 ,1, 敖英
,1, 敖英 ,1, 李斌1,2, 万阳1, 汪晖1
,1, 李斌1,2, 万阳1, 汪晖1Developmental disorder of podocytes and the related renal diseases
Ya'nan Zhu ,1, Ying Ao
,1, Ying Ao ,1, Bin Li1,2, Yang Wan1, Hui Wang1
,1, Bin Li1,2, Yang Wan1, Hui Wang1编委: 陈雁
收稿日期:2017-10-11修回日期:2017-12-5网络出版日期:2017-12-15
| 基金资助: |
Received:2017-10-11Revised:2017-12-5Online:2017-12-15
| Fund supported: |
作者简介 About authors
作者简介:朱亚男,硕士研究生,专业方向:药物毒理学E-mail:
通讯作者:敖英,博士,副教授,硕士生导师,专业方向:肾病的发育起源及药物防治,肾脏发育毒理E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (635KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
朱亚男, 敖英, 李斌, 万阳, 汪晖. 足细胞发育异常及相关肾脏疾病研究进展. 遗传[J], 2018, 40(2): 116-125 doi:10.16288/j.yczz.17-221
Ya'nan Zhu, Ying Ao, Bin Li, Yang Wan, Hui Wang.
足细胞即肾小球脏层上皮细胞,附着于肾小球基底膜(glomerular basement membrane, GBM)的外侧,连同GBM、有窗孔的肾小球内皮细胞、内皮细胞表面蛋白多糖和足细胞下区共同构成肾小球滤过屏障[1,2]。足细胞作为肾小球滤过膜的最外层屏障,在肾小球滤过功能中发挥着重要作用[3]。
足细胞的发育起始于孕中晚期,受到遗传因素(先天)与孕期环境(后天)的影响。遗传因素如足细胞特异性编码基因NPHS突变可以导致足细胞功能基因表达异常和功能障碍[4,5]。孕期不良环境因素是指孕期对母体与胎儿产生不利的因素,包括孕期外源物暴露、母体营养缺乏、胎盘功能不全、缺氧等[6,7,8,9],可引起足细胞发育不良,致足细胞数量的减少。因此,遗传或孕期不良环境都可能通过影响足细胞发育导致成年后相关肾脏疾病的发生。本文将对足细胞结构功能和发育过程、足细胞发育异常的病因和机制、以及足细胞发育异常所致肾脏疾病这几方面进行综述,拟展现有关足细胞发育异常的发生机制和远期危害的最新研究进展。
1 足细胞生理发育及功能调节
足细胞具有特异的结构和功能,并表达一些重要的标志基因。其发育受到调控因子和信号通路的调节。1.1 足细胞结构、功能及发育
足细胞是一种终末分化细胞,由细胞体、主足突和次足突组成。足突由胞体依次伸出,两相邻足突之间的裂隙称为裂孔,宽度约为30~40 nm,其上覆有一层拉链状蛋白结构—裂孔隔膜,为足细胞特有的细胞间连接。足细胞的功能可以概括为以下五个方面:(1)构成蛋白滤过的分子和电荷屏障[10];(2)改变超滤系数,调节肾小球滤过功能[11];(3)抵抗肾小球内压力,维持肾小球毛细血管袢的空间结构;(4)分泌GBM的组成成分和降解酶,参与其代谢;(5)合成分泌血管内皮生长因子,维持肾小球内皮细胞功能的完整性[2]。肾脏发育分为前、中、后肾3个阶段。前肾和中肾在胚胎发育中相继退化,后肾则发育为永久性肾脏。人类妊娠32天(大鼠12.5天)时,中肾导管尾端向背侧伸出输尿管芽,在后肾间充质诱导下,输尿管芽逐级分支,并诱导后肾间充质细胞分化形成肾单位、基质和微脉管系统,最终形成发育成熟的肾脏[12,13](图1)。足细胞发育起始于胎肾S形小体期,由后肾间充质细胞分化来的祖足细胞排列于S形小体的近端,并开始表达标志蛋白WT1和ZO-1。继而,祖足细胞移行至毛细血管袢外层,细胞骨架蛋白重排,逐渐形成足细胞的特有结构,即伸出指状足突并形成裂孔隔膜,此阶段开始表达裂孔隔膜标志蛋白Nephrin、Podocin、CD2相关蛋白(CD2- associated protein, CD2AP)等[14]。足细胞特有结构的形成及裂孔隔膜标志基因的表达标志着足细胞的分化成熟[15]。
1.2 足细胞功能的标志基因
裂孔隔膜是足细胞的特异蛋白连接结构,对于维持足细胞正常功能具有举足轻重的作用。裂孔隔膜主要由蛋白Nephrin、Podocin及 CD2AP[10]参与构成。Nephrin是免疫球蛋白超家族成员,编码基因为NPHS1[4]。它是一种跨膜蛋白,其胞内段与细胞骨架蛋白相结合,参与维持足突的正常形态;胞外段通过二硫键与相邻Nephrin蛋白的胞外段相结合,建立足突之间的细胞连接,对于维持足细胞裂孔隔膜的完整性以及正常的滤过屏障功能具有重要意 义[16,17]。同时,Nephrin通过与CD2AP和podocin相互作用,在足细胞间信号传导中起重要作用[18]。
图1
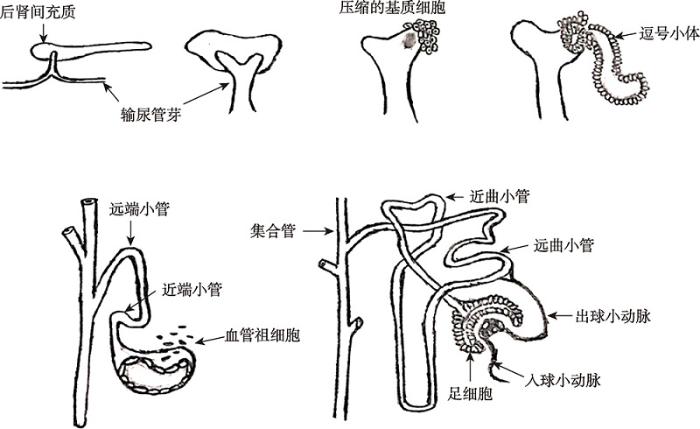 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1肾脏发育过程图
肾脏发生是由输尿管芽与后肾间充质相互作用而产生的。主要是中肾导管尾端向背侧伸出输尿管芽,在后肾间充质诱导下,输尿管芽逐级分支,并诱导后肾间充质细胞分化形成肾单位(肾小体与肾小管)、基质和微脉管系统(入球与出球动脉),最终形成发育熟的肾脏。参考文献[12]修改绘制。
Fig. 1Schematic illustration of nephrogenesis
Podocin是脂质筏相关蛋白Stomatin家族的新成员,编码基因为NPHS2[5]。Podocin蛋白特异性表达于足细胞上[10],呈发卡样结构插入细胞膜。其羧基末端与Nephrin蛋白的胞内羧基末端及CD2AP相互作用,形成蛋白复合体,一方面参与维持滤过屏障正常结构[19],另一方面可明显增强Nephrin诱导的信号转导,参与维持裂孔隔膜结构的正常功能[20]。
CD2AP是一种胞浆蛋白,主要表达于足细胞的裂孔隔膜处[21]。CD2AP含有639个氨基酸残基,氨
基末端有三个连续的SH3结构域,羧基末端有一个螺旋结构域,这些结构与介导蛋白间相互作用及信号转导功能有关[22]。CD2AP具有衔接的功能,其羧基末端连接Nephrin的羧基末端,从而将足细胞裂孔隔膜与足突内细胞骨架蛋白连接起来,稳定裂孔隔膜结构。
1.3 足细胞发育相关信号通路
胎肾以及足细胞的发育受到发育基因神经胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor, GDNF)、Wnt以及Notch通路的调控。其中,GDNF与Wnt通路在肾脏发育早期发挥作用,间接影响足细胞发育,而Notch通路足细胞的形成过程中起了关键性的调控作用(图2)。GDNF/c-Ret信号通路是后肾发育的主要诱导者[23]。后肾间充质细胞分泌GDNF,后者可先与输尿管芽尖端细胞的共同受体神经胶质细胞源性神经营养因子家族受体α-1(GDNF family receptor α-1,
GFRα-1)结合形成GDNF/GFR复合物,然后与c-Ret酪氨酸激酶受体(tyrosine kinase receptor, c-Ret)结合,激活下游Ras/Erk MAP kinase、PI3K/AKT、PLC- C/calcium等信号通路,从而诱导输尿管芽分支和肾单位的形成[24],进而促进足细胞发育[25]。
Wnt家族蛋白是分泌型糖蛋白,其受体为卷曲受体蛋白(Frizzled)和低密度脂蛋白受体相关蛋白5/6(low density lipoprotein receptor-related protein, LRP5/6)协同构成的跨膜异源二聚体。β-catenin是Wnt的下游蛋白,Wnt未激活时β-catenin被降解,不发挥功能;Wnt配体与其受体结合后,Wnt通路活化,LRP5/6阻断β-catenin降解,增多的β-catenin进入细胞核,与共转录因子T细胞因子/淋巴增强因子形成复合物,从而激活Wnt靶基因的转录[26]。在肾脏发育过程中,Wnt信号通路能调控包括基质细胞压缩、帽状基质干细胞自我更新、基质-表皮转化以及集合管生长等过程,因而在胎肾发育中起重要作用。Wnt9b和Wnt4是Wnt家族中两个重要的脂修饰分泌糖蛋白,主要在帽状间充质诱导形成肾单位的早期阶段发挥作用[27]。Wnt9b是Wnt4的上游因子,诱导Wnt4在肾脏中的表达,进而促进足细胞发育[28]。Wnt家族中另一重要蛋白Wnt11主要表达于输尿管芽的尖端,它与GDNF/c-Ret形成一个正反馈回路,有助于产生足够数量的输尿管芽分支以形成集合管系统,并诱导相应数量的肾单位生成[27]。
图2
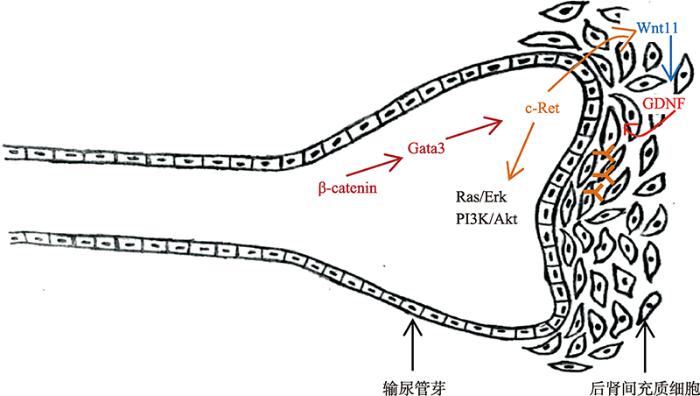 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2足细胞发育相关信号通路图
GDNF/c-Ret通路受发育中的输尿管芽上皮调控以保证分支形态发生的进展。持续表达β-catenin的细胞通过激活Gata3促进c-Ret的表达,GDNF/c-Ret激活Wnt11表达,Wnt11反过来保证GDNF/c-Ret活性,形成一个正反馈环,共同促进输尿管芽形成。GDNF/c-Ret的持续表达激活下游Ras/Erk MAP kinase、PI3K/AKT等信号通路,从而诱导输尿管芽分支和肾单位的发育,进而促进足细胞发育。参考文献[29]修改绘制。
Fig. 2Podocyte development related signaling pathway
已有文献报道,Gata3是β-catenin的下游基因,其可促进c-Ret表达[29]。β-catenin缺乏的中肾管细胞中c-Ret表达缺失,不利于输尿管发育;相反,持续表达β-catenin的细胞通过激活Gata3保留c-Ret的表达。总之,β-catenin是中肾管细胞中c-Ret表达的重要调节因子[29]。此研究也表明,GDNF/c-Ret激活Wnt11表达,Wnt11反过来保证GDNF/c-Ret活性,共同促进输尿管芽和肾单位的发育[29]。
Notch信号通路是一高度保守的信号途径,可精确调节细胞的增殖、分化过程,在三维结构器官(如肾脏、血管、神经系统等)的发育中起重要作用[30]。
Notch信号通路的表达贯穿整个肾脏的发育过程,尤其是在足细胞的形成过程中起了关键性的调控作 用[31,32]。哺乳动物体内存在4种Notch受体(Notch1- 4),其下游基因是Hesr1,主要由配体Jagged1/Delta1维持Notch信号通路活性。Notch1在S型小体形成时对近端小管和足细胞的分化起重要作用[31,32]。在后肾发育时期,Notch2持续表达于后肾间充质中,促进后肾间充质细胞向足细胞分化[33],此外,研究表明,Notch2参与维持足细胞的生存[34]。可见Notch2通路对于足细胞的发育也至关重要。而Notch3-4在足细胞发育过程中发挥的作用较少报道。
1.4 足细胞发育调控因子
在足细胞发育的不同时期,其调控因子也会有所不同。参与后肾间充质向足细胞诱导和分化、肾单位分化和肾小球形成(足细胞形成)等过程的主要调控因子如表1所示[35]。足细胞形成前期,Wnt4/ Wnt9b促进后肾间充质干细胞分化;而足细胞形成期,Notch2/WT-1/Pax-2及Nephrin/Podocin/ CD2AP等因子促进肾单位与肾小球的发育,并促进足细胞的发育成熟。Table 1
表1
表1 肾脏发育不同阶段的关键分子
Table 1
| 肾脏发育不同阶段 | 关键分子 |
|---|---|
| 后肾间充质诱导与分化 | Wnt 4 (MM)/ Wnt 9b (UB)、 (Wnts 1,3,7,11) β-catenin、 BMP4/7、FGF2、Leukemia inhibitory factor (LIF)、TGFβ2/Smad4、FGF7,8/ FGFR1/2、Pax 2/8、WT-1、Pax 2/8、Lim1 |
| 肾单位分化与肾小球发育 | |
| 肾单位分化 | Notch2、BMP7 |
| 足细胞分化 | WT-1、Pax-2、Lmx1b、 Pod-1(Tcf21)、GLEPP1、lim1、Pod1、Kreisler (Mafb)、Podocalyxin、FAT1 |
| 裂孔隔膜蛋白表达 | Nephrin、Neph 1,2,3、Podocin、CD2AP |
新窗口打开|下载CSV
2 足细胞发育异常的病因及发生机制
足细胞发育异常的常见病因有遗传因素(先天因素),也有孕期不良环境(后天因素)等,其发生机制比较复杂。足细胞发育异常的发生必然伴随着重要信号通路的异常,同时已有研究表明,表观遗传修饰在足细胞的发育异常的过程中也可能扮演重要角色。2.1 足细胞发育异常的常见病因
遗传因素是足细胞发育异常的常见病因之一。基因NPHS1和NPHS2分别编码足细胞标志基因Nephrin与Podocin[4,5],上述基因的突变导致Nephrin或Podocin表达异常,进而影响足细胞发育。胚胎时期,NPHS1表达缺失是足细胞发育不良的标志之一,可导致足突和裂孔隔膜的形成障碍,造成动物出生后早期的蛋白尿和死亡[36]。有研究通过定位克隆技术发现,NPHS1突变是芬兰型先天性肾病综合征累及的主要基因,通过破坏裂孔隔膜结构,造成足细胞发育异常[4]。NPHS2基因突变在激素抵抗性肾病综合征、原发性肾小球硬化等肾小球疾病中有明确诊断,通过破坏足突结构,致足突数量减少与融合,最终导致滤过屏障受损,并引发蛋白尿[5]。
孕期不良环境包括孕期外源物暴露、孕期营养不当等。孕期外源物暴露可引起低出生体重与早产,因而影响足细胞发育[37]。本实验室动物实验显示,孕期咖啡因、尼古丁等外源物暴露导致子代IUGR和低出生体重,并引起胎肾结构与功能的破坏,足细胞的标志基因Nephrin、Podocin表达降低,致胎儿肾脏发育不良,并导致成年子代蛋白尿和肾小球硬化的发生[38,39]。也有研究报道,孕期二-(2-乙基己基)酯(DEHP)暴露导致后代断奶后的肾单位数量减少,肾小球体积增大,缩小的鲍曼氏(Bowman)囊,以及在成年肾脏纤维化和足细胞的足突消失[40]。此外也有研究报道,孕期烟雾暴露可致后代足细胞数量急剧下降,但体积并未发生大的变化[41]。由此可见,孕期外源物暴露导致子代足细胞发育异常及子代成年肾脏疾病的易感。
孕期营养不当,无论营养缺陷或是营养过剩,都会对子代成年的健康造成威胁[42,43,44],并与子代成年高胰岛素、蛋白尿、肾小球硬化易感密切相关[45,46]。研究显示,母亲孕期给予高脂或高糖饮食,不管出生后子代的饮食方式如何,均导致子代足细胞标志基因Nestin和Podocin的表达降低,并伴有蛋白尿[45,46]。也有研究报道,孕期低蛋白暴露的胎鼠出生后给予高蛋白饮食,会造成成年后肾小球数量和足细胞数量的减少[47],并导致蛋白尿的产生。可见,孕期营养不当也是造成足细胞损伤的病因之一。
2.2 足细胞发育相关信号通路异常
Wnt是肾脏发育早期调节信号通路,其表达或功能异常,会影响足细胞的发育。有研究表明,在肾脏中构建条件性敲除β-catenin小鼠后,导致输尿管芽分支减少,并影响足细胞的分化[48]。Notch通路在足细胞发育阶段发挥主要作用。Cheng等[31]在逗号形和S型小体中发现Notch1的表达,之后用Notch1的抑制剂DAPT培养后发现,肾脏上皮结构减少,近端小管和肾小球足细胞严重缺陷,并伴随着非上皮细胞的增多。另有研究显示,基因敲除Notch2或降低Notch2的表达导致足细胞数量的减少和肾单位的形成障碍[48]。此外,Notch2基因突变可致遗传缺陷病阿拉吉欧综合症(Alagille syndrome)的发生,而足细胞和肾单位发育不良是其主要的病症之一[49]。可见,Wnt或Notch通路的异常均会对足细胞发育产生不良后果。2.3 足细胞发育异常的表观遗传机制
表观遗传(epigenetics)是指DNA序列不发生变化但基因表达却出现了可遗传的改变[50]。表观遗传修饰(epigenetic modification)存在于高等真核细胞的正常发育之中。已发现的表观遗传修饰有DNA甲基化、组蛋白甲基化和乙酰化、微小核糖核酸(microRNAs, miRNAs)[51]等。DNA甲基化相对稳定,并且可稳定存在于DNA复制期间,虽然不改变核苷酸序列,却能调控基因的转录,在表观遗传学中起着重要作用。启动子区DNA甲基化可以抑制基因的表达,并且可改变染色体结构[52]。此外,组蛋白乙酰化是最早发现的与转录有关的组蛋白修饰方式之一[53]。促进组蛋白乙酰化修饰的组蛋白乙酰化酶(histone acetyltransferases, HATs)和具有降低乙酰化水平的组蛋白去乙酰化酶(histone deacetylases, HDACs)通过改变目的基因启动子区组蛋白乙酰化水平以调控其转录[54]。HATs能将乙酰辅酶A的乙酰基转移到组蛋白的赖氨酸残基上,使其ε-氨基基团乙酰化,致染色体结构松散,促进基因转录;HDACs能水解乙酰化的赖氨酸,使其脱乙酰化,因而染色质呈致密结构,抑制转录[53,54]。
已有研究发现,Nephrin基因的启动子区存在DNA甲基化和组蛋白修饰调控,并且其表遗传修饰水平受到转录因子Kruppel样因子4(kruppel-like factor4, KLF4)的调节[55,56]。KLF4是一种具有锌指结构的转录因子,在肾脏发育及分化时期发挥重要的转录调控作用,与足细胞标志基因表达密切相关[56]。Nephrin是KLF4的下游靶基因,二者的结合位点位于Nephrin启动子区CpG岛区域内。在游离状态下Nephrin启动子区与DNA甲基转移酶1(DNA methyltransferase1, DNMT1)相结合,导致启动子区高甲基化及低表达。而KLF4与Nephrin结合后,促使DNMT1与其启动子解离,从而降低启动子区DNA甲基化水平并促进Nephrin表达。KLF4也可通过招募组蛋白H3赖氨酸9(histone H3 lysine 9, H3K9),增加Nephrin启动子区组蛋白乙酰化,促进Nephrin表达[55,56]。本实验室近期的研究结果发现,孕期外源物暴露的胎鼠肾脏Nephrin表达均降低,并且该现象持续到成年,提示表观遗传学途径可能参与介导孕期不良环境所致的足细胞标志基因的表达异常和足细胞发育不良。
3 足细胞发育异常相关的疾病
研究表明,足细胞损伤是多种肾小球疾病的共同病理基础,包括肾小球硬化症、先天性肾病综合征、膜性肾病、糖尿病肾病等[57]。3.1 足细胞发育异常与肾小球硬化症
肾小球硬化症(glomerulosclerosis, GS)作为一种以肾小球病变为主的临床病理综合征,以肾小球系膜细胞增殖及细胞外基质过量沉积为特征,以大量蛋白尿和肾功能进行性恶化为主要的临床表现,是许多慢性肾病走向终末期肾衰竭和肾功能丧失的共同病理途径和必经阶段[58,59,60]。肾小球硬化症的发病率逐年提高,尤其是在黑人中的发病率更高[61]。目前数据表明,美国多达3%的终末期肾病是由肾小球硬化引起的[62]。有研究报道,遗传因素或孕期不良环境所致的足细胞发育异常会导致足细胞数量的减少或功能缺失,造成肾小球滤过屏障破坏,最终引起蛋白尿与肾小球硬化[63]。研究表明,NPHS2基因突变(Podocin缺失,染色体lq25-31)与儿童原发性局灶性肾小球硬化密切相关[64,65],表现为足细胞结构与数量的丢失,弥漫性系膜硬化。此外,流行病学资料显示,孕期不良环境所致IUGR,也是肾小球硬化症的危险因素,低出生体重患儿的肾小球硬化症发生率显著高于正常出生体重者[37,66,67]。动物实验研究也表明,IUGR个体成年后可出现肾小球滤过功能下降和肾小球硬化症易感的现象[68]。
3.2 足细胞发育异常与肾病综合征
肾病综合征(nephrotic syndrome)可由多种病因引起,以肾小球基膜通透性增加为特征,表现为大量蛋白尿、低蛋白血症、高度水肿、高脂血症等临床症候群,是最常见的儿童原发性肾小球疾病[69]。肾病综合征的平均发病率为每10万名儿童有2~16.9名患病[70]。研究表明,肾病综合征的发生与Nephrin、Podocin的基因突变或表达异常密切相关。有40%~ 60%婴儿慢性肾病综合征的发生与NPHS1基因突变有关[71,72];部分儿童激素抵抗性肾病综合征中也证实存在NPHS2基因突变[73]。同时,也有文献报道,孕期不良环境导致足细胞发育不良和标志基因Nephrin表达降低,伴随着成年蛋白尿和肾病综合征的易感[74]。3.3 足细胞发育异常与慢性肾病
慢性肾病(chronic kidney disease, CKD)被定义为从GFR降低到发生终末期肾病和蛋白尿的一段疾病进程[75]。肾脏病预后质量倡议(the Kidney Disease Out-comes Quality Initiative, KDOQI)将慢性肾脏疾病定义为肾脏损伤超过3个月伴或不伴肾小球滤过率(glomerular filtration rate, GFR)的降低,或者GFR下降超过3个月伴或不伴肾脏损害[76]。研究报道,慢性肾病在成人中的发病率为10%~15%[77]。CKD病理机制涉及细胞损伤、炎症、足细胞消失、蛋白尿与肾小球硬化[78]。研究表明,孕期不良环境可造成低出生体重和出生时肾单位数量的减少,足细胞数量减少,从而增加成年期慢性肾病的易感性[75]。动物实验也证实,孕期低蛋白饮食摄入,会导致胎鼠及成年仔鼠肾小球数量的减少,足细胞的损失[47]等,进而导致成年蛋白尿的产生,而慢性蛋白尿的持续丢失,必然导致慢性肾病的产生。因此,出生前、后不良环境所致的足细胞发育不良与慢性肾病关系密切。4 结语与展望
足细胞是肾小球滤过屏障的重要组成部分,其数量的减少及功能的异常将导致多种肾小球疾病的发生。足细胞为不可再生性细胞,因此足细胞的正常发育对于维持其数量和功能具有重要的意义,这也使得足细胞发育异常与胎源性肾小球疾病之间的关系成为近年来****关注的研究热点之一。现有的研究表明,遗传学因素(基因突变)和孕期不良环境是导致足细胞发育异常的主要病因,可通过表观遗传学等机制影响足细胞功能基因的表达,导致足细胞数量和/或功能的异常,并最终引起相关肾小球疾病的发生。然而,该研究领域仍然存在着诸多空白。比如,在足细胞发育过程中存在许多复杂的调控通路,而胚胎足细胞发育不良时有哪些调控通路和关键因子的参与,这些调控通路或关键因子又影响足细胞发育的哪些环节,以及能否产生编程性改变持续影响足细胞的功能并造成肾脏疾病的易感,是否具有跨代遗传现象,这些问题尚未充分阐明。因而,目前也缺乏胎源性肾小球疾病的早期预警、早期干预靶标和有效的早期治疗手段,这也为将来足细胞发育异常的相关研究提供一定的研究方向。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
PMID:19365184 [本文引用: 1]

PURPOSE OF REVIEW: Three structures (glomerular endothelial fenestrae, glomerular basement membrane and podocyte interfoot process/slit diaphragms) have traditionally been considered as the major determinants of glomerular permeability. We review recent work demonstrating the functional importance of two additional layers: the endothelial surface layer (ESL) and the subpodocyte space (SPS). RECENT FINDINGS: Removing glomerular endothelial cell monolayer ESL in vitro significantly alters monolayer permeability, supporting previous in-vivo demonstrations of the importance of the ESL in determining glomerular permeability. Whether fenestral diaphragms are present to support the ESL in healthy adult glomeruli has been examined in a recent report. On the downstream side of the glomerular filtration barrier, the SPS is a recently described structure that covers approximately two-thirds of the barrier, has highly restrictive dimensions and contributes to the hydraulic resistance and ultrafiltration characteristics of the glomerulus. Different layers of the barrier have also been shown to influence the permeability characteristics of one another, either through biophysical interactions, or through the activities of ligand-receptor axes that cross the various layers of the barrier. SUMMARY: The structure and function of the glomerular filtration barrier remains an area of significant new discovery, and recent work continues to highlight the complexity of this dynamic multilayered watershed.
URLPMID:15740933 [本文引用: 2]

The glomerular barrier is the kidney’s physical block to the unrestricted flow of molecules from the plasma into the urinary space. Its exquisite selectivity allows solutes and water in the glomerular capillaries to pass through but it prevents the bulk of plasma proteins, most notably albumin, froom crossing. Classically the barrier consists of three components: glomerular endothelium, glomerular basement membrane and glomerular epithelium (podocytes) with slit diaphragm. A lot of investigations are experimental but they are sufficient to show the pivotal role of endothelium in glomerular proteinuria. In this study the author discuss glomerular endothelium with particular emphasis on the barrier presumed to be imparted by endothelium-glomerular basement membrane–podocyte interactions. By specialized glomerular endothelial structure (caveolae, tight junctions, endothelium glycocalyx) and by circulating permeability factors (albumin, orosomucoid, apolipoproteins, Amadori’s products). Concluding remarks underline the significance to study the glomerular vascular endothelial dysfunction.
[本文引用: 1]
URLPMID:27019444 [本文引用: 4]

Since the identification of the NPHS1 gene, which encodes nephrin, various investigators have demonstrated that the NPHS1 mutation is a frequent cause of congenital nephrotic syndrome (CNS); it is found in 98% of Finnish children with this syndrome and in 39-80% of non-Finnish cases. In China, compound heterozygous mutations in the NPHS1 gene have been identified in two Chinese families with CNS. To our knowledge, however, whether or not NPHS1 is the causative gene in sporadic Chinese CNS cases has not been established. We identified a homozygous mutation of NPHS1, 3250insG (V1084fsX1095), in a Chinese child with sporadic CNS. This finding leads us to suggest that NPHS1 mutations are also present in sporadic Chinese CNS cases. This gives additional support for the necessity for genetic examination of mutations in the NPHS1 gene in Chinese children with sporadic CNS.
URLPMID:10802674 [本文引用: 4]

Elsevier’s Scopus, the largest abstract and citation database of peer-reviewed literature. Search and access research from the science, technology, medicine, social sciences and arts and humanities fields.
URLPMID:4076357 [本文引用: 1]

Environmental conditions during perinatal development such as maternal undernutrition, maternal glucocorticoids, placental insufficiency, and maternal sodium overload can program changes in renal Na(+) excretion leading to hypertension. Experimental studies indicate that fetal exposure to an adverse maternal environment may reduce glomerular filtration rate by decreasing the surface area of the glomerular capillaries. Moreover, fetal responses to environmental insults during early life that contribute to the development of hypertension may include increased expression of tubular apical or basolateral membrane Na(+) transporters and increased production of renal superoxide leading to enhanced Na(+) reabsorption. This review will address the role of these potential renal mechanisms in the fetal programming of hypertension in experimental models induced by maternal undernutrition, fetal exposure to glucocorticoids, placental insufficiency, and maternal sodium overload in the rat.
URLPMID:23322082 [本文引用: 1]

Abstract Maternal alcohol use during pregnancy is prevalent, with as many as 12% of pregnant women consuming alcohol. Alcohol intake may vary from an occasional drink, to weekly binge drinking, to chronic alcohol use throughout pregnancy. Whereas there are certain known consequences from fetal alcohol exposure, such as fetal alcohol syndrome, other effects are less well defined. Craniofacial dysmorphologies, abnormalities of organ systems, behavioral and intellectual deficits, and fetal death have all been attributed to maternal alcohol consumption. This review article considers the theoretical mechanisms of how alcohol affects the fetus, including the variable susceptibility to fetal alcohol exposure and the implications of ethanol dose and timing of exposure. Criteria for diagnosis of fetal alcohol syndrome are discussed, as well as new methods for early detection of maternal alcohol use and fetal alcohol exposure, such as the use of fatty acid ethyl esters. Finally, current and novel treatment strategies, both in utero and post utero, are reviewed.
URLPMID:24275070 [本文引用: 1]

Prenatal ethanol exposure (PEE) induces dyslipidemia and hyperglycemia in fetus and adult offspring. However, whether PEE increases the susceptibility to non-alcoholic fatty liver disease (NAFLD) in offspring and its underlying mechanism remain unknown. This study aimed to demonstrate an increased susceptibility to high-fat diet (HFD)-induced NAFLD and its intrauterine programming mechanisms in female rat offspring with PEE. Rat model of intrauterine growth retardation (IUGR) was established by PEE, the female fetus and adult offspring that fed normal diet (ND) or HFD were sacrificed. The results showed that, in PEE+ND group, serum corticosterone (CORT) slightly decreased and insulin-like growth factor-1 (IGF-1) and glucose increased with partial catch-up growth; In PEE+HFD group, serum CORT decreased, while serum IGF-1, glucose and triglyceride (TG) increased, with notable catch-up growth, higher metabolic status and NAFLD formation. Enhanced liver expression of the IGF-1 pathway, gluconeogenesis, and lipid synthesis as well as reduced expression of lipid output were accompanied in PEE+HFD group. In PEE fetus, serum CORT increased while IGF-1 decreased, with low body weight, hyperglycemia, and hepatocyte ultrastructural changes. Hepatic IGF-1 expression as well as lipid output was down-regulated, while lipid synthesis significantly increased. Based on these findings, we propose a "two-programming" hypothesis for an increased susceptibility to HFD-induced NAFLD in female offspring of PEE. That is, the intrauterine programming of liver glucose and lipid metabolic function is "the first programming", and postnatal adaptive catch-up growth triggered by intrauterine programming of GC-IGF1 axis acts as "the second programming".
URLPMID:25102264 [本文引用: 1]

We have previously shown that adult rat offspring born intrauterine growth restricted (IUGR) as a result of a prenatal hypoxic insult exhibit several cardiovascular characteristics that are compatible with common manifestations of chronic iron toxicity. As hypoxia is one of the major regulators of iron absorption and metabolism, we hypothesized that hypoxia-induced IUGR offspring will have long-term changes in their ability to regulate iron metabolism leading to myocardial iron deposition and induction of myocardial oxidative stress. Pregnant Sprague Dawley rats were randomized to control (n = 8) or maternal hypoxia (11.5% oxygen; n = 8) during the last 6 days of pregnancy. At birth, litters were reduced to eight pups (four male and four female). At 4 or 12 months of age, offspring were euthanatized and samples (blood and myocardium) were collected. In only the male offspring, IUGR and aging were associated with an increase in myocardial markers of oxidative stress such as oxidized/reduced glutathione ratio and malondialdehyde. Aged male IUGR offspring also exhibited interstitial myocardial remodeling characterized by myocyte loss and disrupted extracellular matrix.Contrary to our hypothesis, however, neither IUGR nor aging were associated with changes in any systemic or local markers of iron metabolism. Our results suggest that hypoxic insults leading to IUGR produce long-term effects on the levels of oxidative stress and connective tissue distribution in the myocardium of male but not female offspring.
URLPMID:19478094 [本文引用: 3]

Slit diaphragms are essential components of the glomerular filtration apparatus, as changes in these junctions are the hallmark of proteinuric diseases. Slit diaphragms, considered specialized adherens junctions, contain both unique membrane proteins (e.g., nephrin, podocin, and Neph1) and typical adherens junction proteins (e.g., P-cadherin, FAT, and catenins). Whether slit diaphragms also contain tight junction proteins is unknown. Here, immunofluorescence, immunogold labeling, and cell fractionation demonstrated that rat slit diaphragms contain the tight junction proteins JAM-A (junctional adhesion molecule A), occludin, and cingulin. We found these proteins in the same protein complexes as nephrin, podocin, CD2AP, ZO-1, and Neph1 by cosedimentation, coimmunoprecipitation, and pull-down assays. PAN nephrosis increased the protein levels of JAM-A, occludin, cingulin, and ZO-1 several-fold in glomeruli and loosened their attachment to the actin cytoskeleton. These data extend current information about the molecular composition of slit diaphragms by demonstrating the presence of tight junction proteins, although slit diaphragms lack the characteristic morphologic features of tight junctions. The contribution of these proteins to the assembly of slit diaphragms and potential signaling cascades requires further investigation.
URLPMID:3141719 [本文引用: 1]

By immunoelectron microscopy the podocyte foot processes of the rat and human kidney have been shown to contain three major proteins of the contractile apparatus in muscle, i.e., actin, myosin, and the Z-line protein, alpha-actinin. Gel electrophoresis and immunoblot analysis of isolated glomeruli suggests that these proteins constitute an important part of the total glomerular protein contents. In the chicken kidney, the plasmalemmal portion of the foot processes that abuts the glomerular basement membrane was specifically labeled with antibodies against chicken gizzard vinculin and talin, two proteins thought to be important for the linkage of actin filaments to the lipid bilayer and to the receptor for fibronectin and laminin. Such a linkage may not only be important for the attachment of actin filaments to the plasma membrane, but could also be of functional significance for restricting the fibronectin-laminin receptor in its lateral diffusion in the plane of the lipid bilayer and to localize it at the basis of the podocytic foot processes. Assuming that actin, myosin, and alpha-actinin are arranged in a way that would allow the foot processes to generate contractile force this filament system might help the glomerular capillaries to resist the high intraluminal hydrostatic pressure as well as to actively modify the surface area for filtration. Vimentin and tubulin, the main protein subunits of intermediate filaments and microtubules, respectively, were confined to the podocyte cell body and the major processes but were virtually absent from the foot processes. This suggests that both proteins and their polymers are not important for the structure and function of the foot processes.
[本文引用: 2]
URL [本文引用: 1]

肾脏发育是一个复杂的过程,需要在输尿管芽细胞和基质细胞相互诱导下,引起细胞生长、增殖、分化,从而产生肾单位及各种管状结构,最终发育为成熟的肾脏。在肾脏发育过程中,GDNF/Ret、Wnt、BMP 等一系列信号通路参与了发育的调控过程。这些信号通路在肾脏发育的不同阶段或不同位置发挥着重要的调控作用,并且通路之间存在相互的调控,从而形成了一个复杂而精细的调控网络,保证了肾脏的正常发育。文章概括了肾脏发育的过程,总结了肾脏发育过程中相关信号通路对肾脏发育的调控作用以及信号通路之间的相互调控。
URL [本文引用: 1]

肾脏发育是一个复杂的过程,需要在输尿管芽细胞和基质细胞相互诱导下,引起细胞生长、增殖、分化,从而产生肾单位及各种管状结构,最终发育为成熟的肾脏。在肾脏发育过程中,GDNF/Ret、Wnt、BMP 等一系列信号通路参与了发育的调控过程。这些信号通路在肾脏发育的不同阶段或不同位置发挥着重要的调控作用,并且通路之间存在相互的调控,从而形成了一个复杂而精细的调控网络,保证了肾脏的正常发育。文章概括了肾脏发育的过程,总结了肾脏发育过程中相关信号通路对肾脏发育的调控作用以及信号通路之间的相互调控。
URLPMID:3600372 [本文引用: 1]

Abstract As an integral member of the filtration barrier in the kidney glomerulus, the podocyte is in a unique geographical position: It is exposed to chemical signals from the urinary space (Bowman's capsule), it receives and transmits chemical and mechanical signals to/from the glomerular basement membrane upon which it elaborates, and it receives chemical and mechanical signals from the vascular space with which it also communicates. As with every cell, the ability of the podocyte to receive signals from the surrounding environment and to translate them to the intracellular milieu is dependent largely on molecules residing on the cell membrane. These molecules are the first-line soldiers in the ongoing battle to sense the environment, to respond to friendly signals, and to defend against injurious foes. In this review, we take a membrane biologist's view of the podocyte, examining the many membrane receptors, channels, and other signaling molecules that have been implicated in podocyte biology. Although we attempt to be comprehensive, our goal is not to capture every membrane-mediated pathway but rather to emphasize that this approach may be fruitful in understanding the podocyte and its unique properties.
URLPMID:4181233 [本文引用: 1]

The podocyte is one of two cell types that contribute to the formation of the glomerular filtration barrier (GFB). It is a highly specialized cell with a unique structure. The key feature of the podocyte is its foot processes that regularly interdigitate. A structure known as the slit diaphragm can be found bridging the interdigitations. This molecular sieve comprises the final layer of the GFB. It is well accepted that the podocyte is the target cell in the pathogenesis of nephrotic syndrome. In nephrotic syndrome the glomerular filtration barrier no longer restricts the passage of macromolecules and protein is lost into the urine. A number of phenotypic and morphological changes are seen in the diseased podocyte and in the literature these have been described as an Epithelial-Mesenchymal Transition (EMT). However, there is a growing appreciation that this term does not accurately describe the changes that are seen. Definitions of type 2 EMT are based on typical epithelial cells. Whilst the podocyte is known as a visceral epithelial cell, it is not a typical epithelial cell. Moreover, podocytes have several features that are more consistent with mesenchymal cells. Therefore we suggest that the term Podocyte Disease Transformation (PDT) is more appropriate.
URL [本文引用: 1]

While the mechanisms that regulate actin dynamics in cellular motility are intensively studied, relatively little is known about signaling events that transmit outside-in signals and direct assembly and regulation of actin polymerization complexes at the cell membrane. The kidney podocyte provides a unique model for investigating these mechanisms since deletion of Nephrin or Neph1, two interacting components of the specialized podocyte intercellular junction, results in abnormal podocyte morphogenesis and junction formation. We provide evidence that extends the existing model by which the Nephrin-Neph1 complex transduces phosphorylation-mediated signals that assemble an actin polymerization complex at the podocyte intercellular junction. Upon engagement, Neph1 is phosphorylated on specific tyrosine residues by Fyn, which results in the recruitment of Grb2, an event that is necessary for Neph1-induced actin polymerization at the plasma membrane. Importantly, Neph1 and Nephrin directly interact and, by juxtaposing Grb2 and Nck1/2 at the membrane following complex activation, cooperate to augment the efficiency of actin polymerization. These data provide evidence for a mechanism reminiscent of that employed by vaccinia virus and other pathogens, by which a signaling complex transduces an outside-in signal that results in actin filament polymerization at the plasma membrane.
URLPMID:16630808 [本文引用: 1]

Two papers, one in Nature (Jones et al., 2006) and the other in the Journal of Clinical Investigation (Verma et al., 2006) show that Nck adaptor proteins connect phosphorylated nephrin with actin polymerization in podocyte foot processes, structures important for slit-diaphragm formation in the kidney. Their results further our understanding of podocyte development and repair in glomerular disease.
[本文引用: 1]
URLPMID:12089372 [本文引用: 1]

The interpodocyte slit diaphragm is an essential structure for maintaining the functional glomerular filtration barrier. The slit diaphragm is proposed to consist of an interacting meshwork of nephrin molecules. Earlier studies with tagged proteins have suggested that the part of nephrin interacts with (). This study was addressed to show by coimmunoprecipitation and pulldown assays an interaction of endogenously expressed nephrin and in the kidney-derived epithelial M-1 cell line, to provide evidence of the domain(s) of involved in the interaction, and to show the of the respective proteins by immunoelectron microscopy in kidney cortex. In addition, the of , , , and nephrin was studied in kidney glomeruli and in M-1 cells by immunofluorescence microscopy. The results indicate an endogenous interaction between nephrin and in M-1 cells and suggest that this interaction is mediated by the third homology 3 (SH3) domain of . We also show by immunoelectron microscopy that nephrin and are detected at the slit diaphragm area, supporting their interaction in the glomeruli in vivo. In addition, nephrin was found to partially colocalize with and in double immunofluorescence microscopy, confirming the close proximity of these proteins and proposing that these proteins may belong to nephrin-associated protein complex in glomeruli. The existence of nephrin, , , and enables further characterization of their relationship in M-1 cells.
URLPMID:17766183 [本文引用: 1]

Since the discovery of nephrin, the first integral component of the slit diaphragm to be identified, the podocyte slit pore has become a major focus in research concerning the glomerular filtration barrier. Nephrin is a central component of the glomerular ultrafilter, with both structural and signaling functions. The extracellular domain of nephrin and other components of the slit diaphragm seem to form a porous molecular sieve. The intracellular domain of nephrin is associated with linker proteins, such as CD2-associated protein and Nck proteins that can connect nephrin to the actin cytoskeleton. Alterations in nephrin interactions with other proteins during development or injury can lead to complex signaling reactions aimed at establishing or restoring the filter function.
URLPMID:24511139 [本文引用: 1]

Abstract CD2-associated protein (CD2AP) is a multidomain scaffolding protein that has a critical role in renal function. CD2AP is expressed in glomerular podocytes at the slit diaphragm, a modified adherens junction that comprises the protein filtration barrier of the kidney, and interacts with a number of protein ligands involved in cytoskeletal remodeling, membrane trafficking, cell motility, and cell survival. The structure of CD2AP is unknown. We used electron microscopy and single particle image analysis to determine the three-dimensional structure of recombinant full-length CD2AP and found that the protein is a tetramer in solution. Image reconstruction of negatively stained protein particles generated a structure at 21 resolution. The protein assumed a roughly spherical, very loosely packed structure. Analysis of the electron density map revealed that CD2AP consists of a central coiled-coil domain, which forms the tetramer interface, surrounded by four symmetry-related motifs, each containing three globular domains corresponding to the three SH3 domains. The spatial organization exposes the binding sites of all 12 SH3 domains in the tetramer, allowing simultaneous binding to multiple targets. Determination of the structure of CD2AP provides novel insights into the biology of this slit diaphragm protein and lays the groundwork for characterizing the interactions between key molecules of the slit diaphragm that control glomerular filtration.
URLPMID:11195047 [本文引用: 1]

During the past 3 years there have been significant advances in our understanding of the biology of the glomerular podocyte. In particular, two , and , have been identified as critical podocyte that are both required for normal . In addition to supporting the idea that the plays a crucial role in glomerular function, these results suggest novel insights into the of . The present review addresses these recent advances and discusses the implications of the findings.
URLPMID:3615609 [本文引用: 1]

Genetically engineered mice have provided much information about gene function in the field of developmental biology. Recently, conditional gene targeting using the Cre/loxP system has been developed to control the cell type and timing of the target gene expression. The increase in number of kidney-specific Cre mice allows for the analysis of phenotypes that cannot be addressed by conventional gene targeting. The mammalian kidney is a vital organ that plays a critical homeostatic role in the regulation of body fluid composition and excretion of waste products. The interactions between epithelial and mesenchymal cells are very critical events in the field of developmental biology, especially renal development. Kidney development is a complex process, requiring inductive interactions between epithelial and mesenchymal cells that eventually lead to the growth and differentiation of multiple highly specialized stromal, vascular, and epithelial cell types. Through the use of genetically engineered mouse models, the molecular bases for many of the events in the developing kidney have been identified. Defective morphogenesis may result in clinical phenotypes that range from complete renal agenesis to diseases such as hypertension that exist in the setting of grossly normal kidneys. In this review, we focus on the growth and transcription factors that define kidney progenitor cell populations, initiate ureteric bud branching, induce nephron formation within the metanephric mesenchyme, and differentiate stromal and vascular progenitors in the metanephric mesenchyme.
URLPMID:16916378 [本文引用: 1]

Abstract The ureteric bud (UB) is an outgrowth of the Wolffian duct, which undergoes a complex process of growth, branching, and remodeling, to eventually give rise to the entire urinary collecting system during kidney development. Understanding the mechanisms that control this process is a fascinating problem in basic developmental biology, and also has considerable medical significance. Over the past decade, there has been significant progress in our understanding of renal branching morphogenesis and its regulation, and this review focuses on several areas in which there have been recent advances. The first section deals with the normal process of UB branching morphogenesis, and methods that have been developed to better observe and describe it. The next section discusses a number of experimental methodologies, both established and novel, that make kidney development in the mouse a powerful and attractive experimental system. The third section discusses some of the cellular processes that are likely to underlie UB branching morphogenesis, as well as recent data on cell lineages within the growing UB. The fourth section summarizes our understanding of the roles of two groups of growth factors that appear to be particularly important for the regulation of UB outgrowth and branching: GDNF and FGFs, which stimulate this process via tyrosine kinase receptors, and members of the TGF family, including BMP4 and Activin A, which generally inhibit UB formation and branching.
URLPMID:25587123 [本文引用: 1]

Abstract Podocyte injury and loss directly cause proteinuria and the progression to glomerulosclerosis. Elucidation of the mechanisms of podocyte survival and recovery from injury is critical for designing strategies to prevent the progression of glomerular diseases. Glial cell line-derived neurotrophic factor (GDNF) and its receptor tyrosine kinase, Ret, are upregulated in both nonimmune and immune-mediated in vitro and in vivo models of glomerular diseases. We investigated whether Ret, a known receptor tyrosine kinase critical for kidney morphogenesis and neuronal growth and development, is necessary for glomerular and podocyte development and survival in vivo. Since deletions of both GDNF and Ret result in embryonic lethality due to kidney agenesis, we examined the role of Ret in vivo by generating mice with a conditional deletion of Ret in podocytes (Ret(flox/flox); Nphs2-Cre). In contrast to the lack of any developmental and maintenance deficits, Ret(flox/flox); Nphs2-Cre mice showed a significantly enhanced susceptibility to adriamycin nephropathy, a rodent model of focal segmental glomerulosclerosis. Thus, these findings demonstrated that the Ret signaling pathway is important for podocyte survival and recovery from glomerular injury in vivo.
URLPMID:3140915 [本文引用: 1]

The Wnt/β-catenin signaling pathway is one of several key conserved intercellular signaling pathways in animals, and plays fundamental roles in the proliferation, regeneration, differentiation, and function of many cell and tissue types. This pathway is activated in a dynamic manner during the morphogenesis of oral organs, including teeth, taste papillae, and taste buds, and is essential for these processes to occur normally. Conversely, forced activation of Wnt/β-catenin signaling promotes the formation of ectopic teeth and taste papillae. In this review, we discuss our current understanding of the roles of Wnt/β-catenin signaling in oral tissue development and in related human diseases, and the potential of manipulating this pathway for therapeutic purposes.
URLPMID:24445433 [本文引用: 2]

Several Wnt proteins are expressed in the embryonic kidney during various stages of development. Gene knockout models and ex vivo studies have provided strong evidence that Wnt-mediated signals are essential in renal ontogeny. Perhaps the most critical factors, Wnt9b and Wnt4, function during the early phase when the cap mesenchyme is induced to undergo morphogenesis into a nephron. Wnt11 controls early ureteric bud branching and contributes to the final kidney size. In addition to its inductive role, later on Wnt9b plays a significant role in the convergent extension of the tubular epithelial cells, while Wnt4 signaling controls smooth muscle cell fates in the medulla. Wnt7b has a specific function together with its likely antagonist Dkk1 in controlling the morphogenesis of the renal medulla. The signal-transduction mechanisms of the Wnts in kidney ontogeny have not been resolved, but studies characterizing the downstream signaling pathways are emerging. Aberrant Wnt signaling may lead to kidney diseases ranging from fatal kidney agenesis to more benign phenotypes. Wnt-mediated signaling regulates several critical aspects of kidney development from the early inductive stages to later steps of tubular epithelial maturation.
URLPMID:21852398 [本文引用: 1]

Abstract The primary excretory organ in vertebrates is the kidney, which is responsible for blood filtration, solute homeostasis and pH balance. These functions are carried out by specialized epithelial cells organized into tubules called nephrons. Each of these cell types arise during embryonic development from a mesenchymal stem cell pool through a process of mesenchymal-to-epithelial transition (MET) that requires sequential action of specific Wnt signals. Induction by Wnt9b directs cells to exit the stem cell niche and express Wnt4, which is both necessary and sufficient for the formation of epithelia. Without either factor, MET fails, nephrons do not form and newborn mice die owing to kidney failure. Ectopic Notch activation in stem cells induces mass differentiation and exhaustion of the stem cell pool. To investigate whether this reflected an interaction between Notch and Wnt, we employed a novel gene manipulation strategy in cultured embryonic kidneys. We show that Notch activation is capable of inducing MET in the absence of both Wnt4 and Wnt9b. Following MET, the presence of Notch directs cells primarily to the proximal tubule fate. Only nephron stem cells have the ability to undergo MET in response to Wnt or Notch, as activation in the closely related stromal mesenchyme has no inductive effect. These data demonstrate that stem cells for renal epithelia are uniquely poised to undergo MET, and that Notch activation can replace key inductive Wnt signals in this process. After MET, Notch provides an instructive signal directing cells towards the proximal tubule lineage at the expense of other renal epithelial fates.
URLPMID:2783241 [本文引用: 3]

Abstract Epithelial branching morphogenesis is critical to the formation of various organs such as the vasculature, mammary glands, lungs, and kidneys in vertebrate embryos. One fascinating aspect of branching morphogenesis is to understand how a simple epithelial tube grows by reiterative branching to form a complex epithelial tree structure. Recent studies combining mouse genetics and chimeric analysis with live imaging have uncovered the molecular networks and interactions that govern kidney branching morphogenesis. This review focuses on ureteric bud (UB) formation and epithelial branching during kidney development. The invasion of the metanephric mesenchyme by the UB is a fundamental step toward establishing the cyto-architecture of the kidney and determining the number of nephrons, which form the filtration units of the adult kidney.
[本文引用: 1]
URLPMID:16221173 [本文引用: 3]

Abstract Notch genes encode transmembrane receptors that mediate intercellular interaction by binding to the ligands on the adjacent cells. Due to early embryonic lethality in mice deficient for some Notch pathway genes, the role of Notch signaling for kidney development has not yet been defined. Using an antibody specific to the N-terminal end of gamma-secretase-cleaved Notch 1, we found evidence for Notch 1 activation in the comma-shaped and S-shaped bodies. We therefore cultured embryonic (E) day E12.5 mouse metanephroi in the presence of a gamma-secretase inhibitor, N-S-phenyl-glycine-t-butyl ester (DAPT), to block Notch signaling. Fewer renal epithelial structures were observed, with a severe deficiency in proximal tubules and glomerular podocytes. Distal tubules were present but at a reduced number, and this was accompanied by an increase in intervening, nonepithelial cells. By culturing day E14.5 metanephroi, we observed the formation of podocyte clusters after 3 days of DAPT treatment. These observations suggest that gamma-secretase activity, probably through activation of Notch, is not essential for podocyte formation beyond the stage of S-shaped body but is required for the proximal tubule and podocyte fates when S-shaped bodies are forming.
URLPMID:12930775 [本文引用: 2]

Mammalian presenilins consist of two highly homologous proteins, PSEN1 and PSEN2, which share redundant activities in Notch processing and signaling. To bypass the early lethality of the Psen1- and Psen2-double (PSEN) null embryos, we used a human PSEN1 transgene to rescue the somite patterning defects in PSEN-null animals and to allow a determination of the function of presenilins in late embryogenesis. We report here that expression of the human PSEN1 transgene supported the survival of PSEN-null embryos to the perinatal stage. However, presenilin deficiency in the kidney led to severe nephrogenesis defects and virtually no comma- or S-shaped bodies, or mature glomeruli were formed. We document that the mesenchyme was induced which could further progress to renal vesicles in the PSEN-null kidney, indicating that the presenilins are not essential for the inductive interactions and mesenchyme to epithelium transition. However, renal vesicles failed to pattern to form proximal tubules and glomerular epithelium. A presenilin-dependent, signaling-competent form of Notch1 was detected in mesenchymal derivatives but not in the ureteric buds of wild-type mice. Consistent with an obligatory role of presenilins in Notch processing and activation, the active form of Notch1 and its downstream target Hesr1 were absent in the PSEN-null kidney. Importantly, sustained Notch1 signaling was required for the maintenance of Notch ligand Jag1 expression. These results identify presenilins as one determinant of renal vesicle patterning in the developing mouse kidney, and we hypothesize that they act through the Notch signaling pathway.
[本文引用: 1]
URLPMID:24526233 [本文引用: 1]

Activation of Notch1 and Notch2 has been recently implicated in human glomerular diseases. Here we show that Notch2 prevents podocyte loss and nephrosis. Administration of a Notch2 agonistic monoclonal antibody ameliorates proteinuria and glomerulosclerosis in a mouse model of nephrosis and focal segmental glomerulosclerosis. In vitro, the specific knockdown of Notch2 increases apoptosis in damaged podocytes, while Notch2 agonistic antibodies enhance activation of Akt and protect damaged podocytes from apoptosis. Treatment with triciribine, an inhibitor of Akt pathway, abolishes the protective effect of the Notch2 agonistic antibody. We find a positive linear correlation between the number of podocytes expressing activated Notch2 and the number of residual podocytes in human nephrotic specimens. Hence, specific activation of Notch2 rescues damaged podocytes and activating Notch2 may represent a novel clinical strategy for the amelioration of nephrosis and glomerulosclerosis.
URLPMID:2789488 [本文引用: 1]

Abnormalities of kidney and urinary tract development are the most common cause of end-stage kidney failure in childhood in the United States. Over the past 20 years, the advent of mutant and transgenic mice and the manipulation of gene expression in other animal models has resulted in major advances in identification of the cellular and molecular mechanisms that direct kidney morphogenesis, providing insights into the pathophysiology of renal and urologic anomalies. This review focuses on the molecular mechanisms that define kidney progenitor cell populations, induce nephron formation within the metanephric mesenchyme, initiate and organize ureteric bud branching, and participate in terminal differentiation of the nephron. Highlighted are common signaling pathways that function at multiple stages during kidney development, including signaling via Wnts , bone morphogenic proteins , fibroblast growth factor , sonic hedgehog , RET/glial cell鈥揹erived neurotrophic factor , and notch pathways. Also emphasized are the roles of transcription factors Odd1 , Eya1 , Pax2 , Lim1 , and WT-1 in directing renal development. Areas requiring future investigation include the factors that modulate signaling pathways to provide temporal and site-specific effects. The evolution of our understanding of the cellular and molecular mechanisms of kidney development may provide methods for improved diagnosis of renal anomalies and, hopefully, targets for intervention for this common cause of childhood end-stage kidney disease.
URLPMID:18046313 [本文引用: 1]

Abstract Nephrin, a major component of the glomerular slit diaphragm (SD), is both a structural protein as well as a signaling molecule influencing foot process (FP) formation and maintenance of podocyte integrity. Analyses of near-term embryonic kidneys showed normal cellular viability and no apoptosis in glomeruli from nephrin knockout mice. Moreover, expression and location of other SD or glomerular basement membrane components were similar in wild-type and mutant mice as was the location and levels of most podocyte-specific proteins. Transcriptional profiling showed that the lack of nephrin had minor impact on the expression of genes for FPs and SD proteins. Claudin 3, a tight-junction protein normally absent in glomeruli, was upregulated threefold in the knockout mice, suggesting a role of nephrin in claudin 3 gene expression within the glomeruli. Our results suggest that nephrin is expressed late in the process of podocyte differentiation and is a locus for the formation of SD and FP maintenance and physical integrity in vivo. Nephrin does not seem to have a primary role in cell survival but has a small impact on gene regulation during glomerular development.
URLPMID:23920104 [本文引用: 2]

Recent reports suggest that low birthweight (LBW) is a risk factor for kidney diseases, including focal segmental glomerulosclerosis (FSGS), although the underlying pathological mechanism remains unknown. Podocyte loss triggers glomerulosclerosis; however, whether FSGS in LBW children is associated with podocytopenia is unclear.We reviewed the birthweights and gestational age of all patients who underwent renal biopsies from 1995 to 2011 at our Institute. Sixteen patients had FSGS, of which 6 (37.5%) had LBW; this LBW rate was significantly higher than the overall LBW rate in Japan (9.7%). The incidence of LBW was also high in patients with minimal change nephrotic syndrome (MCNS; 12.5%). The glomerular cell numbers in biopsy sections were calculated using computer image analysis and compared with FSGS of normal birthweight (NBW-FSGS). Biopsy specimens from age-matched patients with MCNS were also compared. Wilms' tumor-1 (WT1) immunohistochemistry was performed to enumerate the podocytes.All patients in the LBW-FSGS group were also preterm, with an average gestational age of 25.8 weeks. The number of podocytes per glomerulus in the LBW-FSGS patients was 34 and 24% lower as compared to that in the MCNS patients (p < 0.01) and the NBW-FSGS patients (p < 0.05), respectively. Similar results were observed for the WT1-positive glomerular cell number.LBW and premature birth were associated with FSGS development. The possibility that LBW and premature birth may be predisposing factors for severe podocytopenia in children with FSGS warrants further investigation.
URLPMID:25986755 [本文引用: 1]

However, whether PCE could induce glomerulosclerosis and its underlying mechanisms remain unknown. This study aimed to demonstrate the induction to glomerulosclerosis in adult offspring by PCE and its intrauterine programming mechanisms. A rat model of IUGR was established by PCE, male fetuses and adult offspring at the age of postnatal week 24 were euthanized. The results revealed that the adult offspring kidneys in the PCE group exhibited glomerulosclerosis as well as interstitial fibrosis, accompanied by elevated levels of serum creatinine and urine protein. Renal angiotensin II receptor type 2 (AT 2 R) gene expression in adult offspring was reduced by PCE, whereas the renal angiotensin II receptor type 1a (AT 1a R)/AT 2 R expression ratio was increased. The fetal kidneys in the PCE group displayed an enlarged Bowman's space and a shrunken glomerular tuft, accompanied by a reduced cortex width and an increase in the nephrogenic zone/cortical zone ratio. Observation by electronic microscope revealed structural damage of podocytes; the reduced expression level of podocyte marker genes, nephrin and podocin, was also detected by q-PCR. Moreover, AT 2 R gene and protein expressions in fetal kidneys were inhibited by PCE, associated with the repression of the gene expression of glial-cell-line-derived neurotrophic factor (GDNF)/tyrosine kinase receptor (c-Ret) signaling pathway. These results demonstrated that PCE could induce dysplasia of fetal kidneys as well as glomerulosclerosis of adult offspring, and the low functional programming of renal AT 2 R might mediate the developmental origin of adult glomerulosclerosis.
URL [本文引用: 1]

This study aimed at investigating whether prenatal nicotine exposure (PNE) could disrupt fetal kidney development and then induce glomerulosclerosis in adult offspring, and to explore its underlying mechanisms. A rat model of intrauterine growth retardation (IUGR) was established by subcutaneous injection with nicotine (1 mg kg 1) to pregnant rats twice a day from gestational day (GD) 9 to GD20. Male fetuses and adult offspring were sacrificed at GD20 and postnatal week 24 (PW24) respectively, and blood and kidneys were collected for analysis. The results indicated that, in the PNE fetuses, the widths of the renal cortical zone and the nephrogenic zone were both significantly reduced. Meanwhile, some glomeruli presented a dilated Bowman's space and a shrunken glomerular tuft, accompanied by the ultrastructural changes, such as extensive fusion and effacement of podocyte foot processes as well as swollen and vacuolar degeneration of the mitochondria. Moreover, in fetal kidneys, the mRNA and protein expression of AT1R and AT2R were both inhibited by PNE, associated with the repression of glial cell line derived neurotrophic factor/c-Ret tyrosine kinase receptor (GDNF/c-Ret) signaling pathway-related genes and GDNF protein. In adult offspring of the PNE group, the body weight and kidney weight were decreased, whereas the levels of serum creatinine and urea nitrogen were increased or exhibited an increasing trend. The histological examinations revealed glomerular sclerosis, and q-PCR analysis indicated a marked alternation of renal AT1aR mRNA abundance as well as the AT1aR/AT2R ratio. These results suggested that PNE could induce fetal renal dysplasia associated with the suppression of the GDNF/c-Ret pathway and adult glomerulosclerosis in male offspring, and the dysregulation of renal ATR might be involved in the underlying mechanisms.
URLPMID:22677342 [本文引用: 1]

Di-(2-ethylhexyl)phthalate (DEHP) is a widely used industrial plasticizer to which humans are widely exposed. We investigated the consequences of maternal exposure to DEHP on nephron formation, examined the programming of renal function and blood pressure and explored the mechanism in offspring. Maternal rats were treated with vehicle, 0.25 and 6.25 mg/kg body weight/day DEHP respectively from gestation day 0 to postnatal day 21. Maternal DEHP exposure resulted in lower number of nephrons, higher glomerular volume and smaller Bowman's capsule in the DEHP-treated offspring at weaning, as well as glomerulosclerosis, interstitial fibrosis and effacement of podocyte foot processes in adulthood. In the DEHP-treated offspring, the renal function was lower and the blood pressure was higher. The renal protein expression of renin and angiotensin II was reduced at birth day and increased at weaning. Maternal DEHP exposure also led to reduced mRNA expression of some renal development involved genes at birth day, including Foxd1 , Gdnf , Pax2 and Wnt11 . While, the mRNA expression of some genes was raised, including Bmp4 , Cdh11 , Calm1 and Ywhab . These data show that maternal DEHP exposure impairs the offspring renal development, resulting in a nephron deficit, and subsequently elevated blood pressure later in life. Our findings suggest that DEHP exposure in developmental periods may affect the development of nephrons and adult renal disease through inhibition of the reninngiotensin system.
[本文引用: 1]
URLPMID:19103681 [本文引用: 1]

Abstract Significant alterations in maternal nutrition may induce long-term metabolic consequences in offspring, in particular obesity and leptin and insulin resistance. Although maternal nutrient deprivation has been well characterized in this context, there is a relative paucity of data on how high fat (HF) nutrition impacts on the subsequent generation. The present study investigated the effects of maternal HF nutrition either throughout the mother's life up to and including pregnancy and lactation or HF nutrition restricted to pregnancy and lactation, on growth and metabolic parameters in male and female offspring. Virgin Wistar rats were assigned to one of three experimental groups: (1) controls (Cont): dams fed a standard chow diet throughout their life and throughout pregnancy and lactation; (2) maternal high fat (MHF) group: dams fed a HF diet from weaning up to and throughout pregnancy and lactation; and (3) pregnancy and lactation high fat (PLHF): dams fed a chow diet through their life until conception and then fed a HF diet throughout pregnancy and lactation. At weaning, all offspring were fed either a chow or HF diet for the remainder of the study (160 days). Litter size and sex ratios were not significantly different between the groups. MHF and PLHF offspring had significantly lower body weights and were hypoleptinaemic and hypoinsulinaemic at birth compared to Cont offspring. As adults however, chow-fed MHF and PLHF offspring were significantly more obese than Cont offspring (DEXA scanning at day 150, P < 0.001 for maternal HF diet). As expected a postweaning HF diet resulted in increased adiposity in all groups; MHF and PLHF offspring, however, always remained significantly more obese than Cont offspring. Increased adiposity in MHF and PLHF offspring was paralleled by hyperinsulinaemia and hyperleptinaemia (P < 0.001; MHF and PLHF versus Cont). It is of interest that a lifetime of HF nutrition produced a similar offspring phenotype to HF nutrition restricted to pregnancy and lactation alone, thus suggesting that the postnatal sequelae of maternal HF nutrition occurs independent of preconceptional diet. These data further reinforce the importance of maternal nutrition during these critical windows of development and show that maternal HF feeding can induce a markedly obese phenotype in male and female offspring completely independent of postnatal nutrition.
URLPMID:15308487 [本文引用: 1]

Abstract Epidemiological and animal studies suggest that diet-induced epigenetic modifications in early life can contribute to development of the metabolic syndrome in adulthood. We previously reported features of the metabolic syndrome in adult offspring of rats fed a diet rich in animal fat during pregnancy and suckling. We now report a study to compare the relative effects of high-fat feeding during 1) pregnancy and 2) the suckling period in the development of these disorders. As observed previously, 6-mo-old female offspring of fat-fed dams suckled by the same fat-fed dams (OHF) demonstrated raised blood pressure, despite being fed a balanced diet from weaning. Female offspring of fat-fed dams "cross fostered" to dams consuming a control diet during suckling (OHF/C) demonstrated raised blood pressure compared with controls (OC) [systolic blood pressure (SBP; mmHg) means +/- SE: OHF/C, 132.5 +/- 3.0, n = 6 vs. OC, 119.0 +/- 3.8, n = 7, P < 0.05]. Female offspring of controls cross fostered to dams consuming the fat diet (OC/HF) were also hypertensive [SBP (mmHg) 131.0 +/- 2.5 mmHg, n = 6 vs. OC, P < 0.05]. Endothelium-dependent relaxation (EDR) of male and female OHF and OHF/C mesenteric small arteries was similar and blunted compared with OC (P < 0.001). OC/HF arteries showed profoundly impaired EDR (OC/HF vs. OHF, P < 0.001). OHF/C and OC/HF demonstrated hyperinsulinemia and increased adiposity. Features of the metabolic syndrome in adult offspring of fat-fed rats can be acquired both antenatally and during suckling. However, exposure during pregnancy confers adaptive protection against endothelial dysfunction induced by maternal fat feeding during suckling.
URLPMID:19750488 [本文引用: 1]

Abstract BACKGROUND: The developmental environment is thought to determine, in part, lifelong metabolic parameters and risk of adult disease. Effects of maternal malnutrition on fetal growth have been studied extensively, and the role of poor prenatal diet in elevating lifelong risk of cardiovascular and metabolic disease has been well characterized (www.thebarkertheory.com). However, the contribution of gestational high saturated fat diet (HFD) to adult-onset metabolic disease and skeletal dysfunction has only recently been recognized, and as such is incompletely understood. METHODS: The present study evaluates the pathophysiologic mechanisms linking gestational HFD (approximating the macronutrient content of fast food) and elevated oxidative stress (OS) to adult-onset skeletal, cardiovascular, and metabolic dysfunction. RESULTS: Results of this study demonstrate that adult offspring of dams fed HFD during pregnancy exhibited adult hyperglycemia, insulin resistance, obesity, and hypertension, despite being fed healthy standard rodent chow throughout postnatal life. These offspring also showed significantly lower femoral epiphyseal average bone mineral density (ABMD) at 6 months of age, and dysregulation of distal femoral trabecular architecture at 12 months of age, characteristic of osteoporosis. Incidence of these adult-onset adverse skeletal and metabolic effects was reduced by supplementing the pregnant dam with the antioxidant (quercetin, Q) during pregnancy. CONCLUSIONS: Collectively, these data suggest that offspring of dams who consume a diet rich in saturated fats during pregnancy are at increased risk of adult-onset chronic disease. Additionally, these chronic diseases were determined to be in-part OS-mediated, and preventable by increasing a prenatal dietary antioxidant; this knowledge offers both a putative mechanism of disease pathogenesis and suggests a potential preventive strategy. (c) 2009 Wiley-Liss, Inc.
URLPMID:23255587 [本文引用: 2]

Abstract Accumulating evidence suggests that both an adverse prenatal and early postnatal environment increase susceptibility to renal and metabolic dysfunction later in life; however, whether exposure to adverse conditions during both prenatal and postnatal development act synergistically to potentiate the severity of renal and metabolic injury remains unknown. Sprague-Dawley rats were fed either a standard diet or a diet high in fat/fructose throughout pregnancy and lactation. After being weaned, female offspring were randomized to either standard diet or the high-fat/high-fructose diet, resulting in the following treatment groups: NF-NF, offspring of mothers fed a standard diet and fed a standard diet postnatally; NF-HF, offspring of mothers fed a standard diet and fed a high-fat/fructose diet postnatally; HF-NF, offspring of mothers fed a high-fat/fructose diet and fed a standard diet postnatally; HF-HF, offspring of mothers fed a high-fat/fructose diet and fed a high-fat/fructose diet postnatally. At the time of euthanasia (17 wk of age), HF-HF offspring weighed 30% more and had 110% more visceral fat than NF-NF offspring. The HF-HF offspring also had elevated blood glucose levels, glucose intolerance, 286% increase in urine albumin excretion, and 60% increase in glomerulosclerosis compared with NF-NF. In addition, HF-HF offspring exhibited a 100% increase in transforming growth factor-尾 protein expression and 116% increase in the abundance of infiltrated macrophages compared with the NF-NF offspring. These observations suggest that high-fat/fructose feeding during prenatal and throughout postnatal life increases the susceptibility to renal and metabolic injury later in life.
URLPMID:22160775 [本文引用: 2]

Overnutrition during pre- and postnatal development both confer increased susceptibility to renal and metabolic risks later in life; however, whether they have an additive effect on the severity of renal and metabolic injury remains unknown. The present study tested the hypothesis that a combination of a pre- and postnatal diet high in fat/fructose would exacerbate renal and metabolic injury in male offspring later in life. Male offspring born to high fat/high-fructose-fed mothers and fed a high-fat/high-fructose diet postnatally (HF-HF) had increased urine albumin excretion (450%), glomerulosclerosis (190%), and tubulointerstitial fibrosis (101%) compared with offspring born to mothers fed a standard diet and fed a standard diet postnatally (NF-NF). No changes in blood pressure or glomerular filtration were observed between any of the treatment groups. The HF-HF offspring weighed 鈭23% more than offspring born to mothers fed a high-fat/high-fructose diet and fed a normal diet postnatally (HF-NF), as well as offspring born to mothers fed a standard diet regardless of their postnatal diet. The HF-HF rats also had increased (and more variable) blood glucose levels over 12 wk of being fed a high-fat/high-fructose diet. A combination of exposure to a high-fat/high-fructose diet in utero and postnatally increased plasma insulin levels by 140% compared with NF-NF offspring. Our data suggest that the combined exposure to overnutrition during fetal development and early postnatal development potentiate the susceptibility to renal and metabolic disturbances later in life.
URLPMID:20453715 [本文引用: 2]

Abstract Poor fetal growth is linked with long-term detrimental effects on health in late life. We have previously shown that maternal protein restriction leads to hypertension and a reduced number of glomeruli in adult offspring. The aim of this study was to investigate the influence of a postnatal high-protein (HP) diet on renal development and renal function in rats subjected to a low-protein (LP) diet in fetal life. Sprague-Dawley rats were fed an LP diet throughout pregnancy. Male pups were given either a normal-protein (NP) diet (LP/NP) or HP diet (LP/HP), and normal male pups as control (NP/NP). At 12 wk, LP/HP offspring displayed no increase in glomerular number but showed elevated blood pressure and proteinuria compared with the LP/NP group. There was minimal fusion of foot processes in LP/NP rats compared with a moderate fusion of foot processes and hyperplasia of mesangial cells in LP/HP rats. Renal desmin mRNA levels were elevated in both LP/NP and LP/HP groups but more significantly in the LP/HP group. This study suggests that postnatal HP diet amplifies the renal damage induced by fetal under-nutrition. Podocyte injury may be one of the mechanisms by which fetal protein restriction leads to proteinuria.
URLPMID:17229764 [本文引用: 2]

The Notch pathway regulates cell fate determination in numerous developmental processes. Here we report that Notch2 acts non-redundantly to control the processes of nephron segmentation through an Rbp-J-dependent process. Notch1 and Notch2 are detected in the early renal vesicle. Genetic analysis reveals that only Notch2 is required for the differentiation of proximal nephron structures (podocytes and proximal convoluted tubules) despite the presence of activated Notch1 in the nuclei of putative proximal progenitors. The inability of endogenous Notch1 to compensate for Notch2 deficiency may reflect sub-threshold Notch1 levels in the nucleus. In line with this view, forced expression of a gamma-secretase-independent form of Notch1 intracellular domain drives the specification of proximal fates where all endogenous, ligand-dependent Notch signaling is blocked by a gamma-secretase inhibitor. These results establish distinct (non-redundant), instructive roles for Notch receptors in nephron segmentation.
[本文引用: 1]
URLPMID:23463313 [本文引用: 1]

Cell identity is determined by specific gene expression patterns that are conveyed by interactions between transcription factors and DNA in the context of chromatin. In development, epigenetic modifiers are thought to stabilize gene expression and ensure that patterns of DNA methylation and histone modification are reinstated in cells as they divide. Global erasure of epigenetic marks occurs naturally at two stages in the mammalian life cycle, but it can also be artificially engineered using a variety of reprogramming strategies. Here we review some of the recent advances in understanding how epigenetic remodeling contributes to conversion of cell fate in vivo and in vitro. We summarize current models of epigenetic erasure and discuss the various enzymes and mechanisms that may operate in cellular reprogramming.
URL [本文引用: 1]

胎盘介于胎儿与母体之间,是维持胎儿宫内生长发育的重要器官.在胎盘的正常发育过程中,子宫正常蜕膜化、滋养层细胞粘附与侵袭、胎盘血管生成与形成、胎盘印记基因表达都受到表观遗传修饰(如DNA甲基化、组蛋白修饰、非编码RNA等)的调控.研究已经证实环境因素如重金属、化合物、现代辅助生殖技术、营养物质均可导致胎盘上多种基因的表观遗传修饰异常.此外,胎盘基因表达存在性别差异也可能与表观遗传修饰有关.目前,在临床上可运用产前DNA甲基化水平分析技术检测异常的表观遗传修饰,并在疾病早期发现并做出诊断,从而为疾病预防及治疗提供依据.本文对胎盘正常发育过程中表观遗传修饰的调控及环境因素所致的胎盘基因表观遗传改变进行了综述,以期对胎盘相关疾病的诊断与治疗提供借鉴和参考.
URL [本文引用: 1]

胎盘介于胎儿与母体之间,是维持胎儿宫内生长发育的重要器官.在胎盘的正常发育过程中,子宫正常蜕膜化、滋养层细胞粘附与侵袭、胎盘血管生成与形成、胎盘印记基因表达都受到表观遗传修饰(如DNA甲基化、组蛋白修饰、非编码RNA等)的调控.研究已经证实环境因素如重金属、化合物、现代辅助生殖技术、营养物质均可导致胎盘上多种基因的表观遗传修饰异常.此外,胎盘基因表达存在性别差异也可能与表观遗传修饰有关.目前,在临床上可运用产前DNA甲基化水平分析技术检测异常的表观遗传修饰,并在疾病早期发现并做出诊断,从而为疾病预防及治疗提供依据.本文对胎盘正常发育过程中表观遗传修饰的调控及环境因素所致的胎盘基因表观遗传改变进行了综述,以期对胎盘相关疾病的诊断与治疗提供借鉴和参考.
Magsci [本文引用: 1]

在真核生物中, DNA甲基化是一种非常重要的表观遗传学标记, 能影响染色质的结构和基因的表达。随着全基因组甲基化测序的发展, 全基因组范围内的DNA甲基化水平得以了解。文章概述了基因组中启动子、基因本体、增强子、沉默子和转座子等不同元件的DNA甲基化的研究进展, 以及DNA甲基化与基因表达调控间的关系。启动子的DNA甲基化对基因的表达有抑制作用, 而基因本体的DNA甲基化与基因的表达关系因物种或细胞类型不同而异。增强子的DNA甲基化状态与基因活性呈反比关系, 沉默子则相反呈正相关。转座子的DNA高度甲基化抑制其转座活性, 从而维持基因组的稳定性。文章还探讨了DNA甲基化与组蛋白甲基化间的相互作用及其对基因表达、可变剪切、转录的调控作用, 以及本领域的未来研究方向。
Magsci [本文引用: 1]

在真核生物中, DNA甲基化是一种非常重要的表观遗传学标记, 能影响染色质的结构和基因的表达。随着全基因组甲基化测序的发展, 全基因组范围内的DNA甲基化水平得以了解。文章概述了基因组中启动子、基因本体、增强子、沉默子和转座子等不同元件的DNA甲基化的研究进展, 以及DNA甲基化与基因表达调控间的关系。启动子的DNA甲基化对基因的表达有抑制作用, 而基因本体的DNA甲基化与基因的表达关系因物种或细胞类型不同而异。增强子的DNA甲基化状态与基因活性呈反比关系, 沉默子则相反呈正相关。转座子的DNA高度甲基化抑制其转座活性, 从而维持基因组的稳定性。文章还探讨了DNA甲基化与组蛋白甲基化间的相互作用及其对基因表达、可变剪切、转录的调控作用, 以及本领域的未来研究方向。
[本文引用: 2]
URLPMID:26344726 [本文引用: 2]

Chronic hyperglycemia early in the course of diabetes confers a sustained increase in the risk of complications development. In recent years, efforts to understand the molecular basis for this 鈥渕etabolic memory鈥 have focused on epigenetic mechanisms as a means by which transient high glucose can cause persistent and propagated changes in cell function. For instance, in vascular endothelial cells, smooth muscle cells and peripheral blood cells, temporary exposure to high glucose causes changes in epigenetic marks that promote a shift towards a pro-inflammatory phenotype. However, the influence of epigenetic processes in complications development extends beyond their contribution to metabolic memory. Podocytes, for example, are terminally differentiated cells of the renal glomerulus whose injury is a major contributor to the pathogenesis of nephropathy. Over recent months, several reports have emerged describing the essential actions of histone-modifying enzymes and DNA methylation patterns (the two principal epigenetic mechanisms) in maintaining podocyte integrity, especially under diabetic conditions. Here, we review the known and potential role of epigenetic processes within podocytes, focusing on the evidence linking these processes to oxidative stress, crosstalk with tubule cells, autophagy and slit-pore protein expression. Whether podocytes themselves exhibit a metabolic memory awaits to be seen.
URLPMID:26108068 [本文引用: 2]

Proteinuria is a central component of chronic kidney disease and an independent risk factor for cardiovascular disease. Kidney podocytes have an essential role as a filtration barrier against proteinuria. Kruppel-like Factor 4 (KLF4) is expressed in podocytes and decreased in glomerular diseases leading to methylation of the nephrin promoter, decreased nephrin expression and proteinuria. Treatment with an angiotensin receptor blocker (ARB) reduced methylation of the nephrin promoter in murine glomeruli of an adriamycin nephropathy model with recovery of KLF4 expression and a decrease in albuminuria. In podocyte-specific KLF4 knockout mice, the effect of ARB on albuminuria and the nephrin promoter methylation was attenuated. In cultured human podocytes, angiotensin II reduced KLF4 expression and caused methylation of the nephrin promoter with decreased nephrin expression. In patients, nephrin promoter methylation was increased in proteinuric kidney diseases with decreased KLF4 and nephrin expression. KLF4 expression in ARB-treated patients was higher in patients with than without ARB treatment. Thus, angiotensin II can modulate epigenetic regulation in podocytes and ARB inhibits these actions in part via KLF4 in proteinuric kidney diseases. This study provides a new concept that renin鈥揳ngiotensin system blockade can exert therapeutic effects through epigenetic modulation of the kidney gene expression.
URLPMID:4089466 [本文引用: 3]

The transcription factor Kruppel-like factor 4 (KLF4) has the ability, along with other factors, to reprogram somatic cells into induced pluripotent stem (iPS) cells. Here, we determined that KLF4 is expressed in kidney glomerular podocytes and is decreased in both animal models and humans exhibiting a proteinuric. Transient restoration of KLF4 expression in podocytes of diseased glomeruli in vivo, either by gene transfer or transgenic expression, resulted in a sustained increase in nephrin expression and a decrease in albuminuria. In mice harboring podocyte-specific deletion of Klf4, adriamycin-induced proteinuria was substantially exacerbated, although these animals displayed minimal phenotypical changes prior to adriamycin administration. KLF4 overexpression in cultured human podocytes increased expression of nephrin and other epithelial markers and reduced mesenchymal gene expression. DNA methylation profiling and bisulfite genomic sequencing revealed that KLF4 expression reduced methylation at the nephrin promoter and the promoters of other epithelial markers; however, methylation was increased at the promoters of genes encoding mesenchymal markers, suggesting selective epigenetic regulation of podocyte gene expression. Together, these results suggest that KLF4 epigenetically modulates podocyte phenotype and function and that the podocyte epigenome can be targeted for direct intervention and reduction of proteinuria.
URLPMID:21904677 [本文引用: 1]

Nephrotic syndrome is an heterogeneous disease characterized by increased permeability of the glomerular filtration barrier for macromolecules. Podocytes, the visceral epithelial cells of glomerulus, play critical role in ultrafiltration of plasma and are involved in a wide number of inherited and acquired glomerular diseases. The identification of mutations in nephrin and other podocyte genes as causes of genetic forms of nephrotic syndrome has revealed new important aspects of the pathogenesis of proteinuric kidney diseases and expanded our knowledge of the glomerular biology. Moreover, a novel concept of a highly dynamic slit diaphragm proteins is emerging. The most significant discoveries in our understanding of the structure and function of the glomerular filtration barrier are reviewed in this paper.
URLPMID:24151433 [本文引用: 1]

Background: The activation of the renin-angiotensin system (RAS) and lipid disorders are major risk factors in progressive chronic kidney disease. This study aimed to investigate the potential synergistic mechanisms of RAS activation and lipid disorders that contribute to glomerulosclerosis. Materials and methods: Human renal mesangial cells (HMCs) were treated with 10(-7) mol/L angiotensin II (Ang II) or with 30 mu g/ml cholesterol and 1 mu g/ml 25-hydroxycholesterol (lipid loading) for 24 hours. Lipid accumulation in the cells was evaluated by Oil Red O staining and intracellular cholesterol quantitative assays. The gene and protein expression of molecules in the low-density lipoprotein receptor (LDLr) pathway, the RAS family, and the extracellular matrix were examined by real-time polymerase chain reaction and Western blotting. The translocation of sterol regulatory element-binding protein (SREBP) cleavage activating protein (SCAP), which escorts SREBP-2 from the endoplasmic reticulum (ER) to the Golgi, was examined by immuno-fluorescent staining. Results: Ang II increased lipid droplet accumulation in HMCs. Further analysis revealed that Ang II increased the mRNA and protein expression of LDLr, SCAP, and SREBP-2. This increase was correlated with an enhanced translocation of the SCAP/SREBP-2 complex from the ER to the Golgi in HMCs that was induced by Ang II, thereby activating LDLr gene transcription. Interestingly, lipid loading increased the mRNA and protein expression of angiotensinogen, Ang II, renin, angiotensin-converting enzyme, angiotensin II type 1 receptor, and type 2 receptor in HMCs with increased mRNA and protein expression of collagen I, alpha-smooth muscle actin, and fibronectin. Conclusions: This study demonstrates that the interaction of RAS activation and lipid disorders accelerates the progression of glomerulosclerosis.
URLPMID:4036477 [本文引用: 1]

Focal and segmental glomerulosclerosis (FSGS) is one of the most important renal diseases related to end-stage renal failure. Bradykinin has been implicated in the pathogenesis of renal inflammation, whereas the role of its receptor 2 (B2RBK; also known as BDKRB2) in FSGS has not been studied. FSGS was induced in wild-type and B2RBK-knockout mice by a single intravenous injection of Adriamycin (ADM). In order to further modulate the kinin receptors, the animals were also treated with the B2RBK antagonist HOE-140 and the B1RBK antagonist DALBK. Here, we show that the blockage of B2RBK with HOE-140 protects mice from the development of FSGS, including podocyte foot process effacement and the re-establishment of slit-diaphragm-related proteins. However, B2RBK-knockout mice were not protected from FSGS. These opposite results were due to B1RBK expression. B1RBK was upregulated after the injection of ADM and this upregulation was exacerbated in B2RBK-knockout animals. Furthermore, treatment with HOE-140 downregulated the B1RBK receptor. The blockage of B1RBK in B2RBK-knockout animals promoted FSGS regression, with a less-inflammatory phenotype. These results indicate a deleterious role of both kinin receptors in an FSGS model and suggest a possible cross-talk between them in the progression of disease.
URLPMID:25168830 [本文引用: 1]

Focal and segmental glomerulosclerosis (FSGS) represents a group of glomerular disorders, identified on kidney biopsy, that progress in the histopathologic pattern of sclerosis in parts of some glomeruli. Damage to podocytes usually marks the beginning of the disease, most evident in primary FSGS. In addition to genetic predisposition, there are many acquired causes that disturb normal podocyte homeostasis and allow for the development of FSGS. The aim of this review was to summarize recent findings of the most relevant circulating permeability factors that may serve as biomarkers of active primary idiopathic FSGS and aid in the diagnosis and prediction of recurrent FSGS after kidney transplantation.
[本文引用: 1]
URLPMID:15492947 [本文引用: 1]

Focal segmental glomerulosclerosis (FSGS) is a clinicopathologic syndrome with a substantial risk for progression to end-stage renal disease (ESRD). Recent studies of renal biopsy archives in the United States suggest that the incidence of FSGS has increased. FSGS has become the leading cause of idiopathic nephrotic syndrome in the United States, with the greatest incidence rates in the black population. In the absence of a population-based estimate of FSGS incidence, we wished to obtain a population-based estimate of incident ESRD cases caused by FSGS (FSGS ESRD) and characterize temporal changes in this group.We examined the incidence of FSGS ESRD during a 21-year period (1980 to 2000) using data from the United States Renal Data System. We excluded patients who were classified as having acquired immunodeficiency syndrome nephropathy.The annual incidence of FSGS ESRD has increased considerably, whether expressed as an absolute number or a fraction of the total incident ESRD population. Thus, the proportion of ESRD attributed to FSGS has increased 11-fold, from 0.2% in 1980 to 2.3% in 2000. The recent increase in incident FSGS ESRD cases likely is multifactorial in origin, with contributions from changes in renal biopsy practices, changes in disease classification, and a real increase in the incidence of FSGS disease. Black individuals have a 4-fold greater risk of FSGS ESRD than white or Asian individuals. The peak decade for FSGS ESRD incidence is 40 to 49 years among black subjects and 70 to 79 years among white and Asian individuals. Males have 1.5- to 2-fold greater risk than females.The incidence of FSGS ESRD has increased considerably in the United States, with black individuals at greatest risk. Idiopathic FSGS now is the most common cause of ESRD caused by primary glomerular disease in the United States in both the black and white populations.
URLPMID:16688120 [本文引用: 1]

Abstract The terminally differentiated podocyte, also called glomerular visceral epithelial cell, are highly specialized cells. They function as a critical size and charge barrier to prevent proteinuria. Podocytes are injured in diabetic and non-diabetic renal diseases. The clinical signature of podocyte injury is proteinuria, with or without loss of renal function owing to glomerulosclerosis. There is an exciting and expanding literature showing that hereditary, congenital, or acquired abnormalities in the molecular anatomy of podocytes leads to proteinuria, and at times, glomerulosclerosis. The change in podocyte shape, called effacement, is not simply a passive process following injury, but is owing to a complex interplay of proteins that comprise the molecular anatomy of the different protein domains of podocytes. These will be discussed in this review. Recent studies have also highlighted that a reduction in podocyte number directly causes proteinuria and glomerulosclerosis. This is owing to several factors, including the relative inability for these cells to proliferate, detachment, and apoptosis. The mechanisms of these events are being elucidated, and are discussed in this review. It is the hope that by delineating the events following injury to podocytes, therapies might be developed to reduce the burden of proteinuric renal diseases.
[本文引用: 1]

Nephrotic syndrome (NS) in children includes a diverse group of diseases that range from genetic diseases without any immunological defects to causes that are primarily due to immunological effects. Recent advances in molecular and genomic studies have resulted in a plethora of genetic defects that have been localized to the podocyte, the basic structure that is instrumental in normal filtration process. Although the disease can manifest from birth and into adulthood, the primary focus of this review would be to describe the novel genes and pathology of primary podocyte defects that cause NS in children. This review will restrict itself to the pathology of congenital NS, minimal change disease (MCD), and its variants and focal segmental glomerulosclerosis (FSGS). The two major types of congenital NS are Finnish type characterized by dilated sausage shaped tubules morphologically and diffuse mesangial sclerosis characterized by glomerulosclerosis. MCD has usually normal appearing biopsy features on light microscopy and needs electron microscopy for diagnosis, whereas FSGS in contrast has classic segmental sclerosing lesions identified in different portions of the glomeruli and tubular atrophy. This review summarizes the pathological characteristics of these conditions and also delves into the various genetic defects that have been described as the cause of these primary podocytopathies. Other secondary causes of NS in children, such as membranoproliferative and membranous glomerulonephritis, will not be covered in this review.
[本文引用: 1]
URLPMID:19019999 [本文引用: 1]

ABSTRACT Low birth weight (LBW), resulting from intrauterine growth retardation (IUGR) or prematurity, is a risk factor for adult hypertension and chronic kidney disease. LBW is associated with reduced nephron endowment and increased glomerular volume; however, the development of secondary focal segmental glomerulosclerosis (FSGS) has not been reported previously. The authors describe six patients with clinical and pathologic findings suggesting a secondary form of FSGS, in whom a history of prematurity and very LBW was obtained. No other known causes of secondary FSGS were identified. The cohort consisted of two women and four men with a mean age of 32 yr. Patients were born at 22 to 30 wk gestation with mean birth weight of 1054 g (range 450 to 1420 g). Mean 24-h urine protein was 3.3 g/d (range 1.3 to 6.0 g/d), mean creatinine clearance 89 cc/min (range 71 to 132 cc/min), mean creatinine 1.2 mg/dl (range 0.9 to 1.5 mg/dl), and mean serum albumin 4.1 g/dl (range 3.4 to 4.8 g/dl). No patient had full nephrotic syndrome. Renal biopsy revealed FSGS involving a minority (mean 8.8%) of glomeruli, with a predominance of perihilar lesions of sclerosis (five of six patients), glomerulomegaly (all six patients), and only mild foot process effacement (mean 32%), all features typical of postadaptive FSGS. Our findings support that very LBW and prematurity promote the development of secondary FSGS. Because birth history is often not obtained by adult nephrologists, this risk factor is likely to be underrecognized.
URLPMID:17051140 [本文引用: 1]

Intrauterine growth retardation (IUGR) aggravates the course of acute mesangioproliferative glomerulonephritis (GN) in the rat. Observational studies in children suggest that IUGR may be associated with a severe course of kidney diseases such as IgA nephropathy. We tested the hypothesis that IUGR leads to aggravation of acute mesangioproliferative GN in former IUGR rats. IUGR was induced in Wistar rats by isocaloric protein restriction in pregnant dams. Litter size was reduced to six male neonates in low protein animals (LP) and normal protein animals (NP). At 8 weeks GN was induced by injection of an anti-Thy-1.1 antibody. Rats were killed on days 4 and 14 after induction of GN and kidneys were investigated for inflammation and sclerosis using real-time polymerase chain reaction and histological methods. On day 4 after induction of GN, LP animals showed more glomerulosclerosis and tubulointerstitial lesions. On day 14, inflammatory markers (expression of monocyte chemoattractant protein 1, osteopontin, tumor necrosis factor and interleukin-6), extracellular matrix accumulation and markers of sclerosis (plasminogen activator inhibitor-1 expression, transforming growth factor- 尾 1 expression, score for glomerulosclerosis, glomerular deposition of collagen I and collagen IV) were more severe in LP animals. Some degree of induction of inflammatory and profibrotic markers was also present in non-nephritic LP animals. However, these rats did not display marked glomerulosclerosis or interstitial fibrosis. We conclude that after IUGR inflammatory damage is aggravated and the reparation of the kidney is impaired during the course of acute mesangioproliferative GN, leading to more sclerotic lesions.
[本文引用: 1]
[本文引用: 1]
URLPMID:58023325222 [本文引用: 1]

Nephrotic syndrome is a common glomerular disease in children with significant variability in both incidence and steroid responsiveness among various ethnic groups. The average incidence of nephrotic syndrome is 2鈥16.9 per 100,000 children worldwide. Understanding the variability by ethnicity may point to potential factors leading to nephrotic syndrome, which remains elusive, and may highlight factors accounting for differences in medication response. The emerging role of genetic factors associated with steroid responsive and steroid-resistant forms of nephrotic syndrome within an ethnic group can provide insight into potential biological mechanisms leading to disease. For example, among African-Americans, the risk variants inAPOL1are associated with a more than 10-fold increase in risk of focal segmental glomerulosclerosis and high-risk carriers have a twofold greater risk of progression to end-stage renal disease. Ongoing collaborative studies should consider capturing data on self-reported ethnicity to understand differences in incidence and outcomes. In the future, the availability of whole-genome data will provide an excellent opportunity for new clinical and translational research in childhood nephrotic syndrome and lead to a better understanding of the disease.
URLPMID:27617138 [本文引用: 1]

Nephrotic syndrome (NS) is a common pediatric kidney disease and is defined as massive proteinuria, hypoalbuminemia, and edema. Dysfunction of the glomerular filtration barrier, which is made up of endothelial cells, glomerular basement membrane, and visceral epithelial cells known as podocytes, is evident in children with NS. While most children have steroid-responsive nephrotic syndrome (SSNS), approximately 20% have steroid-resistant nephrotic syndrome (SRNS) and are at risk for progressive kidney dysfunction. While the cause of SSNS is still not well understood, there has been an explosion of research into the genetic causes of SRNS in the past 15 years. More than 30 proteins regulating the function of the glomerular filtration barrier have been associated with SRNS including podocyte slit diaphragm proteins, podocyte actin cytoskeletal proteins, mitochondrial proteins, adhesion and glomerular basement membrane proteins, transcription factors, and others. A genetic cause of SRNS can be found in approximately 70% of infants presenting in the first 3 months of life and 50% of infants presenting between 4 and 12 months, with much lower likelihood for older patients. Identification of the underlying genetic etiology of SRNS is important in children because it allows for counseling of other family members who may be at risk, predicts risk of recurrent disease after kidney transplant, and predicts response to immunosuppressive therapy. Correlations between genetic mutation and clinical phenotype as well as genetic risk factors for SSNS and SRNS are reviewed in this article.
[本文引用: 1]
[本文引用: 1]
URLPMID:28242136 [本文引用: 1]

Abstract Dabrafenib and trametinib, BRAF and MEK inhibitors, respectively, are effective targeted metastatic melanoma therapies, but little is known about their nephrotoxicity. Although tubulointerstitial injury has been the most widely reported renal side effect of targeted melanoma therapy, nephrotic syndrome has not been reported before. We report on a patient with metastatic melanoma who developed nephrotic syndrome during dabrafenib and trametinib treatment. Kidney biopsy showed diffuse loss of podocyte cytoarchitecture, extensive foot-process effacement, and glomerular endothelial injury. Kidney function and glomerular ultrastructural changes recovered fully after drug withdrawal. In vitro, BRAF inhibition decreased PLC蔚1 expression in podocytes, accompanied by a reduction in nephrin expression and an increase in permeability to albumin. Additionally, these drugs inhibited the podocyte-vascular endothelial growth factor (VEGF) system. In addition to implications for nephrotic syndrome pathophysiology, we suggest that patients given dabrafenib and trametinib be monitored closely for potential glomerular damage. Copyright 漏 2017 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
URLPMID:24073334 [本文引用: 2]

Cardiovascular diseases are one of the leading causes of mortality. Hypertension (HT) is one of the principal risk factors associated with death. Chronic kidney disease (CKD), which is probably underestimated, increases the risk and the severity of adverse cardiovascular events. It is now recognized that low birth weight is a risk factor for these diseases, and this relationship is amplified by a rapid catch-up growth or overfeeding during infancy or childhood. The pathophysiological and molecular mechanisms involved in the ???early programming??? of CKD are multiple and partially understood. It has been proposed that the developmental programming of arterial hypertension and chronic kidney disease is related to a reduced nephron endowment. However, this mechanism is still discussed. This review discusses the complex relationship between birth weight and nephron endowment and how early growth and nutrition influence long term HT and CKD. We hypothesize that fetal environment reduces moderately the nephron number which appears insufficient by itself to induce long term diseases. Reduced nephron number constitutes a ???factor of vulnerability??? when additional factors, in particular a rapid postnatal growth or overfeeding, promote the early onset of diseases through a complex combination of various pathophysiological pathways.
URLPMID:3791974 [本文引用: 1]

Chronic kidney disease affects millions of people worldwide and is associated with an increased morbidity and mortality as a result of kidney failure and cardiovascular disease. Accurate assessment of kidney function is important in the clinical setting as a screening tool and for monitoring disease progression and guiding prognosis. In clinical research, the development of new methods to measure kidney function accurately is important in the search for new therapeutic targets and the discovery of novel biomarkers to aid early identification of kidney injury. This review considers different methods for measuring kidney function and their contribution to the improvement of detection, monitoring and treatment of chronic kidney disease.
URLPMID:19339091 [本文引用: 1]

Existing data indicate that low birth weight is associated with subsequent risk of CKD, although there is scope for additional well-designed population-based studies with accurate assessment of birth weight and kidney function and consideration of important confounders, including maternal and socioeconomic factors.
URLPMID:25966347 [本文引用: 1]

Endothelin-1 (ET-1) is a 21-amino acid peptide with mitogenic and powerful vasoconstricting properties. Under healthy conditions, ET-1 is expressed constitutively in all cells of the glomerulus and participates in homeostasis of glomerular structure and filtration function. Under disease conditions, increases in ET-1 are critically involved in initiating and maintaining glomerular inflammation, glomerular basement membrane hypertrophy, and injury of podocytes (visceral epithelial cells), thereby promoting proteinuria and glomerulosclerosis. Here, we review the role of ET-1 in the function of glomerular endothelial cells, visceral (podocytes) and parietal epithelial cells, mesangial cells, the glomerular basement membrane, stromal cells, inflammatory cells, and mesenchymal stem cells. We also discuss molecular mechanisms by which ET-1, predominantly through activation of the ET A receptor, contributes to injury to glomerular cells, and review preclinical and clinical evidence supporting its pathogenic role in glomerular injury in chronic renal disease. Finally, the therapeutic rationale for endothelin antagonists as a new class of antiproteinuric drugs is discussed.
