不典型的结构[105]。例如对于猪卵母细胞,染色体在GVBD前就定位于靠近皮质区的位置,极性建立过程是否与鼠卵不同?但不可否认的是,小鼠卵母细胞为哺乳动物卵母细胞不对称分裂提供了极大的参考和借鉴意义。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:24062576 [本文引用: 5]

Abstract Mammalian oocyte meiosis encompasses two rounds of asymmetric divisions to generate a totipotent haploid egg and, as by-products, two small polar bodies. Two intracellular events, asymmetric spindle positioning and cortical polarization, are critical to such asymmetric divisions. Actin but not microtubule cytoskeleton has been known to be directly involved in both events. Recent work has revealed a positive feedback loop between chromosome-mediated cortical activation and the Arp2/3-orchestrated cytoplasmic streaming that moves chromosomes. This feedback loop not only maintains meiotic II spindle position during metaphase II arrest, but also brings about symmetry breaking during meiosis I. Prior to an Arp2/3-dependent phase of fast movement, meiotic I spindle experiences a slow and non-directional first phase of migration driven by a pushing force from Fmn2-mediated actin polymerization. In addition to illustrating these molecular mechanisms, mathematical simulations are presented to elucidate mechanical properties of actin-dependent force generation in this system.
URLPMID:12461532 [本文引用: 2]

Articles and research papers on cell division, cell structure, animal and plant cell biology and cell cycles.
URLPMID:22306711 [本文引用: 4]

Abstract OBJECTIVE: To investigate the molecular mechanism of mouse oocyte polarity loss during aging. DESIGN: Experimental study. SETTING: Academic basic research laboratory. ANIMAL(S): Mice. INTERVENTION(S): Oocytes were collected 16 hours after injection of hCG and cultured in M16 medium for an additional 14 hours with or without caffeine. MAIN OUTCOME MEASURE(S): Expression and localizations of actin nucleators actin-related protein 2/3 complex, JMY, and WAVE2 were examined by immunofluorescence staining, and their messenger RNA levels were examined by real-time reverse transcription-polymerase chain reaction. RESULT(S): The protein and messenger RNA levels of actin-related protein 2/3 complex, JMY, and WAVE2 were decreased in aged oocytes, but the levels were normal in caffeine-treated aged oocytes. CONCLUSION(S): Our data indicated that the loss of oocyte polarity may be due to the degradation of actin nucleators in aged oocytes. Copyright 漏 2012 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
URLPMID:23764118 [本文引用: 5]

Abstract In contrast to symmetric division in mitosis, mammalian oocyte maturation is characterized by asymmetric cell division that produces a large egg and a small polar body. The asymmetry results from oocyte polarization, which includes spindle positioning, migration, and cortical reorganization, and this process is critical for fertilization and the retention of maternal components for early embryo development. Although actin dynamics are involved in this process, the molecular mechanism underlying this remained unclear until the use of confocal microscopy and live cell imaging became widespread in recent years. Information obtained through a PubMed database search of all articles published in English between 2000 and 2012 that included the phrases "oocyte, actin, spindle migration," "oocyte, actin, polar body," or "oocyte, actin, asymmetric division" was reviewed. The actin nucleation factor actin-related protein 2/3 complex and its nucleation-promoting factors, formins and Spire, and regulators such as small GTPases, partitioning-defective/protein kinase C, Fyn, microRNAs, cis-Golgi apparatus components, myosin/myosin light-chain kinase, spindle stability regulators, and spindle assembly checkpoint regulators, play critical roles in asymmetric cell division in oocytes. This review summarizes recent findings on these actin-related regulators in mammalian oocyte asymmetric division and outlines a complete signaling pathway.
URLPMID:3723057 [本文引用: 2]

The influence of mouse oocyte chromosomes on their immediate environment has been investigated following their dispersal by dissolution of the metaphase spindle with nocodazole. Small clusters of chromosomes become redistributed around the egg cortex in a microfilament-dependent process. Each cluster has the capacity, on removal from nocodazole, to organize a spindle that rotates to yield a polar body. In this process of spindle formation, the chromosome clusters are able both to promote tubulin polymerization in their vicinity and to recruit microtubule-organizing centres (MTOCs) which organize the polymerized tubulin into spindles. In addition each oocyte chromosome cluster, as well as the non-dispersed sperm-derived haploid group of chromosomes, induces a focal accumulation of subcortical actin (corresponding to a filamentous area devoid of organelles) and a loss of surface Concanavalin A binding activity (corresponding to a loss of surface microvilli) in the overlying cortex. This induction ceases with the formation of pronuclei whether or not the pronuclei migrate centrally. Pronuclear formation is sensitive to the action of nocodazole for up to 2-4 h postinsemination, and pronuclear migration is totally sensitive to the drug. If pronuclei are blocked in a peripheral location by nocodazole they are associated with an elevation in Con A binding activity of the overlying membrane which corresponds to an area of the surface rich in blebby microvilli.
URLPMID:21874009 [本文引用: 3]

Nat Cell Biol. 2011 Aug 28;13(10):1252-8. doi: 10.1038/ncb2320. Research Support, N.I.H., Extramural
URLPMID:3072972

Mammalian oocyte meiotic maturation involves oocyte polarization and a unique asymmetric division, but until now, the underlying mechanisms have been poorly understood. Arp2/3 complex has been shown to regulate actin nucleation and is widely involved in a diverse range of processes such as cell locomotion, phagocytosis and the establishment of cell polarity. Whether Arp2/3 complex participates in oocyte polarization and asymmetric division is unknown. The present study investigated the expression and functions of Arp2/3 complex during mouse oocyte meiotic maturation. Immunofluorescent staining showed that the Arp2/3 complex was restricted to the cortex, with a thickened cap above the meiotic apparatus, and that this localization pattern was depended on actin. Disruption of Arp2/3 complex by a newly-found specific inhibitor CK666, as well as by Arpc2 and Arpc3 RNAi, resulted in a range of effects. These included the failure of asymmetric division, spindle migration, and the formation and completion of oocyte cytokinesis. The formation of the actin cap and cortical granule-free domain (CGFD) was also disrupted, which further confirmed the disruption of spindle migration. Our data suggest that the Arp2/3 complex probably regulates oocyte polarization through its effect on spindle migration, asymmetric division and cytokinesis during mouse oocyte meiotic maturation.
URLPMID:23851486

Abstract At mitosis onset, cortical tension increases and cells round up, ensuring correct spindle morphogenesis and orientation. Thus, cortical tension sets up the geometric requirements of cell division. On the contrary, cortical tension decreases during meiotic divisions in mouse oocytes, a puzzling observation because oocytes are round cells, stable in shape, that actively position their spindles. We investigated the pathway leading to reduction in cortical tension and its significance for spindle positioning. We document a previously uncharacterized Arp2/3-dependent thickening of the cortical F-actin essential for first meiotic spindle migration to the cortex. Using micropipette aspiration, we show that cortical tension decreases during meiosis I, resulting from myosin-II exclusion from the cortex, and that cortical F-actin thickening promotes cortical plasticity. These events soften and relax the cortex. They are triggered by the Mos-MAPK pathway and coordinated temporally. Artificial cortex stiffening and theoretical modelling demonstrate that a soft cortex is essential for meiotic spindle positioning.
URLPMID:25597399

Abstract Cell mechanics control the outcome of cell division. In mitosis, external forces applied on a stiff cortex direct spindle orientation and morphogenesis. During oocyte meiosis on the contrary, spindle positioning depends on cortex softening. How changes in cortical organization induce cortex softening has not yet been addressed. Furthermore, the range of tension that allows spindle migration remains unknown. Here, using artificial manipulation of mouse oocyte cortex as well as theoretical modelling, we show that cortical tension has to be tightly regulated to allow off-center spindle positioning: a too low or too high cortical tension both lead to unsuccessful spindle migration. We demonstrate that the decrease in cortical tension required for spindle positioning is fine-tuned by a branched F-actin network that triggers the delocalization of myosin-II from the cortex, which sheds new light on the interplay between actin network architecture and cortex tension.
URLPMID:21653611

Abstract Female meiotic divisions are extremely asymmetric, giving rise to a large oocyte and small degenerating polar bodies, keeping the maternal stores for further embryo development. This asymmetry is achieved via off-center positioning of the division spindle. Mouse oocytes have developed a formin-2-dependent actin-based spindle positioning mechanism that allows the meiotic spindle to migrate towards the closest cortex. Using spinning disk microscopy and FRAP analysis, we studied the changes in the organization of the cytoplasmic F-actin meshwork during the first meiotic division. It is very dense in prophase I, undergoes a significant density drop upon meiosis resumption and reforms progressively later on. This meshwork remodeling correlates with endogenous formin 2 regulation. High formin 2 levels at meiosis I entry induce meshwork maintenance, leading to equal forces being exerted on the chromosomes, preventing spindle migration. Hence, the meshwork density drop at meiosis resumption is germane to the symmetry-breaking event required for successful asymmetric meiotic divisions.
URLPMID:20971793

Formin-2 (Fmn2) nucleates actin filaments required for spindle migration during the metaphase of meiosis I in mouse oocytes. While recent studies showed that Fmn2 is involved in the formation of a dynamic actin meshwork on meiotic spindle and the migration of chromosomes, the precise location and the mechanism of action of Fmn2 in the mouse oocyte is not known. In this work, we show that Fmn2 is colocalized with spindle during metaphase I (MI) and this pattern is lost in nocodazole-treated oocytes. Fmn2 directly interacts with polymerized microtubules (MTs) in vitro via a well-conserved domain called formin homology 2 (FH2). Microinjection of mRNA encoding formin homology 1 (FH1)FH2 domains of Fmn2 into Fmn2-/- oocytes partially rescued the defect of polar body extrusion, while mRNAs encoding FH2 domain alone could not rescue the defect. mDia1 and mDia2, Diaphanous (Dia) subfamily of formin proteins, exhibit unique patterns of expression in mouse oocytes. While mDia1 is localized on meiotic spindle, mDia2 localization is confined in spindle poles similar to 纬-tubulin. Collectively, our results suggest that the ability of Fmn2 to directly interact with MTs and to polymerize actins via the conserved FH1FH2 domains is crucial for chromosomal migration in MI oocytes. We also show that mDia1 and mDia2 are dynamic components of meiotic spindle and pole complex during meiotic maturation of oocytes.
URLPMID:4383420

Abstract Dynamic actin reorganization is the main driving force for spindle migration and asymmetric cell division in mammalian oocytes. It has been reported that various actin nucleators including Formin-2 are involved in the polarization of the spindle and in asymmetric cell division. In mammals, the formin family is comprised of 15 proteins. However, their individual roles in spindle migration and/or asymmetric division have not been elucidated yet. In this study, we employed a newly developed inhibitor for formin family proteins, small molecule inhibitor of formin homology 2 domains (SMIFH2), to assess the functions of the formin family in mouse oocyte maturation. Treatment with SMIFH2 during in vitro maturation of mouse oocytes inhibited maturation by decreasing cytoplasmic and cortical actin levels. In addition, treatment with SMIFH2, especially at higher concentrations (500 脦录M), impaired the proper formation of meiotic spindles, indicating that formins play a role in meiotic spindle formation. Knockdown of the mDia2 formins caused a similar decrease in oocyte maturation and abnormal spindle morphology, mimicking the phenotype of SMIFH2-treated cells. Collectively, these results suggested that besides Formin-2, the other proteins of the formin, including mDia family play a role in asymmetric division and meiotic spindle formation in mammalian oocytes.
URLPMID:21620703

Oocytes mature into eggs by extruding half of their chromosomes in a small cell termed the polar body. Asymmetric oocyte division is essential for fertility [ 1 ], but despite its importance, little is known about its mechanism. In mammals, the meiotic spindle initially forms close to the center of the oocyte. Thus, two steps are required for asymmetric meiotic division: first, asymmetric spindle positioning and second, polar body extrusion. Here, we identify Spire1 and Spire2 as new key factors in asymmetric division of mouse oocytes. Spire proteins are novel types of actin nucleators that drive nucleation of actin filaments with their four WH2 actin-binding domains [ 2 , 3 , 4 , 5 聽and聽 6 ]. We show that Spire1 and Spire2 first mediate asymmetric spindle positioning by assembling an actin network that serves as a substrate for spindle movement. Second, they drive polar body extrusion by promoting assembly of the cleavage furrow. Our data suggest that Spire1 and Spire2 cooperate with Formin-2 (Fmn2) to nucleate actin filaments in mouse oocytes and that both types of nucleators act as a functional unit. This study not only reveals how Spire1 and Spire2 drive two critical steps of asymmetric oocyte division, but it also uncovers the first physiological function of Spire-type actin nucleators in vertebrates.
URLPMID:25447542 [本文引用: 1]

Mammalian diaphanous1 (mDia1) is a homologue of Drosophila diaphanous and belongs to the Formin-homology family of proteins that catalyze actin nucleation and polymerization. Although Formin family proteins, such as Drosophila diaphanous, have been shown to be essential for cytokinesis, whether and how mDia1 functions during meiosis remain uncertain. In this study, we explored possible roles and the signaling pathway involved for mDia1 using a mouse oocyte model. mDia1 depletion reduced polar body extrusion, which may have been due to reduced cortical actin assembly. mDia1 and Profilin1 had similar localization patterns in mouse oocytes and mDia1 knockdown resulted in reduced Profilin1 expression. Depleting FMNL1, another Formin family member, resulted in reduced mDia1 expression, while RhoA inhibition did not alter mDia1 expression, which indicated that there was a FMNL1-mDia1-Profilin1 signaling pathway in mouse oocytes. Additionally, mDia1 knockdown resulted in disrupting oocyte spindle morphology, which was confirmed by aberrant p-MAPK localization. Thus, these results demonstrated indispensable roles for mDia1 in regulating mouse oocyte meiotic maturation through its effects on actin assembly and spindle organization.
URLPMID:26083584

Formin-like 1 (FMNL1) is a member of Formin family proteins which are the actin nucleators. Although FMNL1 activities have been shown to be essential for cell adhesion, cytokinesis, cell polarization and migration in mitosis, the functional roles of mammalian FMNL1 during oocyte meiosis remain uncertain. In this study, we investigated the functions of FMNL1 in mouse oocytes using specific morpholino (MO) microinjection and live cell imaging. Immunofluorescent staining showed that in addition to its cytoplasmic distribution, FMNL1 was primarily localized at the spindle poles after germinal vesicle breakdown (GVBD). FMNL1 knockdown caused the low rate of polar body extrusion and resulted in large polar bodies. Time-lapse microscopic and immunofluorescence intensity analysis indicated that this might be due to the aberrant actin expression levels. Cortical polarity was disrupted as shown by a loss of actin cap and cortical granule free domain (CGFD) formation, which was confirmed by a failure of meiotic spindle positioning. And this might be the reason for the large polar body formation. Spindle formation was also disrupted, which might be due to the abnormal localization of p-MAPK. These results indicated that FMNL1 affected both actin dynamics and spindle formation for the oocyte polar body extrusion. Moreover, FMNL1 depletion resulted in aberrant localization and expression patterns of a cis-Golgi marker protein, GM130. Finally, we found that the small GTPase RhoA might be the upstream regulator of FMNL1. Taken together, our data indicate that FMNL1 is required for spindle organization and actin assembly through a RhoA-FMNL1-GM130 pathway during mouse oocyte meiosis.
URLPMID:21511720

Polar body emission is a specialized cell division throughout the animal kingdom, serving to reduce chromosome ploidy while preserving the egg cytoplasm. Critical to polar body emission are the asymmetric positioning of the meiotic spindle prior to anaphase, with one pole attached to the oocyte cortex, and the simultaneous membrane protrusion during subsequent cytokinesis. We have shown that, during Xenopus oocyte maturation, the small GTPase Cdc42 promotes membrane protrusion while a classical RhoA contractile ring forms and constricts at the base of the protrusion. We report here that treating oocytes with low concentrations of nocodazole diminished the size of metaphase I spindles and prevented polar body emission, and yet an active Cdc42 cap of correspondingly diminished size still developed, on time, atop of the spindle pole. Conversely, treating oocytes with low concentrations of taxol resulted in a spindle with multiple poles attached to the cortex, but still each of these poles were associated with activated cortical Cdc42 at the appropriate time. Therefore, the asymmetric positioning of the meiotic spindle with one pole anchored to the cortex is a prerequisite for Cdc42 activation. Furthermore, we demonstrated that the Cdc42-regulated F-actin nucleator ARP2/3 complex was similarly localized at the cortex of the protruding polar body membrane, suggesting that Cdc42 promotes membrane protrusion through an F-actin meshwork mechanism. Finally, we demonstrated that Cdc42 and RhoA formed similarly complementary activity zones during egg activation and that inhibition of Cdc42 prevented second polar body emission. Therefore, Cdc42 activation likely promotes membrane protrusion during polar body emission in widespread systems.
URLPMID:27401749

Abstract STUDY QUESTION: There is an unexplored physiological role of N-WASP (neural Wiskott-Aldrich syndrome protein) in oocyte maturation that prevents completion of second meiosis. SUMMARY ANSWER: In mice, N-WASP deletion did not affect oocyte polarity and asymmetric meiotic division in first meiosis, but did impair midbody formation and second meiosis completion. WHAT IS KNOWN ALREADY: N-WASP regulates actin dynamics and participates in various cell activities through the RHO-GTPase-Arp2/3 (actin-related protein 2/3 complex) pathway, and specifically the Cdc42 (cell division cycle 42)-N-WASP-Arp2/3 pathway. Differences in the functions of Cdc42 have been obtained from in vitro compared to in vivo studies. STUDY DESIGN, SAMPLES/MATERIALS, METHODS: By conditional knockout of N-WASP in mouse oocytes, we analyzed its in vivo functions by employing a variety of different methods including oocyte culture, immunofluorescent staining and live oocyte imaging. Each experiment was repeated at least three times, and data were analyzed by paired-samples t-test. MAIN RESULTS AND THE ROLE OF CHANCE: Oocyte-specific deletion of N-WASP did not affect the process of oocyte maturation including spindle formation, spindle migration, polarity establishment and maintenance, and homologous chromosome or sister chromatid segregation, but caused failure of cytokinesis completion during second meiosis (P脗聽<脗聽0.001 compared to control). Further analysis showed that a defective midbody may be responsible for the failure of cytokinesis completion. LIMITATIONS, REASONS FOR CAUTION: The present study did not include a detailed analysis of the mechanisms underlying the results, which will require more extensive further investigations. WIDER IMPLICATIONS OF THE FINDINGS: N-WASP may play an important role in mediating and co-ordinating the activity of the spindle (midbody) and actin (contractile ring constriction) when cell division occurs. The findings are important for understanding the regulation of oocyte meiosis completion and failures in this process that affect oocyte quality. LARGE SCALE DATA: None. STUDY FUNDING AND COMPETING INTERESTS: This work was supported by the National Basic Research Program of China (No. 2012CB944404) and the National Natural Science Foundation of China (Nos 30930065, 31371451, 31272260 and 31530049). There are no potential conflicts of interests. 脗漏 The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
URLPMID:21543895 [本文引用: 1]

During oocyte meiotic maturation, meiotic spindles form in the central cytoplasm and then migrate to the cortex to extrude a small polar body, forming a highly polarized cell through a process involving actin and actin-related molecules. The mechanisms underlying oocyte polarization are still unclear. The Arp2/3 complex regulates oocyte polarization but it is not known whether the WASP family of proteins, a known regulator of the Arp2/3 complex, is involved in this context. In the present study, the role of WASP family member WAVE2 in mouse oocyte asymmetric division was investigated. (1) WAVE2 mRNA and protein were detected during mouse oocyte meiosis. (2) siRNA-mediated and antibody-mediated disruption of WAVE2 resulted in the failure of chromosome congression, spindle formation, spindle positioning and polar body extrusion. (3) WAVE2 regulated actin-driven chromosome migration since chromosomes were arrested in the central cytoplasm by WAVE2 RNAi in the absence of microtubules. (4) Localization of 脦鲁-tubulin and MAPK was disrupted after RNAi, confirming the effect of WAVE2 on spindle formation. (5) Actin cap and cortical granule-free domain (CGFD) formation was also disrupted, further confirming the failure of oocyte polarization. Our data suggest that WAVE2 regulates oocyte polarization by regulating meiotic spindle, peripheral positioning, probably via an actin-mediated pathway, and is involved in polar body emission during mouse oocyte meiotic maturation.
URLPMID:21266449

JMY is a transcriptional co-factor of p53. Latest work has revealed that JMY is also an actin nucleation factor that regulates new filament assembly and activates Arp2/3 complex in somatic cells; however, roles of JMY in mouse oocyte are unknown. Here we showed the expression and functions of JMY during mouse oocyte meiotic maturation. JMY mRNA is expressed largely from germinal vesicle to metaphase I stage, and gradually decreased during anaphase I, telophase I (TI) and metaphase II (MII) stages. Immunostaining results showed that JMY localized at the spindle and cytoplasm of oocytes. Depletion of JMY by RNAi resulted in symmetric division, failure of spindle migration and cytokinesis during oocyte meiotic maturation, showing a 2-cell-like MII oocyte and TI stage arrest. Actin cap and cortical granules-free domain formation were also disrupted after JMY RNAi, indicating the failure of spindle migration. JMY antibody injection results were consistent with those of JMY RNAi, further confirming the involvement of JMY in oocyte polarity. Our data indicate that JMY is required for spindle migration, asymmetric division and cytokinesis during mouse oocyte maturation.
URLPMID:4184845

Junction-mediating and regulatory protein(JMY) is a multifunctional protein with roles in the transcriptional co-activation of p53 and the regulation of actin nucleation promoting factors and, hence, cell migration; however, its role in the maturation of porcine oocytes is unclear. In the current study, we investigated functional roles of JMY in porcine oocytes. Porcine oocytes expressed JMY mRNA and protein, and the mRNA expression level decreased during oocyte maturation. Knockdown of JMY by RNA interference decreased the rate of polar body extrusion, validating its role in the asymmetric division of porcine oocytes. JMY knockdown also down-regulated the mRNA and protein levels of actin and Arp2/3. Furthermore, JMY accumulated in the nucleus in response to DNA damage, and JMY knockdown suppressed DNA damage-mediated p53 activation. In conclusion, our results show that JMY has important roles in oocyte maturation as a regulator of actin nucleation-promoting factors and an activator of p53 during DNA damage during DNA damages in porcine oocytes.
URLPMID:23160625

WASP homolog associated with actin, membranes and microtubules (WHAMM) is a newly discovered nucleation-promoting factor that links actin and microtubule cytoskeleton and regulates transport from the endoplasmic reticulum to the Golgi apparatus. However, knowledge of WHAMM is limited to interphase somatic cells. In this study, we examined its localization and function in mouse oocytes during meiosis. Immunostaining showed that in the germinal vesicle (GV) stage, there was no WHAMM signal; after meiosis resumption, WHAMM was associated with the spindle at prometaphase I (Pro MI), metaphase I (MI), telophase I (TI) and metaphase II (MII) stages. Nocodazole and taxol treatments showed that WHAMM was localized around the MI spindle. Depletion of WHAMM by microinjection of specific short interfering (si)RNA into the oocyte cytoplasm resulted in failure of spindle migration, disruption of asymmetric cytokinesis and a decrease in the first polar body extrusion rate during meiotic maturation. Moreover, actin cap formation was also disrupted after WHAMM depletion, confirming the failure of spindle migration. Taken together, our data suggest that WHAMM is required for peripheral spindle migration and asymmetric cytokinesis during mouse oocyte maturation.
URLPMID:4083260

Prior to their fertilization, oocytes undergo asymmetric division, which is regulated by actin filaments. Recently, WASH complex were identified as actin nucleation promoting factors (NPF) that activated Arp2/3 complex. However, the roles of WASH complex remain uncertain, particularly for oocyte polarization and asymmetric division. Here, we examined the functions of two important subunits of a WASH complex, WASH1 and Strumpellin, during mouse oocyte meiosis. Depleting WASH1 or disrupting Strumpellin activity by WASH1 morpholino (MO) injection or Strumpellin antibody injection decreased polar body extrusion and caused oocyte symmetric division, and this may have been due to spindle formation and migration defects. Time lapse microscopy showed that actin filaments distribution and relative amount at the membrane and in the cytoplasm of oocytes was significantly decreased after disrupting WASH complex. In addition, Arp2/3 complex expression was reduced after WASH1 depletion. Thus, our data indicated that WASH complex regulated Arp2/3 complex and were required for cytokinesis and following polar body extrusion during mouse oocyte meiotic maturation.
URLPMID:20856880

The interplay between maturation-promoting factor (MPF), mitogen-activated protein kinase (MAPK) and Rho GTPase during actin-myosin interactions has yet to be determined. The mechanism by which microtubule disrupters induce the formation of ooplasmic protrusion during chemical-assisted enucleation of mammalian oocytes is unknown. Moreover, a suitable model is urgently needed for the study of cytokinesis. We have established a model of chemical-induced cytokinesis and have studied the signaling events leading to cytokinesis using this model. The results suggested that microtubule inhibitors activated MPF, which induced actomyosin assembly (formation of ooplasmic protrusion) by activating RhoA and thus MAPK. While MAPK controlled actin recruitment on its own, MPF promoted myosin enrichment by activating RhoA and MAPK. A further chemical treatment of oocytes with protrusions induced constriction of the actomyosin ring by inactivating MPF while activating RhoA. In conclusion, the present data suggested that the assembly and contraction of the actomyosin ring were two separable steps: while an increase in MPF activity promoted the assembly through RhoA-mediated activation of MAPK, a decrease in MPF activity triggered contraction of the ring by activating RhoA.
URLPMID:25485583 [本文引用: 1]

Mammalian oocyte maturation is distinguished by asymmetric division that is regulated primarily by cytoskeleton, including microtubules and microfilaments. Small Rho GTPase RhoA is a key regulator of cytoskeletal organization which regulates cell polarity, migration, and division. In this study, we investigated the roles of RhoA in mammalian oocyte meiosis and early embryo cleavage. (1) Disrupting RhoA activity or knock down the expression of RhoA caused the failure of polar body emission. This may have been due to decreased actin assembly and subsequent spindle migration defects. The involvement of RhoA in this process may have been though its regulation of actin nucleators ROCK, p-Cofilin, and ARP2 expression. (2) In addition, spindle morphology was also disrupted and p-MAPK expression decreased in RhoA inhibited or RhoA KD oocytes, which indicated that RhoA also regulated MAPK phosphorylation for spindle formation. (3) Porcine embryo development was also suppressed by inhibiting RhoA activity. Two nuclei were observed in one blastomere, and actin expression was reduced, which indicated that RhoA regulated actin-based cytokinesis of porcine embryo. Thus, our results demonstrated indispensable roles for RhoA in regulating porcine oocyte meiosis and cleavage during early embryo development.
URLPMID:3690527 [本文引用: 1]

Asymmetric meiotic divisions in mammalian oocytes rely on the eccentric positioning of the spindle and the remodeling of the overlying cortex, resulting in the formation of small polar bodies. The mechanism of this cortical polarization, exemplified by the formation of a thick F-actin cap, is poorly understood. Cdc42 is a major player in cell polarization in many systems; however, the spatio-temporal dynamics of Cdc42 activation during oocyte meiosis, and its contribution to mammalian oocyte polarization, have remained elusive. In this study, we investigated Cdc42 activation (Cdc42–GTP), dynamics and role during mouse oocyte meiotic divisions. We show that Cdc42–GTP accumulates in restricted cortical regions overlying meiotic chromosomes or chromatids, in a Ran–GTP-dependent manner. This polarized activation of Cdc42 is required for the recruitment of N-WASP and the formation of F-actin-rich protrusions during polar body formation. Cdc42 inhibition in MII oocytes resulted in the release of N-WASP into the cytosol, a loss of the polarized F-actin cap, and a failure to protrude the second polar body. Cdc42 inhibition also resulted in central spindle defects in activated MII oocytes. In contrast, emission of the first polar body during oocyte maturation could occur in the absence of a functional Cdc42/N-WASP pathway. Therefore, Cdc42 is a new protagonist in chromatin-induced cortical polarization in mammalian oocytes, with an essential role in meiosis II completion, through the recruitment and activation of N-WASP, downstream of the chromatin-centered Ran–GTP gradient.
URLPMID:5649100

Abstract The mammalian oocyte undergoes an asymmetric division during meiotic maturation, producing a small polar body and a haploid gamete. This process involves the dynamics of actin filaments, and the guanosine triphosphatase (GTPase) protein superfamily is a major regulator of actin assembly. In the present study, the small GTPase CDC42 was shown to participate in the meiotic maturation of porcine oocytes. Immunofluorescent staining showed that CDC42 was mainly localized at the periphery of the oocytes, and accumulated with microtubules. Deactivation of CDC42 protein activity with the effective inhibitor ML141 caused a decrease in actin distribution in the cortex, which resulted in a failure of polar body extrusion. Moreover, western blot analysis revealed that besides the Cdc42-N-WASP pathway previously reported in mouse oocytes, the expression of ROCK and p-cofilin, two molecules involved in actin dynamics, was also decreased after CDC42 inhibition during porcine oocyte maturation. Thus, our study demonstrates that CDC42 is an indispensable protein during porcine oocyte meiosis, and CDC42 may interact with N-WASP, ROCK, and cofilin in the assembly of actin filaments during porcine oocyte maturation.
URLPMID:3713125

Asymmetric meiotic divisions in mammalian oocytes are driven by the eccentric positioning of the spindle, along with a dramatic reorganization of the overlying cortex, including a loss of microvilli and formation of a thick actin cap. Actin polarization relies on a Ran-GTP gradient centered on metaphase chromosomes; however, the downstream signaling cascade is not completely understood. In a recent study, we have shown that Ran promotes actin cap formation via the polarized activation of Cdc42. The related GTPase Rac is also activated in a polarized fashion in the oocyte cortex and co-localizes with active Cdc42. In other cells, microvilli collapse can be triggered by inactivation of the ERM (Ezrin/Radixin/Moesin) family of actin-membrane crosslinkers under the control of Rac. Accordingly, we show here that Ran-GTP promotes a substantial loss of phosphorylated ERMs in the cortex overlying the spindle in mouse oocytes. However, this polarized phospho-ERM exclusion zone was unaffected by Rac or Cdc42 inhibition. Therefore, we suggest that Ran activates two distinct pathways to regulate actin cap formation and microvilli disassembly in the polarized cortex of mouse oocytes. The possibility of a crosstalk between Rho GTPase and ERM signaling and a role for ERM inactivation in promoting cortical actin dynamics are also discussed.
URLPMID:17276347 [本文引用: 1]

Mammalian meiotic divisions are asymmetrical and generate a large oocyte and two small polar bodies. This asymmetry results from the anchoring of the meiotic spindle to the oocyte cortex and subsequent cortical reorganization, but the mechanisms involved are poorly understood. We investigated the role of Rac in oocyte meiosis by using a fluorescent reporter for Rac-GTP. We find that Rac-GTP is polarized in the cortex overlying the meiotic spindle. Polarization of Rac activation occurs during spindle migration and is promoted by the proximity of chromatin to the cortex. Inhibition of Rac during oocyte maturation caused a permanent block at聽prometaphase I and spindle elongation. In metaphase II-arrested oocytes, Rac inhibition caused the spindle to detach from the cortex and prevented polar body emission after activation. These results demonstrate that Rac-GTP plays a major role in oocyte meiosis, via the regulation of spindle stability and anchoring to the cortex.
URLPMID:20515799

P21-activated kinase 1 (PAK1), an effector of Rho GTPase Rac 1 and Cdc42, is required for mitotic progression. However, its functions in meiosis are unclear. In the present study, we examined the expression, localization and function of PAK1 during mouse oocyte meiotic maturation and found that PAK1 was mainly associated with the meiotic spindle microtubules. Taxol treatment resulted in localization of PAK1 on spindle and aster microtubules, while nocodazole treatment induced the dispersion of PAK1 protein into the cytoplasm. Loss-of-function of PAK1 by both inhibitor treatment and morpholino oligonucleotide injection caused disorganized spindles, decreased polar body extrusion and misaligned chromosomes. In addition, inhibition of PAK1 resulted in abnormal localization of mitogen-activated protein kinase kinase (MEK). Taken together, our results suggest that PAK1 plays an important role in spindle assembly and chromosome alignment during mouse oocyte meiotic maturation.
URLPMID:19005166

Abstract Mouse oocytes undergo two successive meiotic divisions to generate one large egg with two small polar bodies. The divisions are essential for preserving the maternal resources to support embryonic development. Although previous studies have shown that some small guanosine triphosphatases, such as RAC, RAN, and CDC42, play important roles in cortical polarization and spindle pole anchoring, no oocytes undergo cytokinesis when the mutant forms of these genes are expressed in mouse oocytes. Here, we show that the ADP-ribosylation factor 1 (ARF1) plays an important role in regulating asymmetric cell division in mouse oocyte meiosis. Microinjection of mRNA of a dominant negative mutant form of Arf1 (Arf1(T31N)) into fully grown germinal vesicle oocytes led to symmetric cell division in meiosis I, generating two metaphase II (MII) oocytes of equal size. Subsequently, the two MII oocytes of equal size underwent the second round of symmetric cell division to generate a four-cell embryo (zygote) when activated parthenogenetically or via sperm injection. Furthermore, inactivation of mitogen-activated protein kinase (MAPK) but not MDK (also known as MEK) has been discovered in the ARF1 mutant oocytes, and this further demonstrated that ARF1, MAPK pathway plays an important role in regulating asymmetric cell division in meiosis I. Similarly, ARF1(T31N)-expressing, superovulated MII oocytes underwent symmetric cell division in meiosis II when activation was performed. Rotation of the MII spindle for 90 degrees was prohibited in ARF1(T31N)-expressing MII oocytes. Taken together, our results suggest that ARF1 plays an essential role in regulating asymmetric cell division in female meiosis.
URLPMID:3256179

Mammalian oocytes undergo an asymmetrical first meiotic division, extruding half of their chromosomes in a small polar body to preserve maternal resources for embryonic development. To divide asymmetrically, mammalian oocytes relocate chromosomes from the center of the cell to the cortex, but little is known about the underlying mechanisms. Here, we show that upon the elevation of intracellular cAMP level, mouse oocytes produced two daughter cells with similar sizes. This symmetrical cell division could be rescued by the inhibition of PKA, a cAMP-dependent protein kinase. Live cell imaging revealed that a symmetrically localized cleavage furrow resulted in symmetrical cell division. Detailed analyses demonstrated that symmetrically localized cleavage furrows were caused by the inappropriate central positioning of chromosome clusters at anaphase onset, indicating that chromosome cluster migration was impaired. Notably, high intracellular cAMP reduced myosin II activity, and the microinjection of phospho-myosin II antibody into the oocytes impeded chromosome migration and promoted symmetrical cell division. Our results support the hypothesis that cAMP plays a role in regulating asymmetrical cell division by modulating myosin II activity during mouse oocyte meiosis I, providing a novel insight into the regulation of female gamete formation in mammals.
URLPMID:2938384

Abstract Cell division is inherently mechanical, with cell mechanics being a critical determinant governing the cell shape changes that accompany progression through the cell cycle. The mechanical properties of symmetrically dividing mitotic cells have been well characterized, whereas the contribution of cellular mechanics to the strikingly asymmetric divisions of female meiosis is very poorly understood. Progression of the mammalian oocyte through meiosis involves remodeling of the cortex and proper orientation of the meiotic spindle, and thus we hypothesized that cortical tension and stiffness would change through meiotic maturation and fertilization to facilitate and/or direct cellular remodeling. This work shows that tension in mouse oocytes drops about sixfold during meiotic maturation from prophase I to metaphase II and then increases 0908041.6-fold upon fertilization. The metaphase II egg is polarized, with tension differing 0908042.5-fold between the cortex over the meiotic spindle and the opposite cortex, suggesting that meiotic maturation is accompanied by assembly of a cortical domain with stiffer mechanics as part of the process to achieve asymmetric cytokinesis. We further demonstrate that actin, myosin-II, and the ERM (Ezrin/Radixin/Moesin) family of proteins are enriched in complementary cortical domains and mediate cellular mechanics in mammalian eggs. Manipulation of actin, myosin-II, and ERM function alters tension levels and also is associated with dramatic spindle abnormalities with completion of meiosis II after fertilization. Thus, myosin-II and ERM proteins modulate mechanical properties in oocytes, contributing to cell polarity and to completion of meiosis.
URLPMID:24824085 [本文引用: 1]

SUMMARY Mammalian oocyte meiotic maturation involves a unique asymmetric division, but the regulatory mechanisms and signaling pathways involved are poorly understood. Dynamins are ubiquitous eukaryotic GTPases involved in membrane trafficking and actin dynamics, whose roles in mammalian oocyte maturation have not been determined. In this study, we used porcine oocytes to show that Dynamin 2 accumulated at the meiotic spindle and in the cortex of oocytes, with a distribution similar to that of actin. Inhibiting Dynamin 2 activity in porcine oocytes with the specific inhibitor dynasore resulted in failed polar-body extrusion. This phenotype may have been due to aberrant actin distribution and/or spindle positioning as inhibitor treatment disrupted the formation of the actin cap and cortical granule-free domain, which negatively impacted spindle positioning. Moreover, the distribution of ARP2, a key actin-nucleation factor, was severely reduced in the cortex after dynasore treatment. Thus, our results suggest that Dynamin 2 possibly regulates porcine oocyte maturation through its effects on actin-mediated spindle positioning and cytokinesis, and that this may depend on regulating ARP2 localization. Mol. Reprod. Dev . 81: 725鈥734, 2014. 漏 2014 Wiley Periodicals, Inc.
URLPMID:5068415 [本文引用: 1]

LIMKi 3 is a specific selective LIMK inhibitor against LIMK1 and LIMK2, while LIMK1 and LIMK2 are the main regulators of actin cytoskeleton to participate in many cell activities. However, the effect of LIMKi 3 in porcine oocyte meiosis is still unclear. The present study was designed to investigate the effects of LIMKi 3 and potential regulatory role of LIMK1/2 on porcine oocyte meiotic maturation. Immunofluorescent staining of p-LIMK1/2 antibody showed that LIMK1/2 was localized mainly to the cortex of porcine oocyte, which co-localized with actin. After LIMKi 3 treatment, the diffusion of COCs became weak and the rate of polar body extrusion was decreased. This could be rescued by moving oocytes to fresh medium. After prolonging the culture time of oocytes, the maturation rate of porcine oocyte increased in LIMKi 3 groups, indicating that LIMKi 3 may suppress the cell cycle during porcine oocyte maturation. We also found that after LIMKi 3 treatment actin distribution was significantly disturbed at porcine oocyte membranes and cytoplasm, indicating the conserved roles of LIMK1/2 on actin dynamics. Next we examined the meiotic spindle positioning in porcine oocyte, and the results showed that a majority of spindles were not attached to the cortex of porcine oocyte, indicating that LIMKi 3 may affect actin-mediated spindle positioning. Taken together, these results showed that LIMK1/2 inhibitor LIMKi 3 had a repressive role on porcine oocyte meiotic maturation.
URLPMID:15680356

During maturation, the mouse oocyte is transformed into a highly polarized egg, characterized by an actin cap and cortical granule-free domain (CGFD) overlying the meiotic spindle that is in close proximity to the cortex. The presence of spindle/chromosomes or microinjected sperm chromatin in the cortical region initiates this cortical reorganization, but the pathway is unknown. We report that cortical reorganization induced by microinjected sperm chromatin is blocked by inhibitors of microfilament assembly or disassembly. Active mitogen-activated protein kinase (MAPK), which becomes enriched in the region of sperm chromatin, is required for cortical reorganization, because microinjected sperm chromatin fails to induce cortical reorganization in Mos 61/61 eggs, which lack MAPK activity. Last, myosin light chain kinase (MLCK), which can be directly phosphorylated and activated by MAPK, appears involved, because the MLCK inhibitors ML-7 and Peptide 18 prevent sperm chromatin-induced cortical reorganization. These results provide new insights into how cortical reorganization occurs independently of extracellular signals to generate egg polarity.
URLPMID:19062278

An oocyte matures into an egg by extruding half of the chromosomes in a small polar body. This extremely asymmetric division enables the oocyte to retain sufficient storage material for the development of the embryo after fertilization. To divide asymmetrically, mammalian oocytes relocate the spindle from their center to the cortex. In all mammalian species analyzed so far, including human, mouse, cow, pig, and hamster, spindle relocation depends on filamentous actin (F-actin). However, even though spindle relocation is essential for fertility, the involved F-actin structures and the mechanism by which they relocate the spindle are unknown. Here we show in live mouse oocytes that spindle relocation requires a continuously reorganizing cytoplasmic actin network nucleated by Formin-2 (Fmn2). We found that the spindle poles were enriched in activated myosin and pulled on this network. Inhibition of myosin activation by myosin light chain kinase (MLCK) stopped pulling and spindle relocation, indicating that myosin pulling creates the force that drives spindle movement. Based on these results, we propose the first mechanistic model for asymmetric spindle positioning in mammalian oocytes and validate five of its key predictions experimentally.
URLPMID:25904014

Vertebrate eggs are arrested at metaphase of meiosis II, a state classically known as cytostatic factor arrest. Maintenance of this arrest until the time of fertilization and then fertilization-induced exit from metaphase II are crucial for reproductive success. Another key aspect of this meiotic arrest and exit is regulation of the metaphase II spindle, which must be appropriately localized adjacent to the egg cortex during metaphase II and then progress into successful asymmetric cytokinesis to produce the second polar body. This study examined the mitogen-activated protein kinases MAPK3 and MAPK1 (also known as ERK1/2) as regulators of these two related aspects of mammalian egg biology, specifically testing whether this MAPK pathway affected myosin-II function and whether myosin-II perturbation would produce some of the same effects as MAPK pathway perturbation. Inhibition of the MEK1/2-MAPK pathway with U0126 leads to reduced levels of phosphorylated myosin-regulatory light chain (pMRLC) and causes a reduction in cortical tension, effects that are mimicked by treatment with the myosin light chain kinase (MLCK) inhibitor ML-7. These data indicate that one mechanism by which the MAPK pathway acts in eggs is by affecting myosin-II function. We further show that MAPK or MLCK inhibition induces loss of normal cortical spindle localization or parthenogenetic egg activation. This parthenogenesis is dependent on cytosolic and extracellular calcium and can be rescued by hyperloading eggs with zinc, suggesting that these effects of inhibition of MLCK or the MAPK pathway are linked with dysregulation of ion homeostasis.
URLPMID:21734461 [本文引用: 3]

Comment on: Zhang CH, et al. Cell Cycle 2011; 10:1639-54.
URLPMID:21552007

GM130, a cis-Golgi protein, plays key roles in various mitotic events, but its function in mammalian oocyte meiosis remains unknown. In this study, we found that GM130 was localized to the spindle poles at both metaphase I and metaphase II stages and associated with the midbody at telophase I stage. The association of GM130 with spindle poles was further confirmed by its colocalization with the centrosome-associated proteins, MEK1/2. By nocodazole treatment, we clarified that GM130 localization was consistently dependent on spindle assembly. Then we investigated the possible function of GM130 by specific morpholino microinjection. This treatment caused abnormal spindle formation, and decreased first polar body extrusion. Our results showed that knockdown of GM130 impaired the localization of MTOCs proteins 脦鲁-tubulin and Plk1. Using live cell imaging we observed that depletion of GM130 affected spindle migration and resulted in elongated spindle and large polar body extrusion. We further found that depletion of GM130 blocked p-MEK1/2 accumulation at the spindle poles. And, it was shown that GM130 detached from the spindle poles in oocytes treated with MEK specific inhibitor U0126. Taken together, our results suggested that GM130 regulates microtubule organization and might cooperate with the MAPK pathway to play roles in spindle organization, migration and asymmetric division during mouse oocyte maturation.
URLPMID:20841362

Abstract Fertilization of mammalian oocytes triggers their exit from the second meiotic division metaphase arrest. The extrusion of the second polar body (PBII) that marks the completion of meiosis is followed by the first mitotic cleavage of the zygote. Several lines of evidence in somatic cells imply the involvement of Fyn, an Src family kinase (SFK), in cell cycle control and actin functions. In this study, we demonstrate, using live cell confocal imaging and microinjection of Fyn cRNAs, the recruitment of Fyn to the oocyte's cortical area overlying the chromosomes and its colocalization with filamentous actin (F-actin) during exit from the meiotic metaphase. Fyn concentrated asymmetrically at the cortical site designated for ingression of the PBII cleavage furrow, where F-actin had already been accumulated, and then redispersed throughout the entire cortex only to be recruited again to the cleavage furrow during the first mitotic division. Although microinjection of dominant negative Fyn did not affect initiation of the cleavage furrow, it prolonged the average duration of ingression, decreased the rates of PB extrusion and of the first cleavage, and led to the formation of bigger PBs and longer spindles. Extrusion of the PBII was blocked in oocytes exposed to SU6656, an SFK inhibitor. Our results demonstrate, for the first time, a continuous colocalization of Fyn and F-actin during meiosis and imply a role for the SFKs, in general, and for Fyn, in particular, in regulating pathways that involve actin cytoskeleton, during ingression of the meiotic and mitotic cleavage furrows.
URLPMID:20372074

Abstract The process of resumption of the first meiotic division (RMI) in mammalian oocytes includes germinal vesicle breakdown (GVBD), spindle formation during first metaphase (MI), segregation of homologous chromosomes, extrusion of the first polar body (PBI) and an arrest at metaphase of the second meiotic division (MII). Previous studies suggest a role for Fyn, a non-receptor Src family tyrosine kinase, in the exit from MII arrest. In the current study we characterized the involvement of Fyn in RMI. Western blot analysis demonstrated a significant, proteasome independent, degradation of Fyn during GVBD. Immunostaining of fixed oocytes and confocal imaging of live oocytes microinjected with Fyn complementary RNA (cRNA) demonstrated Fyn localization to the oocyte cortex and to the spindle poles. Fyn was recruited during telophase to the cortical area surrounding the midzone of the spindle and was then translocated to the contractile ring during extrusion of PBI. GVBD, exit from MI and PBI extrusion were inhibited in oocytes exposed to the chemical inhibitor SU6656 or microinjected with dominant negative Fyn cRNA. None of the microinjected oocytes showed misaligned or lagging chromosomes during chromosomes segregation and the spindle migration and anchoring were not affected. However, the extruded PBI was of large size. Altogether, a role for Fyn in regulating several key pathways during the first meiotic division in mammalian oocytes is suggested, particularly at the GV and metaphase checkpoints and in signaling the ingression of the cleavage furrow.
URLPMID:21871204

Abstract Several lines of evidence imply the involvement of Fyn, a Src family kinase, in cell-cycle control and cytoskeleton organisation in somatic cells. By live cell confocal imaging of immunostained or cRNA-microinjected mouse oocytes at metaphase of the second meiotic division, membrane localisation of active and non-active Fyn was demonstrated. However, Fyn with a disrupted membrane-binding domain at its N-terminus was targeted to the cytoplasm and spindle in its non-active form and concentrated at the spindle poles when active. During metaphase exit, the amount of phosphorylated Fyn and of spindle-poles Fyn decreased and it started appearing at the membrane area of the cleavage furrow surrounding the spindle midzone, either asymmetrically during polar body II extrusion or symmetrically during mitosis. These results demonstrate that post-translational modifications of Fyn, probably palmitoylation, determine its localisation and function; localisation of de-palmitoylated active Fyn to the spindle poles is involved in spindle pole integrity during metaphase, whereas the localisation of N-terminus palmitoylated Fyn at the membrane near the cleavage furrow indicates its participation in furrow ingression during cytokinesis.
URLPMID:28177917

Abstract Mammalian oocyte meiotic maturation is the precondition of early embryo development. Lots of microtubules (MT)-associated proteins participate in oocyte maturation process. Cytoskeleton-associated protein 5 (CKAP5) is a member of the XMAP215 family that regulates microtubule dynamics during mitosis. However, its role in meiosis has not been fully studied. Here, we investigated the function of CKAP5 in mouse oocyte meiotic maturation and early embryo development. Western blot showed that CKAP5 expression increased from GVBD, maintaining at high level at metaphase, and decreased after late 1-cell stage. Confocal microscopy showed there is no specific accumulation of CKAP5 at interphase (GV, PN or 2-cell stage). However, once cells enter into meiotic or mitotic division, CKAP5 was localized at the whole spindle apparatus. Treatment of oocytes with the tubulin-disturbing reagents nocodazole (induces MTs depolymerization) or taxol (prevents MTs depolymerization) did not affect CKAP5 expression but led to a rearrangement of CKAP5. Further, knock-down of CKAP5 resulted in a failure of first polar body extrusion, serious defects in spindle assembly, and failure of chromosome alignment. Loss of CKAP5 also decreased early embryo development potential. Furthermore, co-immunoprecipitation showed that CKAP5 bound to clathrin heavy chain 1 (CLTC). Taken together, our results demonstrate that CKAP5 is important in oocyte maturation and early embryo development, and CKAP5 might work together with CLTC in mouse oocyte maturation.
URLPMID:17276346 [本文引用: 1]

The molecular basis for asymmetric meiotic divisions in mammalian oocytes that give rise to mature eggs and polar bodies remains poorly understood. Previous studies demonstrated that the asymmetrically positioned meiotic chromosomes provide the cue for cortical polarity in mouse oocytes. Here we show that the chromatin-induced cortical response can be fully reconstituted by injecting DNA-coated beads into metaphase II-arrested eggs. The injected DNA beads induce a cortical actin cap, surrounded by a myosin II ring, in a manner that depends on the number of beads and their distance from the cortex. The Ran GTPase plays a critical role in this process, because dominant-negative and constitutively active Ran mutants disrupt DNA-induced cortical polarization. The Ran-mediated signaling to the cortex is independent of the spindle but requires cortical myosin II assembly. We hypothesize that a Ran GTP gradient serves as a molecular ruler to interpret the asymmetric position of the meiotic chromatin.
URLPMID:4038667 [本文引用: 1]

Experiments were carried out to determine the origin of cortical polarity in mouse eggs and its possible relation to the meiotic apparatus. Cortices of mature eggs overlying the meiotic apparatus (microvillus-free area) were distinguished by an absence of microvilli and a thickened layer of actin. In contrast, the surfaces of immature oocytes were covered entirely with a dense population of microvilli and were subtended by a uniform layer of actin. When induced to undergo maturation, meiotic spindles formed in the center of immature oocytes and then moved peripherally. Coincident with the cortical localization of the meiotic spindle was the formation of a microvillus-free area, i.e., a loss of microvilli and a thickening of the actin layer associated with this region of the egg cortex. If immature oocytes were incubated in cytochalasin B, meiotic spindles formed; however, they failed to move peripherally and microvillus-free areas did not develop. Oocytes incubated in colchicine did not form meiotic spindles, although the chromosomes condensed and became localized to cortices where microvillus-free areas developed. Cytochalasin B-treated mature eggs maintained intact meiotic spindles and exhibited a disappearance of microvillus-free areas and a reduction in cortical actin. The chromosomes of mature eggs treated with colchicine remained associated with microvillus-free areas despite the disappearance of meiotic spindles. Occasionally, colchicine-treated eggs possessed more than one cortically located mass of chromosomes, each of which was associated with a microvillus-free area. These observations indicate that mechanisms involving the movement of the meiotic spindle to the oocyte cortex and development and maintenance of cortical polarity are cytochalasin B sensitive. Commensurate with the localization of meiotic chromosomes to the egg cortex is the reorganization of cortical actin and the formation of a microvillus-free area.
URLPMID:12710965 [本文引用: 3]

A cortical granule-free domain (CGFD) overlies the metaphase chromatin in fully mature mouse eggs. Although a chromatin-induced localized release of cortical granules (CG) during maturation is thought to be a major contributing factor to its formation, there are indications that CG redistribution may also be involved in generating the CGFD. We performed experiments to determine the relative contributions of CG exocytosis and redistribution in generating the CGFD. We found that the CGFD-inducing activity was not specific to female germ cell chromatin and was heat stable but sensitive to DNase and protease treatment. Surprisingly, chelation of egg intracellular Ca 2+ levels did not prevent CGFD formation in response to microinjection of exogenous chromatin, suggesting that development of the CGFD was not a result of CG exocytosis. This finding was confirmed by the lack of CG exudate on the plasma membrane surface of the injected eggs and the absence of conversion of ZP2 to ZP2 f during formation of the new CGFD. Moreover, clamping intracellular Ca 2+ did not prevent the formation of the CGFD during oocyte maturation, but did inhibit the maturation-associated release of CGs between metaphase I and II. Results of these experiments suggest that CG redistribution is the dominant factor in formation of the CGFD.
URL [本文引用: 1]

Spindle formation is essential for stable inheritance of genetic material. Experiments in various systems indicate that Ran GTPase is crucial for meiotic and mitotic spindle assembly. Such an important role for Ran in chromatin-induced spindle assembly was initially demonstrated in Xenopus laevis egg extracts. However, the requirement of RanGTP in living meiotic cells has not been shown. In this study, we used a fluorescence resonance energy transfer probe to measure RanGTP-regulated release of importin beta. A RanGTP-regulated gradient was established during meiosis I and was centered on chromosomes throughout mouse meiotic maturation. Manipulating levels of RanGTP in mice and X. laevis oocytes did not inhibit assembly of functional meiosis I spindles. However, meiosis II spindle assembly did not tolerate changes in the level of RanGTP in both species. These findings suggest that a mechanism common to vertebrates promotes meiosis I spindle formation in the absence of chromatin-induced microtubule production and centriole-based microtubule organizing centers.
URL [本文引用: 1]
URLPMID:24444815 [本文引用: 5]

Assisted reproduction technology (ART) has become an attractive option for infertility treatment and holds tremendous promise. However, at present, there is still room for improvement in its success rates. Oocyte maturation is a process by which the oocyte becomes competent for fertilization and subsequent embryo development. To better understand the mechanism underlying oocyte maturation and for the future improvement of assisted reproduction technology, this review focuses on the complex processes of cytoplasmic organelles and the dynamic alterations of the cytoskeleton that occur during oocyte maturation. Ovarian stimulation and in-vitro maturation are the major techniques used in assisted reproduction technology and their influence on the organelles of oocytes is also discussed. Since the first birth by assisted reproduction treatment was achieved in 1978, numerous techniques involved in assisted reproduction have been developed and have become attractive options for infertility treatment. However, the unsatisfactory success rate remains as a main challenge. Oocyte maturation is a process by which the oocyte becomes competent for fertilization and subsequent embryo development. Oocyte maturation includes both nuclear and cytoplasmic maturation. Nuclear maturation primarily involves chromosomal segregation, which has been well studied, whereas cytoplasmic maturation involves a series of complicated processes, and there are still many parts of this process that remain controversial. Ovarian stimulation and in-vitro maturation (IVM) are the major techniques of assisted reproduction. The effect of ovarian stimulation or IVM on the behaviour of cell organelles of the oocyte has been postulated as the reason for the reduced developmental potential of in-vitro-produced embryos. To further understanding of the mechanism of oocyte maturation and future improvement of assisted reproduction treatment, the complex events of cytoplasmic organelles and the cytoskeleton that occur during oocyte maturation and the influence of ovarian stimulation and IVM on these organelles are described in this review.
URL [本文引用: 1]
URLPMID:5147004 [本文引用: 2]

Oogenesis - the process by which female germ cells develop into mature eggs, or ova - is a complex process involving many important elements of developmental and cellular biology: from cell-cell interactions, complex signalling cascades, specialized cell cycles and cytoskeleton organization. Oocytes from various species (including clam, starfish, xenopus and mouse) are excellent model systems to study the biochemistry of cell division with important implications for basic and clinical research. This book describes the entire process of oogenesis in chronological order with contributions from leading international researchers and chapters covering medical and ethical considerations in oogenic biology. Topics include sex determination and gonadal development, control of meiotic chromosome pairing and homologous recombination, control of meiotic divisions and the remodelling of the oocyte into a totipotent zygote as well as medically-assisted reproduction.
URLPMID:18833336 [本文引用: 1]

Formation of female gametes requires acentriolar spindle assembly during meiosis. Mitotic spindles organize from centrosomes and via local activation of the RanGTPase on chromosomes. Vertebrate oocytes present a RanGTP gradient centred on chromatin at all stages of meiotic maturation. However, this gradient is dispensable for assembly of the first meiotic spindle. To understand this meiosis I peculiarity, we studied TPX2, a Ran target, in mouse oocytes. Strikingly, TPX2 activity is controlled at the protein level through its accumulation from meiosis I to II. By RNAi depletion and live imaging, we show that TPX2 is required for spindle assembly via two distinct functions. It controls microtubule assembly and spindle pole integrity via the phosphorylation of TACC3, a regulator of MTOCs activity. We show that meiotic spindle formation in vivo depends on the regulation of at least a target of Ran, TPX2, rather than on the regulation of the RanGTP gradient itself.
URLPMID:16172205 [本文引用: 1]

Centrosomes act as sites of microtubule growth, but little is known about how the number and stability of microtubules emanating from a centrosome are controlled during the cell cycle. We studied the role of the TACC3-XMAP215 complex in this process by using purified proteins and Xenopus laevis egg extracts. We show that TACC3 forms a one-to-one complex with and enhances the microtubule-stabilizing activity of XMAP215 in vitro. TACC3 enhances the number of microtubules emanating from mitotic centrosomes, and its targeting to centrosomes is regulated by Aurora A-dependent phosphorylation. We propose that Aurora A regulation of TACC3 activity defines a centrosomespecific mechanism for regulation of microtubule polymerization in mitosis.
URLPMID:26378257 [本文引用: 1]

Female meiotic spindles are organized in the absence of centrosomes.Caenorhabditis elegansAurora A (AIR-1) is dispensable for the initial assembly of meiotic microtubules within the oocyte nuclei, but its kinase activity is continuously required for the stabilization of meiotic spindle microtubules after germinal vesicle breakdown. In many animals, female meiotic spindles are assembled in the absence of centrosomes, the major microtubule (MT)-organizing centers. How MTs are formed and organized into meiotic spindles is poorly understood. Here we report that, inCaenorhabditis elegans, Aurora A kinase/AIR-1 is required for the formation of spindle microtubules during female meiosis. When AIR-1 was depleted or its kinase activity was inhibited inC. elegansoocytes, although MTs were formed around chromosomes at germinal vesicle breakdown (GVBD), they were decreased during meiotic prometaphase and failed to form a bipolar spindle, and chromosomes were not separated into two masses. Whereas AIR-1 protein was detected on and around meiotic spindles, its kinase-active form was concentrated on chromosomes at prometaphase and on interchromosomal MTs during late anaphase and telophase. We also found that AIR-1 is involved in the assembly of short, dynamic MTs in the meiotic cytoplasm, and these short MTs were actively incorporated into meiotic spindles. Collectively our results suggest that, after GVBD, the kinase activity of AIR-1 is continuously required for the assembly and/or stabilization of female meiotic spindle MTs.
URLPMID:12527899 [本文引用: 1]

Abstract An analytic strategy was followed to identify putative regulatory genes during the development of human hepatocellular carcinoma (HCC). This strategy employed a bioinformatics analysis that used a database search to identify genes, which are differentially expressed in human HCC and are also under cell cycle regulation. A novel cell cycle regulated gene (HURP) that is overexpressed in HCC was identified. Full-length cDNAs encoding the human and mouse HURP genes were isolated. They share 72 and 61% identity at the nucleotide level and amino-acid level, respectively. Endogenous levels of HURP mRNA were found to be tightly regulated during cell cycle progression as illustrated by its elevated expression in the G(2)/M phase of synchronized HeLa cells and in regenerating mouse liver after partial hepatectomy. Immunofluorescence studies revealed that hepatoma up-regulated protein (HURP) localizes to the spindle poles during mitosis. Overexpression of HURP in 293T cells resulted in an enhanced cell growth at low serum levels and at polyhema-based, anchorage-independent growth assay. Taken together, these results strongly suggest that HURP is a potential novel cell cycle regulator that may play a role in the carcinogenesis of human cancer cells.
URL [本文引用: 1]
URLPMID:22552228 [本文引用: 1]

Abstract It is well established that chromosome segregation in female meiosis I (MI) is error-prone. The acentrosomal meiotic spindle poles do not have centrioles and are not anchored to the cortex via astral microtubules. By Cre recombinase-mediated removal in oocytes of the microtubule binding site of nuclear mitotic apparatus protein (NuMA), which is implicated in anchoring microtubules at poles, we determine that without functional NuMA, microtubules lose connection to MI spindle poles, resulting in highly disorganized early spindle assembly. Subsequently, very long spindles form with hyperfocused poles. The kinetochores of homologs make attachments to microtubules in these spindles but with reduced tension between them and accompanied by alignment defects. Despite this, the spindle assembly checkpoint is normally silenced and the advance to anaphase I and first polar body extrusion takes place without delay. Females without functional NuMA in oocytes are sterile, producing aneuploid eggs with altered chromosome number. These findings establish that in mammalian MI, the spindle assembly checkpoint is unable to sustain meiotic arrest in the presence of one or few misaligned and/or misattached kinetochores with reduced interkinetochore tension, thereby offering an explanation for why MI in mammals is so error-prone.
URLPMID:22753278 [本文引用: 3]

Mammalian oocyte maturation involves two successive rounds of extremely asymmetric cell divisions (known as polar body extrusion) to generate a functional haploid egg. Successful polar body extrusion relies on establishment of an asymmetric spindle position and cortical polarity. Decades of studies using mouse oocytes as a model have revealed critical roles for a dynamic actin cytoskeleton in this process. Here, we review the contribution of actin to the critical events during oocyte meiotic cell divisions with an emphasis on recent advances in understanding the underlying molecular and physical mechanisms. 脗漏 2012 Wiley Periodicals, Inc
URLPMID:27152960 [本文引用: 2]

Actin nucleation factors, which promote the formation of new actin filaments, have emerged in the last decade as key regulatory factors controlling asymmetric division in mammalian oocytes. Actin nucleators such as formin-2, spire, and the ARP2/3 complex have been found to be important regulators of actin remodeling during oocyte maturation. Another class of actin-binding proteins including cofilin, tropomyosin, myosin motors, capping proteins, tropomodulin, and Ezrin-Radixin-Moesin proteins are thought to control actin cytoskeleton dynamics at various steps of oocyte maturation. In addition, actin dynamics controlling asymmetric-symmetric transitions after fertilization is a new area of investigation. Taken together, defining the mechanisms by which actin-binding proteins regulate actin cytoskeletons is crucial for understanding the basic biology of mammalian gamete formation and pre-implantation development.
URLPMID:26301502 [本文引用: 2]

Formins are cytoskeleton regulating proteins characterized by a common FH2 structural domain. As key players in the assembly of actin filaments, formins direct dynamic cytoskeletal processes that influence cell shape, movement and adhesion. The large number of formin genes, fifteen in the human, suggests distinct tasks and expression patterns for individual family members, in addition to overlapping functions. Several formins have been associated with invasive cell properties in experimental models, linking them to cancer biology. One example is FMNL1, which is considered to be a leukocyte formin and is known to be overexpressed in lymphomas. Studies on FMNL1 and many other formins have been hampered by a lack of research tools, especially antibodies suitable for staining paraffin-embedded formalin-fixed tissues. Here we characterize, using bioinformatics tools and a validated antibody, the expression pattern of FMNL1 in human tissues and study its subcellular distribution. Our results indicate that FMNL1 expression is not restricted to hematopoietic tissues and that neoexpression of FMNL1 can be seen in epithelial cancer.
URL [本文引用: 1]
URLPMID:27505246 [本文引用: 1]

In this Review, Cheffingset02al.discuss how eukaryotic cells position and form their actomyosin ring during cytokinesis and then examine potential mechanisms for generating the contractile stress that drives ring constriction and cell division.
URL [本文引用: 1]
URL [本文引用: 2]
URLPMID:18481961 [本文引用: 1]

Cleavage furrow formation in animal cells results from a local increase in cortical contractility. During anaphase, the spindle contains, in addition to astral arrays of microtubules, a set of bundled microtubules known as the central spindle. Each of these populations of microtubules, the astral arrays and the central spindle bundles, is sufficient to direct cleavage furrow formation, yet in wild-type situations these sets of microtubules co-operate to induce furrow formation at the same site, between the segregating chromosomes. These pathways have distinct genetic requirements that reflect their differential control of cortical actomyosin. We review our current understanding of the molecular mechanisms of furrow formation, with particular emphasis on the central spindle-independent pathway.
URLPMID:16989804 [本文引用: 1]

Female meiotic divisions in higher organisms are asymmetric and lead to the formation of a large oocyte and small polar bodies. These asymmetric divisions are due to eccentric spindle positioning which, in the mouse, requires actin filaments. Recently Formin-2, a straight actin filaments nucleator, has been proposed to control spindle positioning, chromosome segregation as well as first polar body extrusion in mouse oocytes. We reexamine here the possible role of Formin-2 during mouse meiotic maturation by live videomicroscopy. We show that Formin-2 controls first meiotic spindle migration to the cortex but not chromosome congression or segregation. We also show that the lack of first polar body extrusion in fmn2(-/-) oocytes is not due to a lack of cortical differentiation or central spindle formation but to a defect in the late steps of cytokinesis. Indeed, Survivin, a component of the passenger protein complex, is correctly localized on the central spindle at anaphase in fmn2(-/-) oocytes. We show here that attempts of cytokinesis in these oocytes abort due to phospho-myosin II mislocalization.
URLPMID:19996184 [本文引用: 1]

Abstract Completion of the first meiosis in oocytes is achieved by the extrusion of the first polar body (PBI), a particular example of cell division. In mitosis, the small GTPase RhoA, which is activated by epithelial cell transforming protein 2 (ECT2), orchestrates contractile ring constriction, thus enabling cytokinesis. However, the involvement of this pathway in mammalian oocytes has not been established. To characterize the role of ECT2 in PBI emission in mouse oocytes, the small interfering RNA approach was employed. We found that ECT2 depletion significantly reduces PBI emission, induces first metaphase arrest, and generates oocytes containing two properly formed spindles of the second metaphase. Moreover, we describe, for the first time, that before PBI emission, RhoA forms a ring that is preceded by a dome-like accumulation at the oocyte cortex, next to the spindle. This unique mode of RhoA translocation failed to occur in the absence of ECT2. We further found that the Rho-dependent kinase, a main RhoA effector, is essential for PBI emission. In addition, we demonstrate herein that ECT2 is subjected to phosphorylation/dephosphorylation throughout meiosis in oocytes and further reveal that PBI emission is temporally associated with ECT2 dephosphorylation. Our data provide the first demonstration that an active cyclin-dependent kinase 1, the catalytic subunit of the maturation-promoting factor, phosphorylates ECT2 during the first meiotic metaphase and that cyclin-dependent kinase 1 inactivation at anaphase allows ECT2 dephosphorylation. In conclusion, our study demonstrates the indispensable role of the maturation-promoting factor/ECT2/RhoA pathway in PBI extrusion in mouse oocytes.
URLPMID:17213284 [本文引用: 1]

Kit receptor and its ligand stem cell factor (SCF) are critical regulators of mast cell production, proliferation, degranulation, and chemotaxis. In this study, we investigated how Fyn kinase regulates chemotaxis of mast cells toward SCF. On beta1-integrin engagement, Fyn-deficient (fyn(-/-)) mast cells displayed a striking defect in cell spreading and lamellipodia formation compared to wild-type mast cells. The hematopoietic-specific Src family kinases (Lyn/Fgr/Hck) were not required for initial SCF-induced cell spreading. Reduced SCF-induced activation of Rac1 and Rac2 GTPases, p38 mitogen-activated protein kinase, and filamentous actin polymerization was observed in fyn(-/-) mast cells compared to wild-type mast cells. Retroviral-mediated expression of Fyn, constitutively active forms of Rac2 or phosphatidylinositol 3-kinase (PI3K) in fyn(-/-) mast cells rescued defects in SCF-induced cell polarization and chemotaxis of Fyn-deficient mast cells. Thus, we conclude that Fyn kinase plays a unique role upstream of PI3K and Rac GTPases to promote the reorganization of the cytoskeleton during mast cell spreading and chemotaxis.
URLPMID:8969217 [本文引用: 1]

Ld proteins (formins) are encoded by the limb deformity (ld) gene and define a family of related gene products regulating establishment of embryonic polarity. In this study we establish that chicken and murine Ld proteins interact directly with Src family kinases (c-Src and c-Fyn). Specific binding is mediated by the proline-rich domain present in Ld proteins and the ligand binding surface of the Src SH3 domain. Co-immunoprecipitation of Ld and c-Src proteins from transfected cells shows that these proteins associate in vivo. Immunolocalization and biochemical fractionation of fibroblasts confirms the predominant nuclear localization of Ld proteins, but unexpectedly identifies a population of Ld proteins associated to cellular membranes. This population of Ld proteins co-localizes with membrane-associated c-Src proteins at both plasma and perinuclear membranes. These studies indicate that the morphoregulatory Ld proteins interact with signal transduction cascades by association to membrane-bound Src family kinases.
URLPMID:10678165 [本文引用: 1]

Abstract We have examined the role of the mouse Diaphanous-related formin (DRF) Rho GTPase binding proteins, mDia1 and mDia2, in cell regulation. The DRFs are required for cytokinesis, stress fiber formation, and transcriptional activation of the serum response factor (SRF). 'Activated' mDia1 and mDia2 variants, lacking their GTPase binding domains, cooperated with Rho-kinase or ROCK to form stress fibers but independently activated SRF. Src tyrosine kinase associated and co-localized with the DRFs in endosomes and in mid-bodies of dividing cells. Inhibition of Src also blocked cytokinesis, SRF induction by activated DRFs, and cooperative stress fiber formation with active ROCK. Our results show that the DRF proteins couple Rho and Src during signaling and the regulation of actin dynamics.
URLPMID:14765113 [本文引用: 1]

Cell movement is driven by the coordinated regulation of cytoskeletal reorganization through Rho GTPases downstream of integrin and growth-factor receptor signaling. We have reported that mDia, a target protein of Rho, interacts with Src and DIP. Here we show that DIP binds to p190RhoGAP and Vav2, and that DIP is phosphorylated by Src and mediates the phosphorylation of p190RhoGAP and Vav2 upon EGF stimulation. When endogenous DIP was inhibited by expressing dominant-negative mutants of DIP or siRNA, phosphorylation of p190RhoGAP and Vav2 upon EGF stimulation was diminished, and EGF-induced actin organization, distribution of p190RhoGAP and Vav2, and cell movement were affected. Therefore, DIP seems to transfer the complex of the three proteins from cytosol to beneath the membrane, and the three proteins, in turn, can be phosphorylated by Src. DIP inactivated Rho and activated Rac following EGF stimulation in the membrane fraction. Thus, DIP acts as a regulatory molecule causing Src kinase-dependent feedback modulation of Rho GTPases downstream of Rho-mDia upon EGF stimulation, and plays an important role in cell motility.
URLPMID:20179713 [本文引用: 1]

The Eph receptor tyrosine kinases and their ephrin ligands have intriguing expression patterns in cancer cells and tumour blood vessels, which suggest important roles for their bidirectional signals in many aspects of cancer development and progression. Eph gene mutations probably also contribute to cancer pathogenesis. Eph receptors and ephrins have been shown to affect the growth, migration and invasion of cancer cells in culture as well as tumour growth, invasiveness, angiogenesis and metastasis in vivo. However, Eph signalling activities in cancer seem to be complex, and are characterized by puzzling dichotomies. Nevertheless, the Eph receptors are promising new therapeutic targets in cancer.
URL [本文引用: 1]

The src family of tyrosine kinases (srcFK) are important upstream regulators of Rho-kinase mediated Ca-sensitization and constriction in rat pulmonary artery (RPA). We hypothesise that srcFK act through modulation of the activity of RhoA. Cytosolic to membrane translocation of RhoA in RPA smooth muscle cells, visualised using over-expressed green fluorescent protein-tagged RhoA was used as an indication of RhoA activation. Translocation was stimulated by prostaglandin-F(20 渭M), the superoxide generating quinone LY83583 (10 渭M), and the RhoA activator calpeptin (0.02 U/ml). This translocation, following all three types of stimulus, was prevented by prior incubation with the srcFK antagonist PP2 (10 渭M). Calpeptin also constricted myograph-mounted RPA, and this constriction was inhibited by PP2. srcFK are likely to be activating RhoA through phosphorylation of one or more of the many proteins that directly activate or inhibit RhoA, namely guanine nucleotide exchange factors (RhoGEF), GTPase activating proteins (RhoGAP), and guanine nucleotide dissociation inhibitors (RhoGDI). We used RT-PCR to confirm endogenous expression of p115-RhoGEF, PDZ-RhoGEF, LARG, Vsm-RhoGEF, SmgGDS, vav-3, p73-RhoGAP, RhoGDI-1 and RhoGDI-2 in RPA. The GEF Vav-2 was not expressed. Our data suggests that srcFK may be contributing to Ca-sensitization and constriction of RPA through activation of RhoA.
URLPMID:16574440 [本文引用: 1]

Abstract At fertilisation, Ca(2+) signals activate embryonic development by stimulating metabolism, exocytosis and endocytosis, cytoskeletal remodelling, meiotic resumption and recruitment of maternal RNAs. Mitochondria present in large number in eggs have long been thought to act as a relay in Ca(2+) signalling at fertilisation. However, only recently have studies on ascidians and mouse proven that sperm-triggered Ca(2+) waves are transduced into mitochondrial Ca(2+) signals that stimulate mitochondrial respiration. Mitochondrial Ca(2+) uptake can substantially buffer cytosolic Ca(2+) concentration and the concerted action of heterogeneously distributed mitochondria in the mature egg may modulate the spatiotemporal pattern of sperm-triggered Ca(2+) waves. Regulation of fertilisation Ca(2+) signals could also be achieved through mitochondrial ATP production and mitochondrial oxidant activity but these hypotheses remain to be explored. A critically poised dynamic interplay between Ca(2+) signals and mitochondrial metabolism is stimulated at fertilisation and may well determine whether the embryo can proceed further into development. The monitoring of Ca(2+) signals and mitochondrial activity during fertilisation in living zygotes of diverse species should confirm the universality of the role for sperm-triggered Ca(2+) waves in the activation of mitochondrial activity at fertilisation.
URLPMID:20578238 [本文引用: 1]

Abstract During mammalian oocyte maturation there are marked changes in the distribution of mitochondria that supply the majority of the cellular ATP. Such redistribution of mitochondria is critical for oocyte quality, as oocytes with a poor developmental potential display aberrant mitochondrial distribution and lower ATP levels. Here we have investigated the dynamics of mitochondrial ATP production throughout spontaneous mouse oocyte maturation, using live measurements of cytosolic and mitochondrial ATP levels. We have observed three distinct increases in cytosolic ATP levels temporally associated with discrete events of oocyte maturation. These changes in cytosolic ATP levels are mirrored by changes in mitochondrial ATP levels, suggesting that mitochondrial ATP production is stimulated during oocyte maturation. Strikingly, these changes in ATP levels correlate with the distribution of mitochondria undergoing translocation to the peri-nuclear region and aggregation into clusters. Mitochondrial clustering during oocyte maturation was concomitant with the formation of long cortical microfilaments and could be disrupted by cytochalasin B treatment. Furthermore, the ATP production bursts observed during oocyte maturation were also inhibited by cytochalasin B suggesting that mitochondrial ATP production is stimulated during oocyte maturation by microfilament-driven, sub-cellular targeting of mitochondria. J. Cell. Physiol. 224: 672鈥680, 2010. 漏 2010 Wiley-Liss, Inc.
URL [本文引用: 1]

A fundamental rule of cell division is that daughter cells inherit half the DNA complement and an appropriate proportion of cellular organelles. The highly asymmetric cell divisions of female meiosis present a different challenge because one of the daughters, the polar body, is destined to degenerate, putting at risk essential maternally inherited organelles such as mitochondria. We have therefore investigated mitochondrial inheritance during the meiotic divisions of the mouse oocyte. We find that mitochondria are aggregated around the spindle by a dynein-mediated mechanism during meiosis I, and migrate together with the spindle towards the oocyte cortex. However, at cell division they are not equally segregated and move instead towards the oocyte-directed spindle pole and are excluded from the polar body. We show that this asymmetrical inheritance in favour of the oocyte is not caused by bias in the spindle itself but is dependent on an intact actin cytoskeleton, spindle-cortex proximity, and cell cycle progression. Thus, oocyte-biased inheritance of mitochondria is a variation on rules that normally govern organelle segregation at cell division, and ensures that essential maternally inherited mitochondria are retained to provide ATP for early mammalian development.
URLPMID:3587830 [本文引用: 1]

Polar body extrusion during oocyte maturation is critically dependent on asymmetric positioning of the meiotic spindle, which is established through migration of the meiosis I (MI) spindle/chromosomes from the oocyte interior to a subcortical location. In this study, we show that MI chromosome migration is biphasic and driven by consecutive actin-based pushing forces regulated by two actin nucleators, Fmn2, a formin family protein, and the Arp2/3 complex. Fmn2 was recruited to endoplasmic reticulum structures surrounding the MI spindle, where it nucleated actin filaments to initiate an initially slow and poorly directed motion of the spindle away from the cell center. A fast and highly directed second migration phase was driven by actin-mediated cytoplasmic streaming and occurred as the chromosomes reach a sufficient-proximity to the cortex to activate the Arp2/3 complex. We propose that decisive symmetry breaking in mouse oocytes results from Fmn2-mediated perturbation of spindle position and the positive feedback loop between chromosome signal-induced Arp2/3 activation and Arp2/3-orchestrated cytoplasmic streaming that transports the chromosomes.
URLPMID:11967185 [本文引用: 3]

We have studied Golgi apparatus dynamics during mouse oocyte in vitro maturation, employing both live imaging with the fluorescent lipid BODIPY-ceramide and immunocytochemistry using several specific markers (beta-COP, giantin, and TGN38). In germinal vesicle oocytes the Golgi consisted of a series of structures, possibly cisternal stacks, dispersed in the ooplasm, but slightly more concentrated in the interior than at the cortex. A similar pattern was detected in rhesus monkey germinal vesicle oocytes. These "mini-Golgis" were functionally active because they were reversibly disrupted by the membrane trafficking inhibitor brefeldin A. However, the drug had no visible effect if the oocytes had been previously microinjected with GTP-gamma-S. During in vitro maturation the large Golgi apparatus structures fragmented at germinal vesicle breakdown, and dispersed homogenously throughout the ooplasm, remaining in a fragmented state in metaphase-II oocytes. Similarly to what has been reported using protein synthesis inhibitors, the presence of brefeldin A blocked maturation at the germinal vesicle breakdown stage before the assembly of the metaphase-I spindle. These results suggest that progression of murine oocyte maturation may require functional membrane trafficking.
URLPMID:17368610 [本文引用: 1]

Oocyte maturation in mouse is associated with a dramatic reorganisation of the endoplasmic reticulum (ER) from a network of cytoplasmic accumulations in the germinal vesicle-stage oocyte (GV) to a network of distinctive cortical clusters in the metaphase II egg (MII). Multiple lines of evidence suggest that this redistribution of the ER is important to prepare the oocyte for the generation of repetitive Ca 2+ transients which trigger egg activation at fertilisation. The aim of the current study was therefore to investigate the timecourse and mechanism of ER reorganisation during oocyte maturation. The ER is first restructured at the time of GV-breakdown (GVBD) into a dense network of membranes which envelop and invade the developing meiotic spindle. GVBD is essential for the initiation of ER reorganisation, since ER structure does not change in GV-arrested oocytes. ER reorganisation is also prevented by the microtubule inhibitor nocodazole and by the inhibition of cytoplasmic dynein, a microtubule-associated motor protein. ER redistribution at GVBD is therefore dynein-driven and cell cycle-dependent. Following GVBD the dense network of ER surrounds the spindle during its migration to the oocyte cortex. Cortical clusters of ER are formed close to the time of, but independently of the metaphase I鈥搈etaphase II transition. Formation of the characteristic ER clusters is prevented by the depolymerisation of microfilaments, but not of microtubules. These experiments reveal that ER reorganisation during oocyte maturation is a complex multi-step process involving distinct microtubule- and microfilament-dependent phases and indicate a role for dynein in the cytoplasmic changes which prepare the oocyte for fertilisation.
URLPMID:13317917 [本文引用: 2]

Disappearance of cortical granules in the hamster egg is therefore concluded to be an early and specific response to spermatozoon penetration. It may not, however, be associated with the formation of a block to polyspermy in the surface of the vitellus.
URLPMID:25291640 [本文引用: 1]

Abstract Female meiosis presents unique opportunities for competition between chromosomes for evolutionary dominance. A new study reveals that centromere strength dictates meiotic success, driving karyotype evolution and reproductive isolation in mice. Copyright 漏 2014 Elsevier Ltd. All rights reserved.
URL [本文引用: 3]

Post-translational modifications of tubulin, such as the removal of the C-terminal tyrosine of 伪-tubulin, have long been proposed to influence the ability of microtubule motors to walk along the microtubule surface. This hypothesis has now been tested for cytoplasmic dynein-1 (dynein), revealing that active dynein-dynactin-adaptor complexes prefer to start moving on tyrosinated microtubules.... [Show full abstract]
URL [本文引用: 1]

Instances are known both from studies of natural populations and laboratory experiments in which heterozygotes produce two kinds of gametes, not with the customary equality, but with unequal frequencies. Such meiotic behavior will profoundly affect gene frequencies in populations, and is referred to as meiotic drive. Some of the consequences of meiotic drive on the genetic structure of natural populations, including those of man, and its evolutionary implications are considered.
[本文引用: 1]
URLPMID:25985139 [本文引用: 1]

F1000Prime Recommended Article: Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates.
URLPMID:26454098 [本文引用: 1]

Meiosis is one of the defining events in gametogenesis. Male and female germ cells both undergo one round of meiotic cell division during their development in order to reduce the ploidy of the gametes, and thereby maintain the ploidy of the species after fertilisation. However, there are some aspects of meiosis in the female germline, such as the prolonged arrest in dictyate, that appear to predispose oocytes to missegregate their chromosomes and transmit aneuploidies to the next generation. These maternally-derived aneuploidies are particularly problematic in humans where they are major contributors to miscarriage, age-related infertility, and the high incidence of Down's syndrome in human conceptions. This review will discuss how events that occur in foetal oocyte development and during the oocytes鈥 prolonged dictyate arrest can influence meiotic chromosome segregation and the incidence of aneuploidy in adult oocytes.
URLPMID:16533738 [本文引用: 2]

The balance between stem cell and differentiating cell populations is critical for the long-term maintenance of tissue renewal for cell types derived from adult stem cell lineages such as blood, skin, intestinal epithelium, and sperm. To keep this balance, stem cells have the potential to divide asymmetrically, producing one daughter cell that maintains stem cell identity and one daughter cell that initiates differentiation. In many adult stem cell systems, the maintenance, proliferation, and number of stem cells appear to be controlled by the microenvironment, or niche. The Drosophila male and female germ line provide excellent model systems in which to study asymmetric stem cell divisions within the stem cell niche. In addition to signals from the niche that specify stem cell self-renewal, the stem cells themselves have elaborate cellular mechanisms to ensure the asymmetric outcome of cell division.
URLPMID:2563045 [本文引用: 1]

Adult stem cells often divide asymmetrically to produce one self-renewed stem cell and one differentiating cell, thus maintaining both populations. The asymmetric outcome of stem cell divisions can be specified by an oriented spindle and local self-renewal signals from the stem cell niche. Here we show that developmentally programmed asymmetric behavior and inheritance of mother and daughter centrosomes underlies the stereotyped spindle orientation and asymmetric outcome of stem cell divisions in the Drosophila male germ line. The mother centrosome remains anchored near the niche while the daughter centrosome migrates to the opposite side of the cell before spindle formation.
URLPMID:23681657 [本文引用: 1]

Sister chromatids are the product of DNA replication, which is assumed to be a very precise process. Therefore, sister chromatids should be exact copies of each other. However, reports have indicated that sister chromatids are segregated nonrandomly during cell division, suggesting that sister chromatids are not the same, although their DNA sequences are the same. Researchers have speculated that stem cells may retain template strands to avoid replication-induced mutations. An alternative proposal is that cells may segregate distinct epigenetic information carried on sister chromatids. Recently, we found that Drosophila male germline stem cells segregate sister chromatids of X and Y chromosomes with a strong bias. We discuss this finding in relation to existing models for nonrandom sister chromatid segregation.
URLPMID:3711665 [本文引用: 1]

Adult stem cells undergo asymmetric cell division to self-renew and give rise to differentiated cells that comprise mature tissue1. Sister chromatids may be distinguished and segregated non-randomly in asymmetrically dividing stem cells2, although the underlying mechanism and the purpose it may serve remain elusive. We developed the CO-FISH (chromosome orientation fluorescence in situ hybridization) technique3 with single-chromosome resolution and show that sister chromatids of X and Y chromosomes, but not autosomes, are segregated non-randomly during asymmetric divisions of Drosophila male germline stem cells (GSCs). This provides the first direct evidence that two sister chromatids containing identical genetic information can be distinguished and segregated non-randomly during asymmetric stem cell divisions. We further show that the centrosome, SUN-KASH nuclear envelope proteins, and Dnmt2 are required for non-random sister chromatid segregation. Our data suggest that the information on X and Y chromosomes that enables non-random segregation is primed during gametogenesis in the parents. Moreover, we show that sister chromatid segregation is randomized in GSC overproliferation and dedifferentiated GSCs. We propose that non-random sister chromatid segregation may serve to transmit distinct information carried on two sister chromatids to the daughters of asymmetrically dividing stem cells.
URLPMID:26522592 [本文引用: 1]

Abstract A long-standing question concerns how stem cells maintain their identity through multiple divisions. Previously, we reported that pre-existing and newly synthesized histone H3 are asymmetrically distributed during Drosophila male germline stem cell (GSC) asymmetric division. Here, we show that phosphorylation at threonine 3 of H3 (H3T3P) distinguishes pre-existing versus newly synthesized H3. Converting T3 to the unphosphorylatable residue alanine (H3T3A) or to the phosphomimetic aspartate (H3T3D) disrupts asymmetric H3 inheritance. Expression of H3T3A or H3T3D specifically in early-stage germline also leads to cellular defects, including GSC loss and germline tumors. Finally, compromising the activity of the H3T3 kinase Haspin enhances the H3T3A but suppresses the H3T3D phenotypes. These studies demonstrate that H3T3P distinguishes sister chromatids enriched with distinct pools of H3 in order to coordinate asymmetric segregation of "old" H3 into GSCs and that tight regulation of H3T3 phosphorylation is required for male germline activity. Copyright 脗漏 2015 Elsevier Inc. All rights reserved.
URLPMID:27337481 [本文引用: 1]

Abstract The nucleus is the most prominent cellular organelle, and its sharp boundaries suggest the compartmentalization of the nucleoplasm from the cytoplasm. However, the recent identification of evolutionarily conserved linkers of the nucleoskeleton to the cytoskeleton (LINC) complexes, a family of macromolecular assemblies that span the double membrane of the nuclear envelope, reveals tight physical connections between the two compartments. Here, we review the structure and evolutionary conservation of SUN and KASH domain-containing proteins, whose interaction within the perinuclear space forms the "nuts and bolts" of LINC complexes. Moreover, we discuss the function of these complexes in nuclear, centrosomal, and chromosome dynamics, and their connection to human disease.
URLPMID:25674669 [本文引用: 2]

Numerous studies in the past years provided definite evidence that the nuclear envelope is much more than just a simple barrier. It rather constitutes a multifunctional platform combining structural and dynamic features to fulfill many fundamental functions such as chromatin organization, regulation of transcription, signaling, but also structural duties like maintaining general nuclear architecture and shape. One additional and, without doubt, highly impressive aspect is the recently identified key function of selected nuclear envelope components in driving meiotic chromosome dynamics, which in turn is essential for accurate recombination and segregation of the homologous chromosomes. Here, we summarize the recent work identifying new key players in meiotic telomere attachment and movement and discuss the latest advances in our understanding of the actual function of the meiotic nuclear envelope.
URLPMID:29097549 [本文引用: 2]

Genetic elements compete for transmission through meiosis, when haploid gametes are created from a diploid parent. Selfish elements can enhance their transmission through a process known as meiotic drive. In female meiosis, selfish elements drive by preferentially attaching to the egg side of the spindle. This implies some asymmetry between the two sides of the spindle, but the molecular mechanisms underlying spindle asymmetry are unknown. Here we found that CDC42 signaling from the cell cortex regulated microtubule tyrosination to induce spindle asymmetry and that non-Mendelian segregation depended on this asymmetry. Cortical CDC42 depends on polarization directed by chromosomes, which are positioned near the cortex to allow the asymmetric cell division. Thus, selfish meiotic drivers exploit the asymmetry inherent in female meiosis to bias their transmission.
URLPMID:24949971 [本文引用: 1]

Polar body genome transfer can effectively prevent the transmission of defective mitochondria DNA across generations, indicating the potential of this procedure to treat inherited mitochondrial diseases.
URLPMID:25472922 [本文引用: 1]

During meiotic cell-cycle progression, unequal divisions take place, resulting in a large oocyte and two diminutive polar bodies. The first polar body contains a subset of bivalent chromosomes, whereas the second polar body contains a haploid set of chromatids. One unique feature of the female gamete is that the polar bodies can provide beneficial information about the genetic background of the oocyte without potentially destroying it. Therefore, polar body biopsies have been applied in preimplantation genetic diagnosis to detect chromosomal or genetic abnormalities that might be inherited by the offspring. Besides the traditional use in preimplantation diagnosis, recent findings suggest additional important roles for polar bodies in assisted reproductive technology. In this paper, we review the new roles of polar bodies in assisted reproductive technology, mainly focusing on single-cell sequencing of the polar body genome to deduce the genomic information of its sibling oocyte and on polar body transfer to prevent the transmission of mtDNA-associated diseases. We also discuss additional potential roles for polar bodies and related key questions in human reproductive health. We believe that further exploration of new roles for polar bodies will contribute to a better understanding of reproductive health and that polar body manipulation and diagnosis will allow production of a greater number of healthy babies.
URLPMID:21930382 [本文引用: 1]

Decisions of when and where to divide are crucial for cell survival and fate, and for tissue organization and homeostasis. The temporal coordination of mitotic events during cell division is essential to ensure that each daughter cell receives one copy of the genome. The spatial coordination of these events is also crucial because the cytokinetic furrow must be aligned with the axis of chromosome segregation and, in asymmetrically dividing cells, the polarity axis. Several recent papers describe how cell shape and polarity are coordinated with cell division in single cells and tissues and begin to unravel the underlying molecular mechanisms, revealing common principles and molecular players. Here, we discuss how cells regulate the spatial and temporal coordination of cell polarity with cell division.
URLPMID:5062000 [本文引用: 1]

Cleavage is a period after fertilization, when a 1-cell embryo starts developing into a multicellular organism. Due to a series of mitotic divisions, the large volume of a fertilized egg is divided into numerous smaller, nucleated cells鈥攂lastomeres. Embryos of different phyla divide according to different patterns, but molecular mechanism of these early divisions remains surprisingly conserved. In the present paper, we describe how polarity cues, cytoskeleton and cell-to-cell communication interact with each other to regulate orientation of the early embryonic division planes in model animals such asCaenorhabditis elegans,Drosophilaand mouse. We focus particularly on the Par pathway and the actin-driven cytoplasmic flows that accompany it. We also describe a unique interplay between Par proteins and the Hippo pathway in cleavage mammalian embryos. Moreover, we discuss the potential meaning of polarity, cytoplasmic dynamics and cell-to-cell communication as quality biomarkers of human embryos.
URLPMID:11222139 [本文引用: 1]

Abstract Studies on the development of aggregated, isolated and rearranged blastomeres have engendered the view that in mammals, unlike most other animals, egg organization has no role in the genesis of asymmetries that are essential for cellular diversification and the specification of embryonic axes. Such asymmetries are assumed to arise post-zygotically through interactions between initially naive cells. However, various findings are difficult to reconcile with this view. Here, a consistent relationship between the structure of the blastocyst and the two-cell stage in the mouse has been found using a strictly non-invasive marking technique: injection of small oil drops into the substance of the zona pellicuda. This has revealed that both the embryonic-abembryonic axis of the blastocyst and its plane of bilateral symmetry are normally orthogonal to the plane of first cleavage. This relationship was also seen when denuded two-cell conceptuses were prevented from rotating during subsequent cleavage by immobilizing them in a gel. Therefore, during normal mouse development the axes of the blastocyst, which have been implicated in establishing those of the fetus, are already specified by the onset of cleavage.
URLPMID:15772664 [本文引用: 1]

One of the unanswered questions in mammalian development is how the embryonic-abembryonic axis of the blastocyst is first established. It is possible that the first cleavage division contributes to this process, because in most mouse embryos the progeny of one two-cell blastomere primarily populate the embryonic part of the blastocyst and the progeny of its sister populate the abembryonic part. However, it is not known whether the embryonic-abembryonic axis is set up by the first cleavage itself, by polarity in the oocyte that then sets the first cleavage plane with respect to the animal pole, or indeed whether it can be divorced entirely from the first cleavage and established in relation to the animal pole. Here we test the importance of the orientation of the first cleavage by imposing an elongated shape on the zygote so that the division no longer passes close to the animal pole, marked by the second polar body. Non-invasive lineage tracing shows that even when the first cleavage occurs along the short axis imposed by this experimental treatment, the progeny of the resulting two-cell blastomeres tend to populate the respective embryonic and abembryonic parts of the blastocyst. Thus, the first cleavage contributes to breaking the symmetry of the embryo, generating blastomeres with different developmental characteristics.
URLPMID:12456621 [本文引用: 1]

Abstract BACKGROUND: Mammalian conceptuses typically have an approximately regular tetrahedral shape at the 4-cell stage. In the rabbit, this has been attributed to both 2-cell blastomeres dividing meridionally, but with the animal-vegetal axis of the second blastomere to divide rotating through roughly 90 degrees before or during cytokinesis. The aim of the present study was to ascertain whether this was also true for the mouse. METHODS AND RESULTS: First, the distribution in regular tetrahedral 4-cell conceptuses of fluorescent microspheres applied to the vegetal polar region of one or both blastomeres at the 2-cell stage was analysed. Second, the ability of 2-cell stages to form regular tetrahedral 4-cell conceptuses after the previtelline space had been gelated to prevent blastomeres from rotating was also investigated. Neither experiment yielded evidence supporting blastomere rotation during second cleavage. Rather, the findings were consistent with the regular tetrahedral form of 4-cell conceptus resulting from meridional division of one blastomere and approximately equatorial division of the other. CONCLUSIONS: Second cleavage in the mouse typically yields 4-cell conceptuses with three distinct types of blastomere. While both products of the meridional division include all axial levels of the zygote, those of the equatorial division acquire only its vegetal or animal half.
URLPMID:2655627 [本文引用: 1]

Setting aside pluripotent cells that give rise to the future body is a central cell fate decision in mammalian development. It requires that some blastomeres divide asymmetrically to direct cells to the inside of the embryo. Despite its importance, it is unknown whether the decision to divide symmetrically versus asymmetrically shows any spatial or temporal pattern, whether it is lineage-dependent or occurs at random, or whether it influences the orientation of the embryonic-abembryonic axis. To address these questions, we developed time-lapse microscopy to enable a complete 3D analysis of the origins, fates and divisions of all cells from the 2- to 32-cell blastocyst stage. This showed how in the majority of embryos, individual blastomeres give rise to distinct blastocyst regions. Tracking the division orientation of all cells revealed a spatial and temporal relationship between symmetric and asymmetric divisions and how this contributes to the generation of inside and outside cells and thus embryo patterning. We found that the blastocyst cavity, defining the abembryonic pole, forms where symmetric divisions predominate. Tracking cell ancestry indicated that the pattern of symmetric/asymmetric divisions of a blastomere can be influenced by its origin in relation to the animal-vegetal axis of the zygote. Thus, it appears that the orientation of the embryonic-abembryonic axis is anticipated by earlier cell division patterns. Together, our results suggest that two steps influence the allocation of cells to the blastocyst. The first step, involving orientation of 2- to 4-cell divisions along the animal-vegetal axis, can affect the second step, the establishment of inside and outside cell populations by asymmetric 8- to 32-cell divisions.
URLPMID:12700771 [本文引用: 1]

Cell polarity is defined as asymmetry in cell shape, protein distributions and cell functions. It is characteristic of single-cell organisms, including yeast and bacteria, and cells in tissues of multi-cell organisms such as epithelia in worms, flies and mammals. This diversity raises several questions: do different cell types use different mechanisms to generate polarity, how is polarity signalled, how do cells react to that signal, and how is structural polarity translated into specialized functions? Analysis of evolutionarily diverse cell types reveals that cell-surface landmarks adapt core pathways for cytoskeleton assembly and protein transport to generate cell polarity.
URLPMID:8870075 [本文引用: 1]

Marsupial oocytes are larger and have a thinner zona than eutherian oocytes; and the ooplasm becomes almost completely filled with empty-looking vacuoles the contents of which have, so far, defied histochemical analysis. In the opossum, Monodelphis domestica, apart from orthodox mitochondria, a 'hooded' form is found occasionally in young primary oocytes, and a novel 'spiked' form-which is very elongate and has longitudinally-running filaments attached to the outer membrane--is found in mature oocytes. On the genesis of the ooplasmic vacuoles in mammals, information is available only for two marsupials. In Monodelphis, the vacuoles originate from endoplasmic, endocytotic and Golgi vesicles which generate multivesicular bodies; these give rise to the vacuoles. For the bandicoot, Isoodon macrourus, evidence is presented for the formation of the vacuoles from enlarged, transformed mitochondria which undergo a complex evolution during development. Primordial oocytes of Isoodon contain three ooplasmic localizations--a paranuclear complex, a vesicle microtubule complex and an aggregate of tubular cistenae-which have not been described for other mammalian oocytes. The origin, fate and function of these organelle localizations is unknown. In this paper, problems with respect to the definition of 'yolk' are described and the extent of our ignorance concerning oocyte organelles is discussed.
