 ,1
,1MYB-like transcription factor SiMYB42 from foxtail millet (Setaria italica L.) enhances Arabidopsis tolerance to low-nitrogen stress
Qingqian Ding1, Xiaoting Wang1,2, Liqin Hu1, Xin Qi1, Linhao Ge1, Weiya XU1, Zhaoshi Xu1, Yongbin Zhou1,2, Guanqing Jia1, Xianmin Diao1, Donghong Min2, Youzhi Ma1, Ming Chen ,1
,1通讯作者:
编委: 储成才
收稿日期:2017-09-25修回日期:2018-01-2网络出版日期:2018-04-20
| 基金资助: |
Editorial board:
Received:2017-09-25Revised:2018-01-2Online:2018-04-20
| Fund supported: |
作者简介 About authors
王小婷,在读博士研究生,专业方向:生物化学与分子生物学E-mail:
丁庆倩,在读硕士研究生,专业方向:生物化学与分子生物学E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (815KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
丁庆倩, 王小婷, 胡利琴, 齐欣, 葛林豪, 徐伟亚, 徐兆师, 周永斌, 贾冠清, 刁现民, 闵东红, 马有志, 陈明. 谷子MYB类转录因子SiMYB42提高转基因拟南芥低氮胁迫耐性. 遗传[J], 2018, 40(4): 327-338 doi:10.16288/j.yczz.17-315
Qingqian Ding, Xiaoting Wang, Liqin Hu, Xin Qi, Linhao Ge, Weiya XU, Zhaoshi Xu, Yongbin Zhou, Guanqing Jia, Xianmin Diao, Donghong Min, Youzhi Ma, Ming Chen.
氮素是作物从土壤中吸收最多的元素,是限制作物产量的关键因素。研究表明,硝酸盐不仅是植物生长发育的主要氮源[1],还是植物生长过程中的重要信号分子,可以调控植物一系列复杂的生理过程来影响植物对氮的利用效率,保证植物的正常生长发育[2]。
转录因子是基因表达的重要调节因子,一般包含DNA结合结构域以及转录激活/抑制结构域,通过调节下游靶基因的转录起始速率来综合调节许多生理和生物化学过程。Myeloblastosis(MYB)类转录因子是高等植物中最大的转录因子家族之一,在植物发育及防御反应过程中发挥重要作用,还参与植物对干旱等非生物胁迫的响应。此外,MYB类转录因子还参与调节细胞的次级代谢[3]、控制细胞形态
建成[4];一些MYB类转录因子还参与植物氮素代谢过程。例如拟南芥MYB转录因子PAP2在低氮胁迫条件下高度诱导表达,该基因参与花青素代谢的过程,进而调控氮循环通路[5]。单细胞红藻MYB转录因子基因CmMYB1可以调节植物的氮素利用[6]。根据MYB保守域种类及数量的不同,MYB类转录因子基因家族分为3个亚家族:MYB1R,R2R3-MYB和MYB3R,这3个亚家族分别含有1个、2个和3个MYB保守域。其中R2R3-MYB是植物MYB类转录因子基因家族中最大的亚家族[7]。因此,在过去10年中,R2R3-MYB亚家族的成员已被广泛研 究[8]。据报道,R2R3-MYB类基因不仅参与一些生理和化学过程,而且还参与胁迫响应[9]、光响应等信号途径[10]。例如:ZmMYBC1主要在种子糊粉层和一些花组织中表达,调节分生组织的形成以及植物的花和种子的发育[11];AtMYB2和AtMYC2互作能够转录激活干旱和ABA诱导基因RD22的表达[12];AtMYB102能够调节干旱和盐胁迫响应过程,并且在损伤信号通路中发挥有重要作用[13]。
谷子具有抗旱、耐贫瘠、适应性广的特点,是研究单子叶植物非生物胁迫响应过程的理想材料之一。目前,谷子功能基因组研究刚刚起步,参与谷子低氮胁迫响应的基因功能还有待深入研究。本研究从低氮胁迫耐性较强的谷子品种郑204克隆一个MYB转录因子基因SiMYB42 (Seita.2G286400.1)。SiMYB42受低氮胁迫诱导表达。基因功能分析表明:转基因植株在低氮条件下表现为主根变长、根系表面积扩大、鲜重增加等特性,证明过表达SiMYB42基因可以提高转基因植物对低氮胁迫的耐性。同时发现SiMYB42基因可以调控下游硝酸盐转运基因的表达影响植物低氮胁迫耐性。本研究揭示了SiMYB42基因的功能及作用机制,为作物氮高效利用改良提供了优异基因资源,也为揭示谷子低氮胁迫耐性调控网络奠定了基础。
1 材料和方法
1.1 材料
谷子品种郑204由中国农业科学院作物科学研究所刁现民研究员课题组提供,野生型Columbia-0拟南芥(WT)种子由本实验室保存。农杆菌GV3101菌株和大肠杆菌TOP10感受态分别购于全式金公司和江苏康为公司,带有CaMV35S启动子无GUS的pBI121载体、16318hGFP载体、AH109载体均由本实验室保存。将清洗好的拟南芥种子均匀撒在MS(4.43 g/L MS粉末、10 g/L蔗糖、1 mL/L 1000X有机成分、5 mL/L 200X肌醇和2.4 g/L植物凝胶)固体培养基上,4℃冰箱春化3 d,然后在16 h光照/8 h黑暗、相对湿度(RH)60%、温度22℃的组培间中培养,生长7 d后移苗处理。对谷子的种子进行次氯酸钠处理打破休眠,然后均匀撒在湿润的滤纸上,30℃培养箱培养生长。待谷子幼苗生长为三叶一心且长势均一时,参考霍冬英等[14]和闵东红等[15]的方法,分别取谷子幼苗进行低氮(低氮水培营养液组分包括0.2 mmol/L NH4NO3、4.0 mmol/L MgCl2、1.0 mmol/L MgSO4、1.5 mmol/L KH2PO4、1.5 mmol/L K2HPO4、2.0 mmol/L K2SO4、40 μmol/L Na2Fe-EDTA、4.0 mmol/L H?BO?、14 μmol/L MnSO4、1.0 μmol/L ZnSO4、0.3 μmol/L Na2MoO4、0.6 μmol/L CuSO4、0.4 μmol/L NiCl2、20 nmol/L CoCl2)、干旱(8%PEG6000)、NaCl(120 mmol/L)和ABA(50 μmol/L)等水培处理。均在处理0 h、1 h、6和24 h后取整个植株于装有钢珠的2.0离心管中,将所有鲜样迅速于液氮速冻,-80℃存放待用。
1.2 谷子SiMYB42基因生物信息学分析
谷子数据来源于Phytozome 数据库(https:// phytozome.jgi.doe.gov/pz/portal.html),利用SMART 数据库(http://smart.embl-heidelberg.de/)在线工具分析谷子SiMYB42蛋白的保守结构域。用Compute pI/Mw tool(http://expasy.org/tools/)计算蛋白质的分子量和等电点,根据SiMYB42氨基酸序列,利用Plant TFDB(http://planttfdb.cbi.pku.edu.cn)搜索谷子中与之相关的MYB基因的氨基酸序列,并在Phytozome数据库中进行BlastP比对,搜索与其同源性较高的其他物种的基因序列,利用ClustalW1.8.3[16]进行多序列比对,并用软件MEGA5.1[17]以相邻法(Bootstrap设为1000)[18]构建系统进化树。1.3 RNA提取和Real-time PCR分析
采用植物总RNA快速提取试剂盒(北京庄盟生物有限公司)提取1.1中各处理的样品以及谷子幼苗不同组织的总RNA,以TransScriptⅡ一步法反转录试剂盒(TransGen, 北京)反转录成cDNA。使用Real MasterMix(SYBR Green)试剂盒,荧光定量PCR仪(ABI7500)进行quantitative real-time PCR(qRT-PCR)扩增。以合成好的cDNA为模板,用SiMYB42的定量特异性引物进行PCR扩增,以SiActin (Si001873m.g)为内参(表1)。qRT-PCR反应体系包含2×SuperReal PreMix Plus12.5 μL、正向引物和反向引物(10 μmol/L)各0.75 μL,cDNA模板(约110 ng/μL) 2 μL、50×ROX Reference Dye 0.5 μL、ddH2O 8.5 μL。采用3步法反应程序进行扩增:95℃ 15 min;95℃ 10 s,60℃ 20 s,72℃ 32 s,40个循环。根据各样品特有的Ct值,采用2-ΔΔCt法计算基因在不同处理条件下的相对表达量。Table 1
表1
表1 本研究所用的引物信息
Table 1
| 引物名称 | 引物序列(5′→3′) | 用途 | 复性温度(℃) |
|---|---|---|---|
| SiMYB42 | F:ATGGGTTCTACAACGGCGA | 基因克隆 | 59 |
| SiMYB42 | R:TCATCGCTTCCCTATAGCATTA | ||
| SiMYB42-pBI121 | F:CTCTAGAGGATCCCCGGGATTTGGGCTTCTCG | pBI121载体构建 | 65 |
| SiMYB42-pBI121 | R:ACTAGTGGATCCCCCGGGTTCACCTGCTGCAGC | ||
| SiMYB42-GFP | F:ATCTCTAGAGGATCCATGGGGCGGCAGC | GFP载体构建 | 60 |
| SiMYB42-GFP | R:GCTCACCATGGATCCGAACTTTGCTCCG | ||
| SiMYB42-BD | F:AGGAGGACCTGCATATGATGGGGCGGCAGCCGTGCT | BD载体构建 | 68 |
| SiMYB42-BD | R:GCCTCCATGGCCATATGGAACTTTGCTCCGTTTGAG | ||
| SiMYB42-RT | F:TGGACGAGTTCAGCAGCAT | 实时定量PCR | 60 |
| SiMYB42-RT | R:CTCCGTTTGAGCCGTTGA | ||
| SiActin | F:GGCAAACAGGGAGAAGATGA | 谷子内参基因扩增 | 60 |
| SiActin | R:GAGGTTGTCGGTAAGGTCACG |
新窗口打开|下载CSV
1.4 SiMYB42基因克隆和表达载体构建
根据谷子低氮处理的转录组差异表达谱分析,鉴定出一个受低氮诱导表达明显上调的MYB类转录因子SiMYB42(Seita.2G286400.1)。根据谷子的CDS序列,设计SiMYB42基因特异性引物SiMYB42-F和SiMYB42-R,以郑204的cDNA为模板扩增SiMYB42基因的完整编码框。利用In-Fusion重组 酶技术将比对正确的目的片段分别连接到16318hGFP、CaMV35S启动子的pBI121和pGBKT7载体上(表1)。1.5 SiMYB42基因转录活性分析
一般情况下,单独的BD(pGBKT7)可以与GAL4上游活化序列(GLA UAS)结合,但是不能引起转录,然而将一段具有转录激活活性的转录因子基因构建到载体BD上,若有转录激活活性,则该基因表达产生的BD单独与UAS 结合也可引起下游报告基因的转录,这种现象为自激活现象,因此,本文利用这种自激活现象来验证该转录因子是否具有转录激活活性。将1 μg SiMYB42-BD质粒和与1 μg对应的BD空载体[19]混合后转化到酵母AH109[20]菌株中。将转化产物涂布于SD/-Trp固体平板上,倒置平板,于30℃培养箱培养3 d。挑取单克隆于SD/-Trp的液体培养基中,静置培养18 h。按照OD600=100、10-1和10-2稀释酵母菌液,将稀释后的酵母菌液分别点滴到SD/-Trp和SD/-Trp/-His/-Ade/X-α-gal的固体平板上,倒置平板,于30℃培养箱培养3 d,观察其在营养缺陷型培养基上的生长及显色情况,判断是否具有转录激活活性。1.6 SiMYB42蛋白亚细胞定位
取两叶一心生长旺盛的谷子叶片,参考Yoo等[21]方法制备原生质体,将融合表达的重组质粒SiMYB42-GFP和16318hGFP空载体质粒作为对照分别转化到原生质体中,在25℃培养箱中黑暗静置18 h以上。将静置后SiMYB42-GFP的原生质体分别进行ABA (20 μmol/L)、LN(0.2 mmol/L)、NaCl(75 mmol/L)和PEG(8%)处理,各处理3.5 h、3 h、2 h和1.5 h,然后在激光共聚焦荧光显微镜(Zeiss LSM700)下观察SiMYB42-GFP蛋白亚细胞定位。1.7 拟南芥转化及纯合转基因植株的获取
参考Beehtold等[22]方法进行SiMYB42转基因的拟南芥遗传转化,采用蘸花法侵染野生型拟南芥(WT),最终得到带有CaMV35S启动子无GUS的pBI121载体的拟南芥转基因植株。将烘干的T0代拟南芥种子撒播于含卡那霉素(50 mg/L)的MS培养基上进行筛苗,最终筛选到3个转基因株系OE1、OE2和OE3,将3个转基因株系进行扩繁,采用纯合的T3代种子进行功能验证。1.8 SiMYB42转基因拟南芥耐低氮分析
将野生型拟南芥及SiMYB42转基因株系OE1、OE2和OE3的种子用70%的酒精处理3 min,ddH2O漂洗5次,每次1 min左右,用1%的NaClO溶液浸泡15 min,ddH2O漂洗3次,每次1 min;在4℃春化3 d后,再将种子分别点播至MS(4.43 g/L MS粉末、10 g/L蔗糖、1 mL/L 1000X有机成分、5 mL/L 200X肌醇和2.4 g/L植物凝胶)固体培养基中。参考Peters等[23]和Wimalasekera等[24]的处理方法,将生长4 d长势均一的WT、OE1、OE2和OE3的幼苗分别转移到含有3mmol/L氮的培养基和0.2 mmol/L的低氮培养基上,每个处理每个株系各放3株幼苗。垂直生长7 d后在EPSON Flatbed Scanner EPSON Expression 10000XL扫描仪上用WINRHIZO proLA2400软件对两种处理下的幼苗进行根系扫描,直接显示根长和根系表面积,采用方差分析软件统计分析数据,用GraphPad Prism 5软件绘制柱状图。2 结果与分析
2.1 SiMYB42基因克隆及基因特性分析
本课题组前期工作通过对谷子低氮处理表达谱进行分析,发现一个MYB类转录因子基因SiMYB42响应低氮胁迫处理。从谷子品种内701中克隆SiMYB42基因,通过测序发现该基因全长5069 bp,开放读码框(ORF)为837 bp,编码278个氨基酸,分子量为30.38 kDa,pI为5.42。为分析谷子及其他物种MYB基因家族的进化关系,在Phytozome数据库中搜索与SiMYB42氨基酸序列相似性最高的MYB类转录因子。将氨基酸序列进行多重比对,构建系统发育树(图1A)。分析结果表明,37个MYB类蛋白分为3个亚族,即MYB1R、R2R3-MYB和MYB3R亚族。SiMYB42属于R2R3-MYB类蛋白,SiMYB20和SiMYB42的同源性最高(57%),同时和AtMYB42在一个分支上。蛋白结构分析显示(图1B),相同亚族的蛋白含有相同的MYB结构域,其中MYB1R亚族蛋白只含有一个MYB结构域,R2R3-MYB亚族蛋白含有2个MYB结构域,MYB3R亚族蛋白含有3个MYB结构域。图1
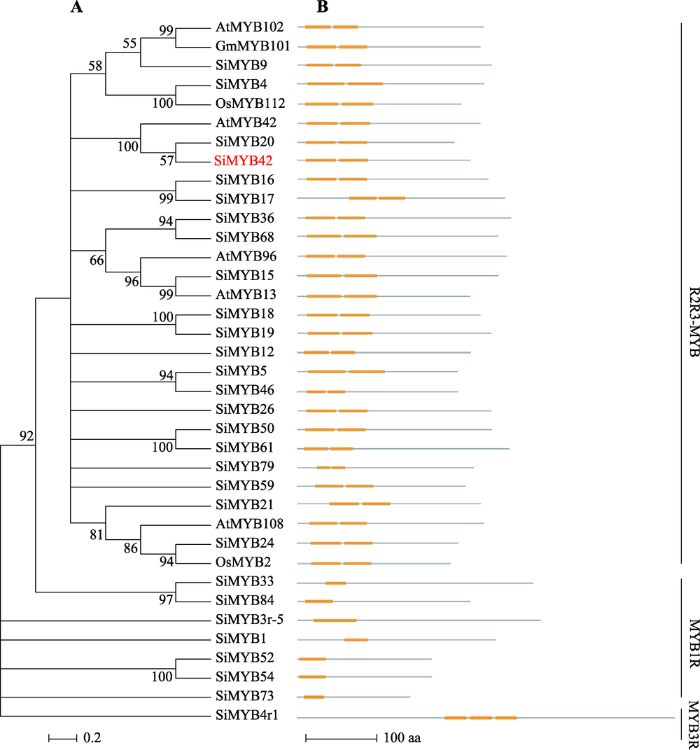 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1MYB基因家族系统进化树和蛋白结构分析
A: MYB基因家族系统进化树分析(红色标注的为SiMYB42);B: 蛋白结构分析(黄色方框代表MYB结构域)。
Fig. 1Phylogenetic tree and proteins structure analysis of MYB gene family
2.2 SiMYB42在不同胁迫条件下的表达模式分析
利用qRT-PCR分别扩增谷子幼苗在正常处理(CK)和胁迫处理下SiMYB42的表达情况(图2)。结果显示,在ABA处理下,SiMYB42的表达量逐渐上升,在24 h时达到极显著差异,表达量约是对照处理的4倍;在低氮胁迫处理下,SiMYB42在6 h表达达到最高值,表达量比对照提高了约3倍;在高盐(NaCl)处理下,SiMYB42的表达量也呈上升趋势,在6 h达到最高值,但在1 h和12 h表达量达到极显著差异;在干旱(PEG)处理下,SiMYB42响应比较敏感,在24 h时其表达量达到最高,但在1 h达到极显著差异,表达量约为对照的7倍。上述结果表明,SiMYB42基因响应谷子ABA、低氮、高盐、干旱等多种胁迫处理。图2
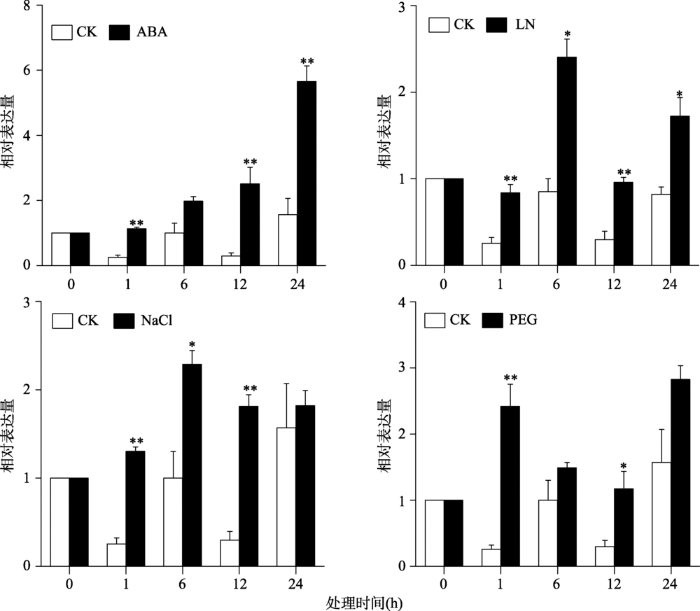 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2SiMYB42在不同胁迫条件下的表达模式
ABA:脱落酸;LN:低氮;NaCl:高盐;PEG:干旱;CK:空白对照。*表示P <0.05,**表示P<0.01。
Fig. 2Expression patterns of the SiMYB42 gene under various treatments
2.3 SiMYB42蛋白亚细胞定位以及组织特异性表达特性
利用瞬时表达法将融合表达载体p35S::SiMYB42-GFP和16318hGFP空载体对照质粒分别转化到谷子叶片的原生质体中,在共聚焦显微镜下观察SiMYB42-GFP融合蛋白亚细胞定位情况。结果显示,对照GFP蛋白在整个细胞中都可以表达,SiMYB42- GFP融合蛋白定位在细胞膜和细胞核中。为探讨SiMYB42在生物逆境下调节功能是如何发挥的,本文分别对瞬时表达后的原生质体进行了ABA、低氮、高盐以及PEG的后处理,观察SiMYB42亚细胞定位的变化。结果显示,SiMYB42蛋白仍定位在细胞膜和细胞核中(图3A)。qRT-PCR结果表明,SiMYB42主要在叶部表达量最高,其次是在根中表达,其他组织的表达量很低(图3B),表明SiMYB42主要在叶部或根部发挥作用。图3
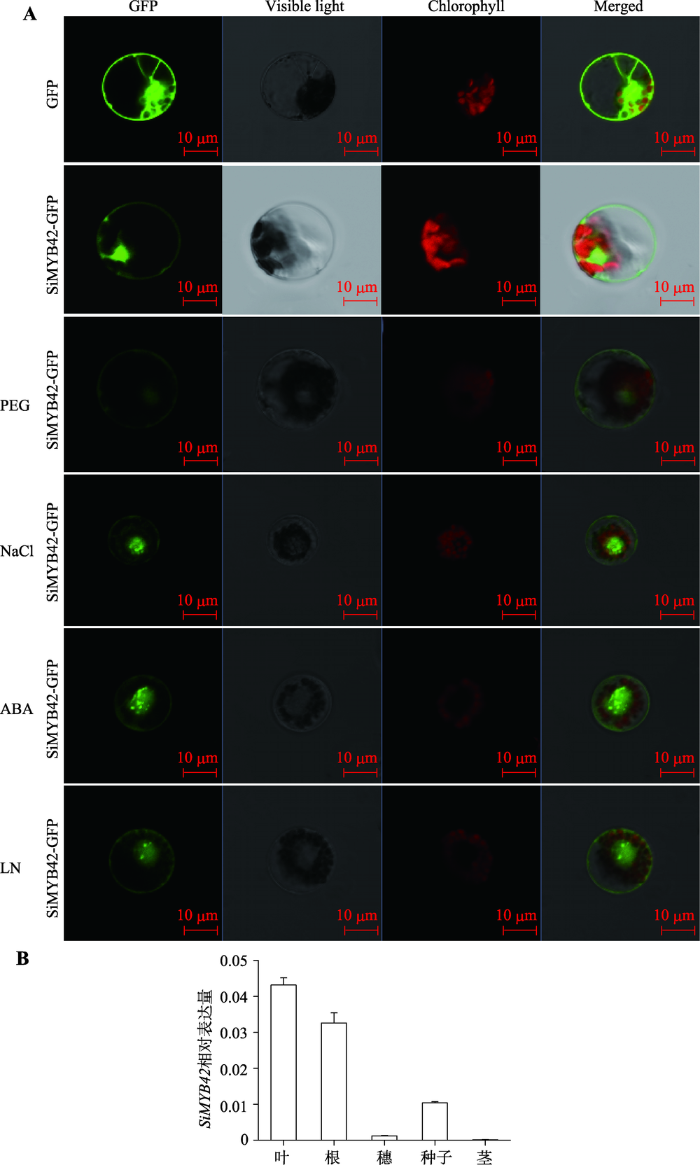 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3SiMYB42蛋白亚细胞定位与组织特异性表达情况
A:SiMYB42蛋白亚细胞定位;B:SiMYB42在谷子不同组织中的表达情况。PEG:干旱处理融合蛋白SiMYB42-GFP;NaCl:盐处理融合蛋白SiMYB42-GFP;ABA:脱落酸处理融合蛋白SiMYB42-GFP;LN:低氮处理融合蛋白SiMYB42-GFP。
Fig. 3Subcellular localization and the tissue specific expression analysis of SiMYB42
2.4 SiMYB42蛋白的转录活性分析
为研究SiMYB42是否具有转录激活活性,将SiMYB42构建到表达载体pGBKT7(BD)的GAL4 DNA结合域。利用LiAc方法将构建好的载体和BD空载体(做为对照)转化到制备好的酵母AH109感受态中,在SD/-Trp单缺平板和 SD/-Trp/-His/-Ade/ X-α-gal三缺平板上验证SiMYB42转录激活活性。结果显示:所有转化子均能在SD/-Trp培养基上正常生长,SiMYB42-BD重组质粒转化的酵母细胞在SD/-Trp/-His/-Ade/X-α-gal平板上生长良好,显淡蓝色,表现出α-半乳糖苷酶活性,而对照组只能在SD/ -Trp平板上生长,并且没有显示α-半乳糖苷酶活性(图4),结果表明,SiMYB42基因具有转录激活活性。
图4
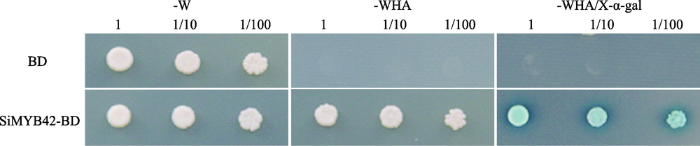 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4转录因子SiMYB42的转录激活活性
-W代表缺陷型氨基酸Trp;-WHA代表缺陷型氨基酸Trp、His和Ade;1,1/10和1/100代表稀释倍数
Fig. 4Transcription activation activity of transcription factor SiMYB42
2.5 SiMYB42转基因拟南芥苗期低氮胁迫耐性鉴定
将SiMYB42转基因拟南芥垂直培养7 d后,在总氮浓度为3 mmol/L的正常培养基上(CK),野生型拟南芥(WT)和SiMYB42转基因株系OE1、OE2、OE3的主根长和根表面积没有明显差异(图5A)。在总氮浓度为0.2 mmol/L的低氮培养基的条件下,WT和SiMYB42过表达植株的地上生长均受到一定的抑制,但是转基因拟南芥的主根长明显高于WT,侧根也比WT多(图5B)。分析WT和转基因拟南芥的主根长和根系表面积发现,在对照处理的条件下,WT和转基因拟南芥的主根长和根系表面积不存在明显差异,而在低氮处理条件下,SiMYB42转基因拟南芥的主根长和根系表面积均显著高于WT(图5:C,D)。经过称量转基因拟南芥和WT的鲜重发现,在对照处理的条件下,SiMYB42转基因拟南芥和WT的鲜重基本一致,但在低氮处理条件下,SiMYB42转基因植株的鲜重显著高于WT(图5E)。同时,在苗期分析转基因拟南芥与WT对干旱和ABA的胁迫抗性发现,SiMYB42转基因拟南芥和WT无明显的差异,证明SiMYB42通过ABA和干旱胁迫非依赖途径调控植物对低氮胁迫的耐性。图5
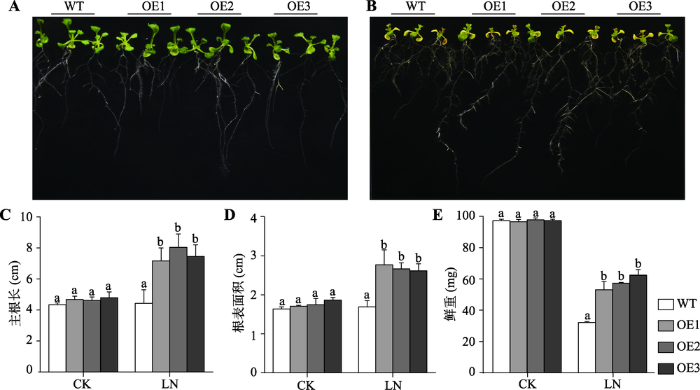 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5SiMYB42转基因拟南芥在低氮胁迫下的抗性鉴定
A:在对照 (3 mmol/L)培养基上SiMYB42转基因植株与WT的生长情况;B:在低氮(0.2 mmol/L) 培养基上SiMYB42转基因植株与WT的生长情况;C:SiMYB42转基因植株与WT的主根长统计结果;D :SiMYB42转基因植株与WT的根表面积统计结果;E:SiMYB42转基因植株与WT的鲜重统计结果。LN:低氮处理。柱上小写字母a和b代表柱值在0.05水平上差异显著。OE1、OE2、OE3分别代表着谷子基因SiMYB42转拟南芥的3个不同转基因株系。
Fig. 5Resistance analysis of SiMYB42 transgenic Arabidopsis under low-nitrogen stress treatment
2.6 SiMYB42转基因植株的表达水平及下游氮胁迫相关基因的表达分析
SiMYB42转基因植株的表达水平结果显示,OE2的表达量最高,约为WT的4.5倍(图6A)。下游基因表达分析结果表明,在SiMYB42过表达植株中,部分硝酸盐转运体基因NRT2.1、NRT2.4和NRT2.5 表达量都表现为不同程度的上调,其中NRT2.1与NRT2.4的表达量在OE2转基因株系中比WT上调了约3.5倍,在OE3转基因株系中NRT2.1与NRT2.4表达比WT上调2倍左右,但OE1转基因株系中NRT2.1和NRT2.4表达均不显著,应该是和自身的表达量低有关(图6B)。但NRT2.5在3个转基因株系中的表达量均很高。根据Lezhneva等[25]报道,NRT2.5是一种高亲和力的NO3转运蛋白,通过确保NRT2.1和NRT2.4共同有效摄取NO3,在严重缺氮的植物中发挥重要作用,通过NO3再活化过程参与NO3的转运。此外,分析NRT2.1、NRT2.4和NRT2.5的启动子序列,发现它们均含有MYB结合元件,核心序列为TAACTG。因此转基因植株中NRT2.5的高表达水平这一结果与转基因拟南芥根长分析结果相符。图6
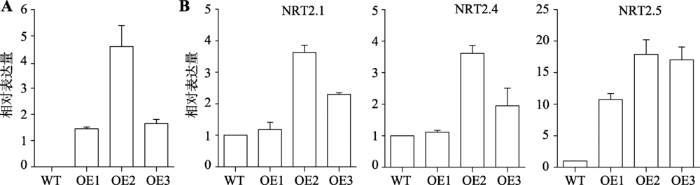 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6SiMYB42转基因植株的表达水平及下游氮胁迫相关基因的表达分析
A:SiMYB42在转基因株系中的表达;B:NRT2.1、NRT2.4和NRT2.5在SiMYB42转基因拟南芥和WT中的相对表达量。OE1、OE2、OE3分别代表着谷子基因SiMYB42转拟南芥的3个不同转基因株系。
Fig. 6Expression levels of SiMYB42 and the nitrate transporters genes in SiMYB42 transgenic plants
3 讨 论
MYB类转录因子在基因的表达调控中发挥着重要的作用。在拟南芥中,MYB类转录因子参与高等植物氮缺乏胁迫的应答调控[26]。此外,氮饥饿可以诱导高亲和力硝酸盐转运体和氮同化相关基因的表达[27],而且也能够诱导激素生物合成和氮同化的过程[28,29]。本研究克隆了一个属于R2R3-MYB 亚家族的基因SiMYB42,该基因在细胞核和细胞膜中均定位。组织特异性表达分析结果表明,该基因在谷子的叶和根中均表现为高表达。在低氮胁迫条件下,SiMYB42基因在谷子中上调表达,并且SiMYB42转基因拟南芥植株的总根长、主根长和根表面积均显著高于WT,侧根数显著增多(图4)。因此,推测该基因参与了谷子低氮胁迫介导的侧根和主根的伸长生长过程,影响根吸收营养的表面积,从而促进植株对氮素的吸收利用。目前,报道的与氮代谢相关的MYB家族转录因子几乎都是R2R3-MYB亚族的基因[30]。例如海棠花(Malus spectabilis (Ait.) Borkh) MYB转录因子MsMYB10 属于R2R3-MYB,通过改变花青苷等类黄酮化合物的积累量来应答低氮胁迫,最大程度保护机体不受损害[28];大豆(Glycine max (Linn.) Merr.)R2R3-MYB转录因子GmMYB101调节类黄酮生物合成来响应氮饥饿胁迫,表明GmMYB101在氮代谢调节中起重要作用[31];火炬松(Pinus taeda)的两个R2R3-MYB类转录因子PtMYB1和PtMYB4能够在酵母、拟南芥和松树细胞中激活PsGS1b启动子的转录,PsGS1b编码谷氨酰胺合成酶,促进氮循环的过程[29]。本文克隆的MYB类转录因子也属于R2R3-MYB亚家族,在MYB3个亚家族中,主要是R2R3-MYB亚族成员参与调控氮代谢吸收相关的基因的表达调控。同时,从基因调控植物氮代谢作用机制看,SiMYB42转录因子调控氮代谢过程的作用方式主要是通过硝酸盐转运体的调节。在SiMYB42转基因拟南芥植株中,硝酸盐转运蛋白基因NRT2.1、NRT2.4和NRT2.5的表达量相对于WT有着明显的提高,但是NRT2.5的在3个转基因拟南芥株系中的表达量均很显著,因此,推测在氮胁迫处理条件 下NRT2.5起主要作用。同时,利用PlantCARE(http: //bioinformatics.psb.ugent.be/webtools/plantcare/html/)预测硝酸盐转运基因NRT2.1、NRT2.4和NRT2.5的启动子元件分析结果显示均具有一个MYB结合位点。由于植物对土壤中氮素胁迫的响应及运输主要发生在根部,因此,推测SiMYB42通过促进氮转运相关的硝酸盐转运蛋白基因的表达,提高植株对氮素的吸收,进而正向调控植物低氮胁迫抗性,这一作用机制与其他MYB类转录因子调控植物氮代谢的作用机制不同。虽然本研究初步阐明了谷子基因 SiMYB42在低氮胁迫下调控途径,但是SiMYB42如何在低营养条件下调控根部发育的分子机制还需要进一步的研究。SiMYB42蛋白主要定位在细胞核与细胞膜上,可能是由于不同时期的SiMYB42蛋白的定位情况不同所造成的。我们推测定位于细胞膜上的SiMYB42蛋白在经过某些蛋白修饰后,会进入细胞核中行使功能,启动下游基因的表达。但是经过处理后的SiMYB42蛋白的定位未发生变化,因此SiMYB42蛋白的定位与胁迫处理的关系还需要进一步探讨。
研究表明,过表达MYB转录因子可提高转基因植株对多种非生物胁迫抗性。除了响应低氮胁迫,SiMYB42虽然也受ABA、高盐和干旱诱导表达,但是SiMYB42过表达植株并没有明显表现提高对ABA、
高盐和干旱胁迫的抗性。这可能因为SiMYB42调控其他胁迫响应过程需要其他转录因子的参与。另外一个原因可能是低氮胁迫与其他胁迫的作用机制不同。低氮胁迫主要与植物的营养物质的吸收及代谢有关。干旱主要是脱水胁迫,高盐包含离子毒害及脱水胁迫。本研究SiMYB42主要调节硝酸盐转运蛋白的表达,这些下游基因的表达对脱水胁迫及离子毒害影响不大,因此SiMYB42转基因植物未出现出抗旱及耐盐的特性。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:9989997 [本文引用: 1]

Frink et al examine the changing ratio of fertilizer nitrogen application to its intended incorporation in crop yield and discuss prospects for more or less nitrogen fertilizer by 2070.
URLPMID:17067284 [本文引用: 1]

Abstract Plants often grow in soils that contain very low concentrations of the macronutrients nitrogen, phosphorus, potassium, and sulfur. To adapt and grow in nutrient-deprived environments plants must sense changes in external and internal mineral nutrient concentrations and adjust growth to match resource availability. The sensing and signal transduction networks that control plant responses to nutrient deprivation are not well characterized for nitrogen, potassium, and sulfur deprivation. One branch of the signal transduction cascade related to phosphorus-deprivation response has been defined through the identification of a transcription factor that is regulated by sumoylation. Two different microRNAs play roles in regulating gene expression under phosphorus and sulfur deprivation. Reactive oxygen species increase rapidly after mineral nutrient deprivation and may be one upstream mediator of nutrient signaling. A number of molecular analyses suggest that both short-term and longer-term responses will be important in understanding the progression of signaling events when the external, then internal, supplies of nutrients become depleted.
URLPMID:8313474 [本文引用: 1]

The maize P gene, which specifies red pigmentation of the kernel pericarp, cob, and other floral organs, has been an important model since the early days of modern genetics. Here we show that P encodes a Myb homolog that recognizes the sequence CCT/AACC, in sharp contrast with the C/TAACGG bound by vertebrate Myb proteins. P binds to and activates transcription of the A1 gene required for 3-deoxy flavonoid and phlobaphene biosynthesis, but not the Bz1 gene required for anthocyanin biosynthesis. The maize C1 gene, which also encodes a Myb homolog, activates both the A1 and Bz1 genes, but only in the presence of a basic-helix-loop-helix coactivator encoded by the maize genes R or B. These results indicate that Myb homologs can differentially regulate gene expression by binding different DNA sequences, through combinatorial interactions with other factors, or both.
URLPMID:1934056 [本文引用: 1]

The GL1 gene is required for the initiation of differentiation of hair cells (trichomes) on the crucifer, Arabidopsis thaliana. This gene has been localized to a 4.5 kb DNA fragment by molecular complementation of gl1 mutants. DNA sequence analysis has shown that the protein encoded by GL1 contains a Myb DNA-binding motif. Southern analysis and subsequence analysis of isolated 位 clones has established that GL1 is a member of an extensive myb gene family in Arabidopsis. The putative GL1 promoter directs the expression of the GUS reporter gene in non-trichome-bearing structures that appear to be stipules. This pattern of expression suggests that GL1 may control the synthesis of a diffusible signal that activates the developmental pathway for trichome diferentation.
URL [本文引用: 1]

Approximately 30鈥45% of plant organic matter is derived from l -phenylalanine, and to a lesser extent from l -tyrosine, through the cinnamate pathway. In the initial step, mediated by phenylalanine ammonia lyase, cinnamic acid and an equimolar amount of ammonium ion is generated. To determine the metabolic fate of the ammonia formed, [ 15 N]- l -phenylalanine was administered to potato discs in the light under aseptic conditions. It was found, using 15 N NMR spectroscopic analyses, that this nitrogen is first incorporated into the amide nitrogen of glutamine and then into glutamate. When incubations were repeated in the presence of methionine- S -sulphoximine, a known inhibitor of glutamine synthetase, only resonances corresponding to 15 NH 4 + and [ 15 N]- l -Phe accumulated. It is proposed that during active phenylpropanoid metabolism, the ammonia released by phenylalanine ammonia lyase/tyrosine ammonia lyase is efficiently recycled back to Phe/Tyr with glutamate serving as aminoreceptor and donor. This is the first evidence for a novel nitrogen cycle in plants.
[本文引用: 1]
URLPMID:15888681 [本文引用: 1]

Abstract The development of complex eukaryotic organisms can be viewed as the selective expression of distinct fractions of the genome in different organs or tissue types in response to developmental and environmental cues. Here, we generated a genome expression atlas of 18 organ or tissue types representing the life cycle of Arabidopsis (Arabidopsis thaliana). We showed that each organ or tissue type had a defining genome expression pattern and that the degree to which organs share expression profiles is highly correlated with the biological relationship of organ types. Further, distinct fractions of the genome exhibited expression changes in response to environmental light among the three seedling organs, despite the fact that they share the same photo-perception and transduction systems. A significant fraction of the genes in the Arabidopsis genome is organized into chromatin domains exhibiting coregulated expression patterns in response to developmental or environmental signals. The knowledge of organ-specific expression patterns and their response to the changing environment provides a foundation for dissecting the molecular processes underlying development.
[本文引用: 1]
[本文引用: 1]
URLPMID:8535141 [本文引用: 1]

Abstract Functional analysis of a barley high-pI alpha-amylase gene promoter has identified a gibberellin (GA) response complex in the region between -174 and -108. The sequence of the central element, TAACAAA, is very similar to the c-Myb and v-Myb consensus binding site. We investigated the possibility that a GA-regulated Myb transactivates alpha-amylase gene expression in barley aleurone cells. A cDNA clone, GAmyb, which encodes a novel Myb, was isolated from a barley aleurone cDNA library. RNA blot analysis revealed that GAmyb expression in isolated barley aleurone layers is up-regulated by GA. The kinetics of GAmyb expression indicates that it is an early event in GA-regulated gene expression and precedes alpha-amylase gene expression. Cycloheximide blocked alpha-amylase gene expression but failed to block GAmyb gene expression, indicating that protein synthesis is not required for GAmyb gene expression. Gel mobility shift experiments with recombinant GAMyb showed that GAMyb binds specifically to the TAACAAA box in vitro. We demonstrated in transient expression experiments that GAMyb activates transcription of a high-pI alpha-amylase promoter fused to a beta-glucuronidase reporter gene in the absence of GA. Our results indicate that the GAMyb is the sole GA-regulated transcription factor required for transcriptional activation of the high-pI alpha-amylase promoter. We therefore postulate that GAMyb is a part of the GA-response pathway leading to alpha-amylase gene expression in aleurone cells.
[本文引用: 1]
[本文引用: 1]
URLPMID:12857823 [本文引用: 1]

Transcript levels of the Arabidopsis R2R3-AtMYB102 transcription factor gene, previously named AtM4, are rapidly induced by osmotic stress or abscisic acid (ABA) treatment. Reporter gene expression studies revealed that in addition, wounding is required for full induction of the gene. Histochemical analysis showed a local beta-glucuronidase induction around the wounding site, especially in veins. In ABA-treated plants, wounding-induced beta-glucuronidase activity could be mimicked by the wound signaling compound methyl jasmonate. In silico studies of the AtMYB102 promoter sequence and its close homolog AtMYB74 demonstrated several conserved putative stress regulatory elements such as an ABA-responsive element, its coupling element 1 (CE1), and a W box. Interestingly, further studies showed that the 5'-untranslated region is essential for the osmotic stress and wounding induced expression of the AtMYB102 gene. This 5'-untranslated region contains putative conserved regulatory elements such as a second W box and an overlapping MYB-binding element. These studies suggest that AtMYB102 expression depends on and integrates signals derived from both wounding and osmotic stress.
URLMagsci [本文引用: 1]

<p><span >蛋白参与细胞周期调控、细胞凋亡及信号转导等多种生命活动</span><span >对维持植物正常生长发育</span><span >和介导非生阿物胁迫响应等过程发挥重要作用。谷子具有显著的耐旱耐瘠薄等特性</span><span >本研究</span><span >根据谷子转录组分析结果</span><span >从</span><span >个</span><span >家族成员中鉴定出</span><span >个在干旱胁迫下表达量上调的</span><span >基因</span><span new="">根据</span><span >序列相似性</span><span >将其分为</span><span >类</span><span >同一类基因具有相似的内含子</span><span >外显子结构</span><span new="">染色体定位分析发现</span><span >这些基因分别分布在谷子的</span><span >条染色体上</span><span >其中</span><span >第</span><span >条染色体上含</span><span >基因最多</span><span >有</span><span >个。结构域分析结果表明</span><span >个</span><span >蛋白均含保守的</span><span >结构域</span><span >而</span><span >端含</span><span >、</span><span >、</span><span >、</span><span >、</span><span >和</span><span >等结构域。启动子元件分析表明</span><span >谷子</span><span >个</span><span >基因均含逆境应答元件</span><span >其中</span><span >和</span><span >元件的数量最多</span><span >个</span><span >说明这些基因对干旱应答反应可能主要受</span><span >、</span><span >转录因子调控。转录组分析结果表明</span><span new="">基因对干旱胁迫的响应远远高于其他</span><span >成员</span><span >对干旱、高盐、</span><span >、</span><span >和</span><span >都有响应</span><span new="">亚细胞定位结果显示</span><span>蛋白定位在细胞核中。本研究为进一步深入了解<em>基因的功能提供了依据。</em></span><span lang="EN-US" >F-box</span><span >蛋白参与细胞周期调控、细胞凋亡及信号转导等多种生命活动</span><span lang="EN-US" >, </span><span >对维持植物正常生长发育</span><span lang="EN-US" >a</span><span >和介导非生阿物胁迫响应等过程发挥重要作用。谷子具有显著的耐旱耐瘠薄等特性</span><span lang="EN-US" >, </span><span >本研究</span><span >根据谷子转录组分析结果</span><span lang="EN-US" >, </span><span >从</span><span lang="EN-US" >525</span><span >个</span><span lang="EN-US" >F-box</span><span >家族成员中鉴定出</span><span lang="EN-US" >19</span><span >个在干旱胁迫下表达量上调的</span><span lang="EN-US" >F-box</span><span >基因</span><span lang="EN-US" >; </span><span >根据</span><span >序列相似性</span><span >将其分为</span><span lang="EN-US" >6</span><span >类</span><span lang="EN-US" >, </span><span >同一类基因具有相似的内含子</span><span lang="EN-US" >-</span><span >外显子结构</span><span lang="EN-US" >; </span><span >染色体定位分析发现</span><span lang="EN-US" >, </span><span >这些基因分别分布在谷子的</span><span lang="EN-US" >8</span><span >条染色体上</span><span lang="EN-US" >, </span><span >其中</span><span lang="EN-US" >, </span><span >第</span><span lang="EN-US" >2</span><span >条染色体上含</span><span lang="EN-US" >F-box</span><span >基因最多</span><span lang="EN-US" >, </span><span >有</span><span lang="EN-US" >6</span><span >个<span >。结构域分析结果表明</span></span><span lang="EN-US" >, 19</span><span >个</span><span lang="EN-US" >F-box</span><span >蛋白均含保守的</span><span lang="EN-US" >F-box</span><span >结构域</span><span lang="EN-US" >, </span><span >而</span><span lang="EN-US" >C</span><span >端含</span><span lang="EN-US" >FBD</span><span >、</span><span lang="EN-US" >WD40</span><span >、</span><span lang="EN-US" >FBA</span><span >、</span><span lang="EN-US" >ZnF</span><span >、</span><span lang="EN-US" >Kelch</span><span >和</span><span lang="EN-US" >LRR</span><span >等结构域。<span >启动子元件分析表明</span></span><span lang="EN-US" >, </span><span >谷子</span><span lang="EN-US" >19</span><span >个</span><span lang="EN-US" >F-box</span><span >基因均含逆境应答元件</span><span lang="EN-US" >, </span><span >其中</span><span lang="EN-US" >, MYB</span><span >和</span><span lang="EN-US" >MYC</span><span >元件的数量最多</span><span lang="EN-US" >(9~78</span><span >个</span><span lang="EN-US" >), </span><span >说明这些基因对干旱应答反应可能主要受</span><span lang="EN-US" >MYB</span><span >、</span><span lang="EN-US" >MYC</span><span >转录因子调控。转录组分析结果表明</span><span lang="EN-US" >, <em >SiF-box18</em></span><span >基因对干旱胁迫的响应远远高于其他</span><span lang="EN-US" >F-box</span><span >成员</span><span lang="EN-US" >, </span><span >对干旱、高盐、</span><span lang="EN-US" >ABA</span><span >、</span><span lang="EN-US" >SA</span><span >和</span><span lang="EN-US" >JA</span><span >都有响应</span><span lang="EN-US" >; </span><span >亚细胞定位结果显示</span><span lang="EN-US" >, SiF-box18</span><span >蛋白定位在细胞核中。本研究为进一步深入了解</span><em ><span lang="EN-US" >SiF-box18</span></em><span >基因的功能提供了依据。</span></p>
URLMagsci [本文引用: 1]

<p><span >蛋白参与细胞周期调控、细胞凋亡及信号转导等多种生命活动</span><span >对维持植物正常生长发育</span><span >和介导非生阿物胁迫响应等过程发挥重要作用。谷子具有显著的耐旱耐瘠薄等特性</span><span >本研究</span><span >根据谷子转录组分析结果</span><span >从</span><span >个</span><span >家族成员中鉴定出</span><span >个在干旱胁迫下表达量上调的</span><span >基因</span><span new="">根据</span><span >序列相似性</span><span >将其分为</span><span >类</span><span >同一类基因具有相似的内含子</span><span >外显子结构</span><span new="">染色体定位分析发现</span><span >这些基因分别分布在谷子的</span><span >条染色体上</span><span >其中</span><span >第</span><span >条染色体上含</span><span >基因最多</span><span >有</span><span >个。结构域分析结果表明</span><span >个</span><span >蛋白均含保守的</span><span >结构域</span><span >而</span><span >端含</span><span >、</span><span >、</span><span >、</span><span >、</span><span >和</span><span >等结构域。启动子元件分析表明</span><span >谷子</span><span >个</span><span >基因均含逆境应答元件</span><span >其中</span><span >和</span><span >元件的数量最多</span><span >个</span><span >说明这些基因对干旱应答反应可能主要受</span><span >、</span><span >转录因子调控。转录组分析结果表明</span><span new="">基因对干旱胁迫的响应远远高于其他</span><span >成员</span><span >对干旱、高盐、</span><span >、</span><span >和</span><span >都有响应</span><span new="">亚细胞定位结果显示</span><span>蛋白定位在细胞核中。本研究为进一步深入了解<em>基因的功能提供了依据。</em></span><span lang="EN-US" >F-box</span><span >蛋白参与细胞周期调控、细胞凋亡及信号转导等多种生命活动</span><span lang="EN-US" >, </span><span >对维持植物正常生长发育</span><span lang="EN-US" >a</span><span >和介导非生阿物胁迫响应等过程发挥重要作用。谷子具有显著的耐旱耐瘠薄等特性</span><span lang="EN-US" >, </span><span >本研究</span><span >根据谷子转录组分析结果</span><span lang="EN-US" >, </span><span >从</span><span lang="EN-US" >525</span><span >个</span><span lang="EN-US" >F-box</span><span >家族成员中鉴定出</span><span lang="EN-US" >19</span><span >个在干旱胁迫下表达量上调的</span><span lang="EN-US" >F-box</span><span >基因</span><span lang="EN-US" >; </span><span >根据</span><span >序列相似性</span><span >将其分为</span><span lang="EN-US" >6</span><span >类</span><span lang="EN-US" >, </span><span >同一类基因具有相似的内含子</span><span lang="EN-US" >-</span><span >外显子结构</span><span lang="EN-US" >; </span><span >染色体定位分析发现</span><span lang="EN-US" >, </span><span >这些基因分别分布在谷子的</span><span lang="EN-US" >8</span><span >条染色体上</span><span lang="EN-US" >, </span><span >其中</span><span lang="EN-US" >, </span><span >第</span><span lang="EN-US" >2</span><span >条染色体上含</span><span lang="EN-US" >F-box</span><span >基因最多</span><span lang="EN-US" >, </span><span >有</span><span lang="EN-US" >6</span><span >个<span >。结构域分析结果表明</span></span><span lang="EN-US" >, 19</span><span >个</span><span lang="EN-US" >F-box</span><span >蛋白均含保守的</span><span lang="EN-US" >F-box</span><span >结构域</span><span lang="EN-US" >, </span><span >而</span><span lang="EN-US" >C</span><span >端含</span><span lang="EN-US" >FBD</span><span >、</span><span lang="EN-US" >WD40</span><span >、</span><span lang="EN-US" >FBA</span><span >、</span><span lang="EN-US" >ZnF</span><span >、</span><span lang="EN-US" >Kelch</span><span >和</span><span lang="EN-US" >LRR</span><span >等结构域。<span >启动子元件分析表明</span></span><span lang="EN-US" >, </span><span >谷子</span><span lang="EN-US" >19</span><span >个</span><span lang="EN-US" >F-box</span><span >基因均含逆境应答元件</span><span lang="EN-US" >, </span><span >其中</span><span lang="EN-US" >, MYB</span><span >和</span><span lang="EN-US" >MYC</span><span >元件的数量最多</span><span lang="EN-US" >(9~78</span><span >个</span><span lang="EN-US" >), </span><span >说明这些基因对干旱应答反应可能主要受</span><span lang="EN-US" >MYB</span><span >、</span><span lang="EN-US" >MYC</span><span >转录因子调控。转录组分析结果表明</span><span lang="EN-US" >, <em >SiF-box18</em></span><span >基因对干旱胁迫的响应远远高于其他</span><span lang="EN-US" >F-box</span><span >成员</span><span lang="EN-US" >, </span><span >对干旱、高盐、</span><span lang="EN-US" >ABA</span><span >、</span><span lang="EN-US" >SA</span><span >和</span><span lang="EN-US" >JA</span><span >都有响应</span><span lang="EN-US" >; </span><span >亚细胞定位结果显示</span><span lang="EN-US" >, SiF-box18</span><span >蛋白定位在细胞核中。本研究为进一步深入了解</span><em ><span lang="EN-US" >SiF-box18</span></em><span >基因的功能提供了依据。</span></p>
URL [本文引用: 1]

PP2Cs(2C type protein phosphatases)是一种单体丝氨酸/苏氨酸蛋白磷酸酶,在真核生物中,PP2Cs在脱落酸(ABA)、茉莉酸(JA)、水杨酸(SA)等激素信号传导途径中起着重要的调控作用。本研究通过序列比对,从谷子基因组中筛选出80个PP2C候选基因,聚类分析将其分为12个亚族(A、B、C、D、E1、E2、F1、F2、G、H、I、J)。与拟南芥PP2C基因家族比对表明,A~I为2个物种共有的亚族,J亚族只存在于谷子基因组中,L亚族只存在于拟南芥中。将谷子A亚族的10个成员命名为SiPP2CA1-10。基因表达谱分析表明,A亚族基因不同程度受ABA、干旱、高盐、低温和低氮诱导表达,其中,SiPP2CA6、SiPP2CA8在5种处理下诱导表达量都高。对10个A亚族成员的启动子分析发现,在这些基因的启动子序列中含有多种参与逆境胁迫应答的顺式作用元件,其中,SiPP2CA5、SiPP2CA6、SiPP2CA7、SiPP2CA8的启动子中含有参与低氮胁迫响应的元件。进一步研究发现,SiPP2CA8主要在根部表达,且在低氮胁迫下一直有较高的表达水平。亚细胞定位结果显示SiPP2CA8定位在细胞膜、细胞质、细胞核中;双分子荧光互补试验(BiFC)结果表明,SiPP2CA8与一个ABA受体类似蛋白SiRCAR3(基因号为Si018317m.g)在细胞膜、细胞质及细胞核上互作,表明SiPP2CA8在谷子中可能参与ABA信号传导过程。
URL [本文引用: 1]

PP2Cs(2C type protein phosphatases)是一种单体丝氨酸/苏氨酸蛋白磷酸酶,在真核生物中,PP2Cs在脱落酸(ABA)、茉莉酸(JA)、水杨酸(SA)等激素信号传导途径中起着重要的调控作用。本研究通过序列比对,从谷子基因组中筛选出80个PP2C候选基因,聚类分析将其分为12个亚族(A、B、C、D、E1、E2、F1、F2、G、H、I、J)。与拟南芥PP2C基因家族比对表明,A~I为2个物种共有的亚族,J亚族只存在于谷子基因组中,L亚族只存在于拟南芥中。将谷子A亚族的10个成员命名为SiPP2CA1-10。基因表达谱分析表明,A亚族基因不同程度受ABA、干旱、高盐、低温和低氮诱导表达,其中,SiPP2CA6、SiPP2CA8在5种处理下诱导表达量都高。对10个A亚族成员的启动子分析发现,在这些基因的启动子序列中含有多种参与逆境胁迫应答的顺式作用元件,其中,SiPP2CA5、SiPP2CA6、SiPP2CA7、SiPP2CA8的启动子中含有参与低氮胁迫响应的元件。进一步研究发现,SiPP2CA8主要在根部表达,且在低氮胁迫下一直有较高的表达水平。亚细胞定位结果显示SiPP2CA8定位在细胞膜、细胞质、细胞核中;双分子荧光互补试验(BiFC)结果表明,SiPP2CA8与一个ABA受体类似蛋白SiRCAR3(基因号为Si018317m.g)在细胞膜、细胞质及细胞核上互作,表明SiPP2CA8在谷子中可能参与ABA信号传导过程。
URL [本文引用: 1]
URLPMID:11751241 [本文引用: 1]

: We have developed a new software package, Molecular Evolutionary Genetics Analysis version 2 (MEGA2), for exploring and analyzing aligned DNA or protein sequences from an evolutionary perspective. MEGA2 vastly extends the capabilities of MEGA version 1 by: (1) facilitating analyses of large datasets; (2) enabling creation and analyses of groups of sequences; (3) enabling specification of domains and genes; (4) expanding the repertoire of statistical methods for molecular evolutionary studies; and (5) adding new modules for visual representation of input data and output results on the Microsoft Windows platform. AVAILABILITY: http://www.megasoftware.net. CONTACT: s.kumar@asu.edu.
URL [本文引用: 1]
URLPMID:1946372 [本文引用: 1]

We describe a method that detects proteins capable of interacting with a known protein and that results in the immediate availability of the cloned genes for these interacting proteins. Plasmids are constructed to encode two hybrid proteins. One hybrid consists of the DNA-binding domain of the yeast transcriptional activator protein GAL4 fused to the known protein; the other hybrid consists of the GAL4 activation domain fused to protein sequences encoded by a library of yeast genomic DNA fragments. Interaction between the known protein and a protein encoded by one of the library plasmids leads to transcriptional activation of a reporter gene containing a binding site for GAL4. We used this method with the yeast SIR4 protein, which is involved in the transcriptional repression of yeast mating type information. (i) We used the two-hybrid system to demonstrate that SIR4 can form homodimers. (ii) A small domain consisting of the C terminus of SIR4 was shown to be sufficient to mediate this interaction. (iii) We screened a library to detect hybrid proteins that could interact with the SIR4 C-terminal domain and identified SIR4 from this library. This approach could be readily extended to mammalian proteins by the construction of appropriate cDNA libraries in the activation domain plasmid.
URLPMID:7940758 [本文引用: 1]

The two-hybrid system is a yeast-based genetic assay for detecting protein-protein interactions. It can be used to identify proteins that bind to a protein of interest, or to delineate domains or residues critical for an interaction. Variations on this methodology have been developed to clone genes that encode DNA-binding proteins, to identify peptides that bind to a protein and, potentially, to screen for drugs.
URLPMID:17585298 [本文引用: 1]

Abstract The transient gene expression system using Arabidopsis mesophyll protoplasts has proven an important and versatile tool for conducting cell-based experiments using molecular, cellular, biochemical, genetic, genomic and proteomic approaches to analyze the functions of diverse signaling pathways and cellular machineries. A well-established protocol that has been extensively tested and applied in numerous experiments is presented here. The method includes protoplast isolation, PEG-calcium transfection of plasmid DNA and protoplast culture. Physiological responses and high-throughput capability enable facile and cost-effective explorations as well as hypothesis-driven tests. The protoplast isolation and DNA transfection procedures take 6-8 h, and the results can be obtained in 2-24 h. The cell system offers reliable guidelines for further comprehensive analysis of complex regulatory mechanisms in whole-plant physiology, immunity, growth and development.
In: Martinez-Zapater JM, Salinas J, eds.
URLPMID:9664431 [本文引用: 1]

Nous présentons une nouvelle méthode de transformation in situ par Agrobacterium. Cette approche est basée sur l'infiltration sous vide de plantes d'Arabidopsis par une souche d'Agrobacterium contenant un vecteur binaire. Les plantes caractérisées après leur developpement présentaient des secteurs végétatifs transformés parmi des secteurs non transformés. En moyenne jusqu'à 5 transformants par paire inoculée ont été sélectionnés dans la descendance des plantes infiltrées. Leur analyse génétique et moléculaire suggère que la transformation a lieu tardivement, lors du dveloppement floral, car tous les transformants sont hémizygotes et possèdent des sites différents d'insertion de l'ADN-T [...]
URLPMID:20699393 [本文引用: 1]

Abstract Diacyglycerol (DAG) is an important class of cellular lipid messengers, but its function in plants remains elusive. Here, we show that knockout of the Arabidopsis thaliana nonspecific phospholipase C (NPC4) results in a decrease in DAG levels and compromises plant response to abscisic acid (ABA) and hyperosmotic stresses. NPC4 hydrolyzes various phospholipids in a calcium-independent manner, producing DAG and a phosphorylated head group. NPC4 knockout (KO) plants display decreased ABA sensitivity in seed germination, root elongation, and stomatal movement and had decreased tolerance to high salinity and water deficiency. Overexpression of NPC4 renders plants more sensitive to ABA and more tolerant to hyperosmotic stress than wild-type plants. Addition of a short-chain DAG or a short-chain phosphatidic acid (PA) restores the ABA response of NPC4-KO to that of the wild type, but the addition of DAG together with a DAG kinase inhibitor does not result in a wild-type phenotype. These data suggest that NPC4-produced DAG is converted to PA and that NPC4 and its derived lipids positively modulate ABA response and promote plant tolerance to drought and salt stresses.
URLPMID:20507939 [本文引用: 1]

Phosphatidylcholine-hydrolyzing phospholipase C (PC-PLC) catalyzes the hydrolysis of phosphatidylcholine(PC) to generate phosphocholine and diacylglycerol (DAG). PC-PLC has a long tradition in animal signal transduction to generate DAG as a second messenger besides the classical phosphatidylinositol splitting phospholipase C (PI-PLC). Based on amino acid sequence similarity to bacterial PC-PLC, six putative PC-PLC genes (NPC1 to NPC6) were identified in the Arabidopsis genome. RT-PCR analysis revealed overlapping expression pattern of NPC genes in root, stem, leaf, flower,and silique. In auxin-treated PNPC3:GUS and PNPC4:GUS seedlings, strong increase of GUS activity was visible in roots, leaves,and shoots and, to a weaker extent, in brassinolide-treated (BL) seedlings. PNPC4:GUS seedlings also responded to cytokinin with increased GUS activity in young leaves. Compared to wild-type, T-DNA insertional knockouts npc3 and npc4 showed shorter primary roots and lower lateral root density at low BL concentrations but increased lateral root densities in response to exogenous 0.05-1.0 渭M BL. BL-induced expression of TCH4 and LRX2, which are involved in cell expansion, was impaired but not impaired in repression of CPD, a BL biosynthesis gene, in BL-treated npc3 and npc4. These observations suggest NPC3 and NPC4 are important in BL-mediated signaling in root growth. When treated with 0.1 渭M BL, DAG accumulation was observed in tobacco BY-2 cell cultures labeled with fluorescent PC as early as 15 min after application. We hypothesize that at least one PC-PLC is a plant signaling enzyme in BL signal transduction and, as shown earlier, in elicitor signal transduction.
URLPMID:25065551 [本文引用: 1]

Summary Nitrogen is a key mineral nutrient playing a crucial role in plant growth and development. Understanding the mechanisms of nitrate uptake from the soil and distribution through the plant in response to nitrogen starvation is an important step on the way to improve nitrogen uptake and utilization efficiency for better growth and productivity of plants, and to prevent negative effects of nitrogen fertilizers on the environment and human health. In this study, we show that Arabidopsis NITRATE TRANSPORTER 2.5 (NRT2.5) is a plasma membrane-localized high-affinity nitrate transporter playing an essential role in adult plants under severe nitrogen starvation. NRT2.5 expression is induced under nitrogen starvation and NRT2.5 becomes the most abundant transcript amongst the seven NRT2 family members in shoots and roots of adult plants after long-term starvation. GUS reporter analyses showed that NRT2.5 is expressed in the epidermis and the cortex of roots at the root hair zone and in minor veins of mature leaves. Reduction of NRT2.5 expression resulted in a decrease in high-affinity nitrate uptake without impacting low-affinity uptake. In the background of the high-affinity nitrate transporter mutant nrt2.4 , an nrt2.5 mutation reduced nitrate levels in the phloem of N-starved plants further than in the single nrt2.4 mutants. Growth analyses of multiple mutants between NRT2.1 , NRT2.2 , NRT2.4 , and NRT2.5 revealed that NRT2.5 is required to support growth of nitrogen-starved adult plants by ensuring the efficient uptake of nitrate collectively with NRT2.1, NRT2.2 and NRT2.4 and by taking part in nitrate loading into the phloem during nitrate remobilization.
URL [本文引用: 1]
URL [本文引用: 1]

Nitrate is an important macronutrient and also acts as a signal for plant growth; however, its levels in the soil solution can vary by three to four orders of magnitude. Consequently, plants have evolved regulated, energy dependent systems for the uptake of nitrate using both high and low affinity transporters. Genes that encode representatives of each class of transport system have been identified and fall into two families: NRT1 and NRT2 . Members of these families are induced in response to nitrate in the environment and are regulated by internal signals including nitrogen metabolites and shoot demand for nitrogen. The evidence to date indicates that the NRT2 transporters contribute specifically to the nitrate-inducible, high affinity nitrate uptake system while the NRT1 transporters contribute more broadly to nitrogen uptake and show both inducible and constitutive expression.
URLPMID:10748256 [本文引用: 2]

Abstract Physiological studies have established that plants acquire their NO(-3) from the soil through the combined activities of a set of high- and low-affinity NO(-3) transport systems, with the influx of NO(-3) being driven by the H(+) gradient across the plasma membrane. Some of these NO(-3) transport systems are constitutively expressed, while others are NO(-3)-inducible and subject to negative feedback regulation by the products of NO(-3) assimilation. Here we review recent progress in the characterisation of the two families of NO(-3) transporters that have so far been identified in plants, their structure and their regulation, and consider the evidence for their roles in NO(-3) acquisition. We also discuss what is currently known about the genetic basis of NO(-3) induction and feedback repression of the NO(-3) transport and assimilatory pathway in higher plants.
[本文引用: 2]
URLPMID:15272871 [本文引用: 1]

Abstract In Scots pine (Pinus sylvestris), ammonium assimilation is catalysed by glutamine synthetase (GS) [EC 6.3.1.2], which is encoded by two genes, PsGS1a and PsGS1b. PsGS1b is expressed in the vascular tissue throughout the plant body, where it is believed to play a role in recycling ammonium released by various facets of metabolism. The mechanisms that may underpin the transcriptional regulation of PsGS1b were explored. The PsGS1b promoter contains a region that is enriched in previously characterized cis-acting elements, known as AC elements. Pine nuclear proteins bound these AC element-rich regions in a tissue-specific manner. As previous experiments had shown that R2R3-MYB transcription factors could interact with AC elements, the capacity of the AC elements in the PsGS1b promoter to interact with MYB proteins was examined. Two MYB proteins from loblolly pine (Pinus taeda), PtMYB1 and PtMYB4, bound to the PsGS1b promoter were able to activate transcription from this promoter in yeast, arabidopsis and pine cells. Immunolocalization experiments revealed that the two MYB proteins were most abundant in cells previously shown to accumulate PsGS1b transcripts. Immunoprecipitation analysis and supershift electrophoretic mobility shift assays implicated these same two proteins in the formation of complexes between pine nuclear extracts and the PsGS1b promoter. Given that these MYB proteins were previously shown to have the capacity to activate gene expression related to lignin biosynthesis, we hypothesize that they may function to co-regulate lignification, a process that places significant demands on nitrogen recycling, and GS, the major enzyme involved in the nitrogen recycling pathway.
URL [本文引用: 1]
