 ,, 张永
,, 张永 ,, 胡景华, 王子龙, 曾志将江西农业大学蜜蜂研究所,南昌 330045
,, 胡景华, 王子龙, 曾志将江西农业大学蜜蜂研究所,南昌 330045Molecular cloning and expression analysis of the tyramine
receptor genes in Apis cerana cerana
Lizhen Zhangreceptor genes in Apis cerana cerana
 ,, Yong Zhang
,, Yong Zhang ,, Jinghua Hu, Zilong Wang, Zhijiang ZengHoneybee Research Institute, Jiangxi Agricultural University, Nanchang 330045, China
,, Jinghua Hu, Zilong Wang, Zhijiang ZengHoneybee Research Institute, Jiangxi Agricultural University, Nanchang 330045, China编委: 任军
收稿日期:2017-03-21修回日期:2017-12-12网络出版日期:2018-01-09
| 基金资助: |
Received:2017-03-21Revised:2017-12-12Online:2018-01-09
| Fund supported: |
作者简介 About authors
作者简介:张丽珍,博士,实验师,研究方向:蜜蜂行为及分子生物学E-mail:
张永,硕士研究生,专业方向:蜜蜂分子生物学E-mail:
张丽珍和张永为并列第一作者。

摘要
关键词:
Abstract
Keywords:
PDF (529KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张丽珍, 张永, 胡景华, 王子龙, 曾志将. 中华蜜蜂酪胺受体基因克隆及表达分析. 遗传[J], 2018, 40(2): 155-161 doi:10.16288/j.yczz.17-096
Lizhen Zhang, Yong Zhang, Jinghua Hu, Zilong Wang, Zhijiang Zeng.
receptor genes in Apis cerana cerana
酪胺(tyramine)属于生物多聚胺类,是昆虫中枢神经系统内重要的神经递质、神经调质和神经激素,参与调控昆虫的多种行为和生理过程,如参与调控求偶[1]、运动[2]、学习记忆[3]和繁殖[4]等。昆虫体内的酪胺相当于脊椎动物体内的去甲肾上腺素[5],其作用途径也是通过结合在细胞膜上的G蛋白偶联受体,从而激活这些受体,导致细胞内第二信使的浓度发生改变(如cAMP和Ca2+),从而引起各种生理生化反应[6,7]。
药理学和行为学研究发现,酪胺受体基因参与调控动物的学习与记忆。2000年,Kutsukake等[8]发现酪氨受体基因突变后的果蝇(Drosophila)个体经PER(proboscis extension reflex)测试后,嗅觉学习能力下降。Blenau等[6]成功克隆了西方蜜蜂(Apis mellifera)酪胺受体基因Amtyr1,发现该基因广泛分布在大脑和食管神经节中,推测该基因可能参与了蜜蜂高级认知能力的信号传导过程。2015年,袁安等[9,10]发现饲料蛋白水平和发育温度过低会抑制西方蜜蜂Amtyr1的表达并造成记忆力的下降。2017年,Scheiner等[11]用药理学方法激活酪胺受体提高了哺育蜂和采集蜂的味觉反应,并且提高了哺育蜂的奖赏学习能力。
中华蜜蜂(Apis cerana cerana)简称中蜂,是东方蜜蜂(Apis cerana)的指名亚种,也是我国饲养的重要蜂种。相比西方蜜蜂(Apis mellifera),中华蜜蜂性情温驯、抗寒、耐热、抗螨能力强、嗅觉灵敏,善于采集零星蜜源或低密度花蜜,对颜色和方向的认知能力更强[12,13,14]。中华蜜蜂养殖生产的健康发展对我国生态环境的保护和农作物产量的提高有着重要作用。但是,目前中华蜜蜂种群数量和分布区域都显著降低,有些地方甚至处于濒危状态,这对依赖蜜蜂授粉的农业造成了巨大的损失。蜂群数量的减少可能与环境的改变有关,也可能与成年工蜂学习记忆能力的下降有关[15]。
中华蜜蜂具有其他蜂种不可替代的资源优势和种质特性,但是对中华蜜蜂酪胺受体基因的研究目前还未见报道。本研究首次克隆获得中华蜜蜂酪胺受体基因Actyr1和Actyr2的全长cDNA序列,分析了这两个基因在大脑中的表达定位情况,为进一步探讨Actyr1和Actyr2对中华蜜蜂学习记忆的功能机制提供基础,为利用和保护我国的特有蜂种资源提供新思路。
1 材料和方法
1.1 材料
中华蜜蜂由江西农业大学蜜蜂研究所饲养提供,均来自同一群健康无病的蜂群。用干净的镊子抓取后足带有花粉的采集蜂,置于无RNA酶的EP管中,-80℃保存待用。1.2 方法
1.2.1 总RNA提取和反转录取20只中华蜜蜂的大脑,经研磨器处理后,利用Trizol裂解提取大脑总RNA,使用微量核酸蛋白测定仪测定RNA纯度。选择纯度合适的RNA,用反转录酶试剂盒(TaKaRa)合成cDNA,-80℃冰箱保存,备用。
1.2.2 PCR扩增及测序
参照GenBank中西方蜜蜂Amtyr1和Amtyr2的cDNA序列,利用生物学软件Primer5.0设计引物(引物序列信息见表1),扩增中华蜜蜂Amtyr1和Amtyr2基因。引物由上海生工生物工程技术服务有限公司合成。
PCR扩增体系为10 μL,包括10×Taq Buffer 1.0 μL、dNTP mixture 0.8 μL、LA-Taq DNA polymerase 0.1 μL、上下游引物各0.2 μL,cDNA 1 μL,加ddH2O至10 μL。PCR扩增条件:94℃预变性5 min;94℃变性1 min,复性(Actyr1 56℃,Actyr2 58℃) 1 min,72℃延伸2 min,30个循环;72℃再延伸10 min,4℃保存1 h。PCR产物经15%的琼脂糖凝胶进行电泳检测,胶回收试剂盒(上海普洛麦格生物公司)回收目的片段。
取胶回收产物1~3 μL与pMD 18-T载体于16℃连接反应30 min,转化到JM109大肠杆菌感受态细胞中,涂布于含有氨苄抗生素的培养基平板上,37℃倒置培养24~30 h。挑取单菌落,利用PCR扩增进行转化后阳性克隆子的鉴定,得到正确的重组子后在恒温培养箱中扩大培养,由上海英杰生物公司进行测序。
1.2.3 生物信息学分析
利用DNAstar软件中的Seqman程序进行序列拼接,获得Actyr1和Actyr2基因全长cDNA序列,并在NCBI网站上用Blast进行序列比对分析;利用ClustalX比较分析中华蜜蜂Actyr1和Actyr2基因氨
Table 1
表1
表1 引物序列信息
Table 1
| 用途 | 基因名称 | 引物序列(5°→3°) |
|---|---|---|
| PCR | Actyr1 | F:GCCAAACCAACCAGCAAATC R:CAATGGAGCGGGGGTAAAG |
| Actyr2 | F:AACAGCAACGGGATGAAGAA R: AGAAACGCACCCTGAAGAGAGT | |
| qRT-PCR | Actyr1 | F:AATCCAAGGAGTGCGAGGTG R:TGCGTGTCAATGGGAAGAAG |
| Actyr2 | F:TCTCTTCAGGGTGCGTTTCT R:GTTAACCTGCGACGTGCTCT |
新窗口打开|下载CSV
基酸序列与其他物种的序列相似性;用MEGA4.1软件采用Neighbor-Joining法构建系统进化树(3000次重抽样)。
1.2.4 qRT-PCR检测Actyr1和Actyr2在中华蜜蜂不同组织中的表达
将刚出房的工蜂用标记笔标记,取约25只同日龄采集工蜂进行组织分离,分别取其触角、头(去除触角)、胸部肌肉、腹部表皮;每种组织分成3个平行样,取样过程均在干冰上操作,取样后迅速放入液氮中,经研磨加入1 mL Trizol裂解液提取RNA。获得的各组织RNA经检测合格后,利用反转录试剂盒(TaKaRa)合成cDNA。
根据中华蜜蜂Actyr1和Actyr2基因的cDNA序列,利用软件Primer5.0设计qRT-PCR定量扩增引物(引物序列见表1),以β-actin为内参基因。
qRT-PCR扩增体系为10 μL,包括:SYBR Premix Ex Taq(2×)5 μL,上、下游引物各 0.4 μL,ROX Reference Dye(50×) 0.2 μL,ddH 2O 3 μL,cDNA 1 μL。qRT-PCR扩增条件:95℃预变性 30 s;95℃ 10 s,58.9℃ 1 min,40个循环。空白对照模板为ddH2O。每个样品进行3次重复。根据每个样品与内参基因β-actin所得的Ct值,采用2-△△Ct法进行数据分析,利用StatView软件进行方差分析。
1.2.5 原位杂交
根据中华蜜蜂Actyr1和Actyr2基因的cDNA序列,利用原位杂交试剂盒(上海罗式公司)合成酪胺受体基因寡核苷酸探针,探针序列如下:
Actyr1探针序列(总长167 bp):ATCTCTTGCTACGTCGCCTCCGGCTGTATATCAACGGTTGAGTTACAGCTAGATACCTGTCTATGGATATGGCGCATAACGATAGTATGCT
GGCAGTACAGAGAAGAATGTCGAGGGAGACCCACGAGTCGCAGAGCATCGGGCCCAACTCCCAGGTACCACCTGTA。
Actyr2探针序列(总长126 bp):ACCACGACAACGGTTTACCAATTCATCGAGGAGAGGCAGAGGATCTCGTTGTCGAAGGAGAGACGAGCCGCGAGGACAT
TGGGCGTGATAATGGGCGTGTTCGTCGTGTGCTGGCTACCGTTCTTT。
将解剖好的中华蜜蜂大脑放在4%中性甲醛固定过夜,再经过脱水、透明、浸蜡、包埋和切片处理
制作石蜡切片;将切片分别用二甲苯、无水乙醇和DEPC水脱蜡两次;经蛋白酶 K于37℃温育 30 min,杂交缓冲液约150 μL孵育 10 min,之后每张切片加入杂交液(稀释的预杂液,探针1:80稀释,10 μL/张),空白对照不含有探针,于42℃杂交18~30 h;杂交后再用5×SSC洗涤浸泡封闭,加 anti-DIG-Ap稀释液(1∶1 000),4℃轻摇过夜;加入按比例配置的NBT、BCIP和稀释液的显色液(1∶1∶250),避光显色0.5~ 4 h,洗涤终止显色,再滴加核固红复染,快速进行脱水透明,中性树胶封片,使用高清显微镜观察拍照。
2 结果与分析
2.1 中华蜜蜂酪胺受体基因的克隆及序列分析
本研究首次克隆获得了中华蜜蜂Actyr1和Actyr2全长cDNA序列。Actyr1序列全长为1241 bp (GenBank登录号:KC814693),推测编码297aa,分子量为33.458 kDa,等电点为9.80。Actyr2序列全长为1270 bp(GenBank登录号:KC814694),推测编码399aa,分子量为44.588 kDa,等电点为8.61。通过SMART(http://smart.embl-heidelberg.de/)数据库分析,Actyr1和Actyr2的氨基酸序列均包括2个结构域。根据中华蜜蜂Actyr1和Actyr2的氨基酸序列,采用MEGA4.0软件构建系统进化树,结果如图1所示。从图1A可见,中华蜜蜂与小蜜蜂(Apis florea)、西方蜜蜂聚成一支;从图1B可见,中华蜜蜂与西方蜜蜂聚成一支。
2.2 中华蜜蜂酪胺受体基因在不同组织中的表达分析
利用qRT-PCR分析了中华蜜蜂Actyr1和Actyr2基因在不同组织中的表达情况,结果见图2所示。由图2可知,Actyr1和Actyr2均在中华蜜蜂头部(去除触角)的表达量最高,显著高于触角、胸部肌肉、腹部表皮中的表达量,Actyr1在胸部肌肉中几乎不表达。2.3 中华蜜蜂酪胺受体基因在大脑中的表达定位
经地高辛原位杂交,Actyr1和 Actyr2在中华蜜蜂大脑蘑菇体的凯尼恩细胞﹑触角叶周围的细胞以及侧前桥神经纤维网细胞均有阳性着色(图3)。另外,Actyr1在髓质与小叶之间的细胞也有阳性着色。图1
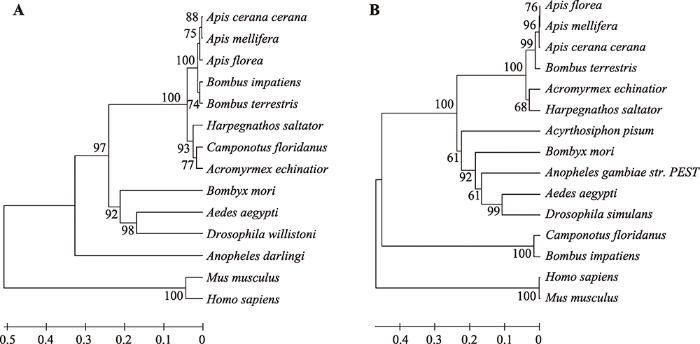 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1中华蜜蜂与其他物种Tyr1、Tyr2氨基酸序列系统进化树
A:中华蜜蜂与其他物种Tyr1系统进化树;B:中华蜜蜂与其他物种Tyr2系统进化树。
Fig. 1Phylogenetic tree analysis of the deduced amino acid sequence of Tyr1、Tyr2 from A. cerana cerana and other species
图2
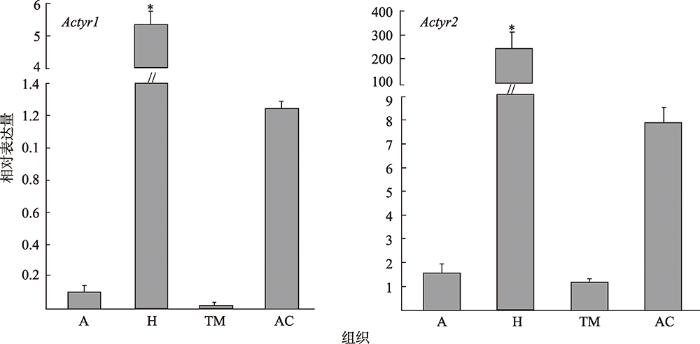 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2Actyr1和Actyr2在中华蜜蜂各组织中的相对表达量
A:触角;H:头;TM:胸部肌肉;AC:腹部表皮。数值均以平均数±SEM计算;*表示Actyr1和Actyr2在不同组织中的表达差异显著(P<0.05)。
Fig. 2Relative expression levels of Actyr1 and Actyr2 in different tissues of A. ceranacerana
图3
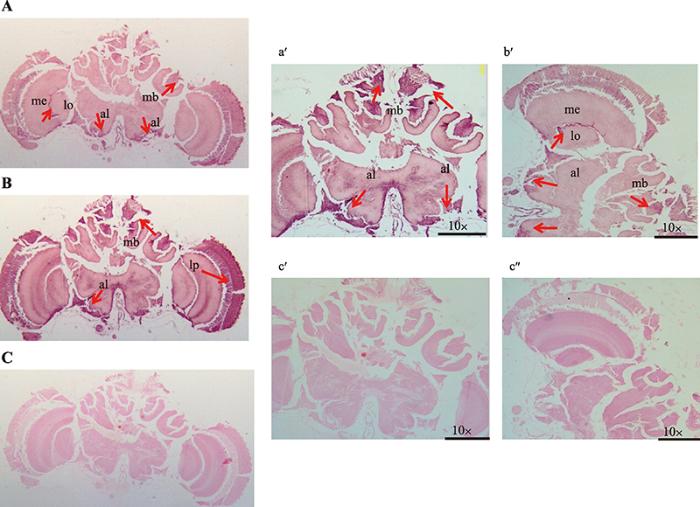 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3 Actyr1和Actyr2在中华蜜蜂大脑中的表达定位
A、B:分别表示Actyr1和Actyr2在中华蜜蜂大脑中的表达部位;a°、b°:分别表示Actyr1和Actyr2在中华蜜蜂大脑中阳性着色较强部位的放大图;C、c°、c?为空白对照。箭头表示阳性着色;mb:蘑菇体;al:触角叶;me:髓质;lo:小叶;lp:侧前桥神经纤维网。
Expression localization of Actyr1 and Actyr2 in the brain of A. ceranacerana
Fig. 3
3 讨论
本研究首次克隆获得中华蜜蜂Actyr1和Actyr2基因全长cDNA序列,其长度分别为1241 bp和1270 bp。系统进化分析结果表明,中华蜜蜂Actyr1和Actyr2氨基酸序列与西方蜜蜂序列相似性较高,且高度同源,而且Amtyr1基因受体与果蝇的Dmtyr同源[6,16]。上述结果表明,Actyr1和Actyr2基因编码酪氨受体。
研究已证实,果蝇和西方蜜蜂的酪胺受体基因主要表达在头部(去除触角)—大脑的蘑菇体,另外在果蝇的心脏肌肉和绛色细胞中特有表达[6,17]。本研究对中华蜜蜂采集蜂的触角、头、胸部肌肉、腹部表皮4个组织中Actyr1和Actyr2的表达量进行了分析,证实Actyr1和Actyr2也主要表达在中华蜜蜂的头部。另外,腹部表皮中的表达量高于触角、胸部肌肉,这可能是由于蜜蜂的心脏位于腹部,有研究表明酪胺受体基因在蜜蜂的心脏中也有表达[5,18]。
原位杂交结果表明,Actyr1和Actyr2基因在中华蜜蜂大脑的神经细胞中均有表达,如蘑菇体凯尼恩细胞、触角叶周围细胞、中心体的神经区域以及髓质和小叶之间的细胞,与西方蜜蜂大脑中的表达定位情况相似[6,19,20]。已有研究证明蘑菇体与昆虫记忆的形成、恢复、储存直接相关[21,22,23],而在触角叶退化了的古老昆虫如蜉蝣目(Ephemeroptera)、蜻蜓目(Odonata)及石蛃目(Microcoryphia)中,嗅觉记忆也几乎完全退化[24,25,26]。另外,章鱼胺受体(octopamine receptors)已被证明参与了蜜蜂感官信号的输入、触觉信号的输出以及大脑的高级认知功能[27]。章鱼胺受体与酪胺受体同属起信号传导作用的G蛋白偶联受体,且具有相似的结构和生理环境[28,29],因此,Actyr1和Actyr2基因可能参与调控了中华蜜蜂的学习记忆过程。
本研究发现Actyr1和Actyr2基因的表达谱一致,均是在头部最高,其次是在腹部表皮、触角和胸部肌肉,且它们均在蘑菇体的凯尼恩细胞和触角叶周围细胞处的阳性着色较强,可见Actyr1和Actyr2基因在大脑中的表达区域有重叠。我们推测酪胺受体基因可能在凯尼恩细胞或者触角叶周围细胞中互相作用,共同调控蜜蜂的部分生物学功能,包括学习记忆。另外,本研究首次发现Actyr1和Actyr2在中华蜜蜂腹部表皮中表达。但是Actyr1和Actyr2基因是如何共同调控蜜蜂的生物学功能,参与了哪些关键信号通路以及如何影响中华蜜蜂学习记忆还有待于深入研究。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
URLPMID:27498566 [本文引用: 1]

Abstract Neuromodulators influence the activities of collections of neurons and have profound impacts on animal behavior. Male courtship drive is complex and subject to neuromodulatory control. Using the fruit fly Drosophila melanogaster, we identified neurons in the brain (inferior posterior slope; IPS) that impact courtship drive and were controlled by tyramine-a biogenic amine related to dopamine, whose roles in most animals are enigmatic. We knocked out a tyramine-specific receptor, TyrR, which was expressed in IPS neurons. Loss of TyrR led to a striking elevation in courtship activity between males. This effect occurred only in the absence of females, as TyrR Gal4 mutant males exhibited a wild-type preference for females. Artificial hyperactivation of IPS neurons caused a large increase in male-male courtship, whereas suppression of IPS activity decreased male-female courtship. We conclude that TyrR is a receptor for tyramine, and suggest that it serves to curb high levels of courtship activity through functioning as an inhibitory neuromodulator. Copyright 漏 2016 Elsevier Ltd. All rights reserved.
[本文引用: 1]
[本文引用: 1]
URLMagsci [本文引用: 2]

<P class=MsoNormal ><FONT size=3><SPAN >章鱼胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">(octopamine, OA)</FONT></SPAN><SPAN >和酪胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">(tyramine, TA)</FONT></SPAN><SPAN >在昆虫体内扮演着各种重要的生理角色。它们协调控制着昆虫的各种器官和行为</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >如调节外周淋巴器官功能和影响昆虫的学习与记忆、昼夜节律等</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >使得昆虫能够以合理的方式来应对外界刺激</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >并被认为在功能上对应于脊椎动物体内的肾上腺素和去甲肾上腺素。虽然都是酪氨酸脱羧基产物</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >且酪胺是章鱼胺的生物合成前体</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >但它们都通过不同的</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">G</FONT></SPAN><SPAN >蛋白偶联受体在昆虫体内发挥不同的神经调控作用。近年来</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >对昆虫体内章鱼胺和酪胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >尤其是它们与对应受体作用的研究</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >日益受到关注。本文对昆虫体内章鱼胺和酪胺的生物合成</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >在神经和非神经组织中的分布</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >被突触前结构的再摄取以及它们在昆虫体内的不同生理功能等方面的研究进展进行了综述</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >特别对章鱼胺和酪胺受体基因的克隆、信号转导途径以及药理作用特性等相关研究的最新进展进行了详细评述。</SPAN></FONT></P>
URLMagsci [本文引用: 2]

<P class=MsoNormal ><FONT size=3><SPAN >章鱼胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">(octopamine, OA)</FONT></SPAN><SPAN >和酪胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">(tyramine, TA)</FONT></SPAN><SPAN >在昆虫体内扮演着各种重要的生理角色。它们协调控制着昆虫的各种器官和行为</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >如调节外周淋巴器官功能和影响昆虫的学习与记忆、昼夜节律等</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >使得昆虫能够以合理的方式来应对外界刺激</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >并被认为在功能上对应于脊椎动物体内的肾上腺素和去甲肾上腺素。虽然都是酪氨酸脱羧基产物</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >且酪胺是章鱼胺的生物合成前体</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >但它们都通过不同的</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">G</FONT></SPAN><SPAN >蛋白偶联受体在昆虫体内发挥不同的神经调控作用。近年来</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >对昆虫体内章鱼胺和酪胺</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >尤其是它们与对应受体作用的研究</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >日益受到关注。本文对昆虫体内章鱼胺和酪胺的生物合成</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >在神经和非神经组织中的分布</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >被突触前结构的再摄取以及它们在昆虫体内的不同生理功能等方面的研究进展进行了综述</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >特别对章鱼胺和酪胺受体基因的克隆、信号转导途径以及药理作用特性等相关研究的最新进展进行了详细评述。</SPAN></FONT></P>
URLPMID:10693920 [本文引用: 5]

Abstract Biogenic amine receptors are involved in the regulation and modulation of various physiological and behavioral processes in both vertebrates and invertebrates. We have cloned a member of this gene family from the CNS of the honeybee, Apis mellifera. The deduced amino acid sequence is homologous to tyramine receptors cloned from Locusta migratoria and Drosophila melanogaster as well as to an octopamine receptor cloned from Heliothis virescens. Functional properties of the honeybee receptor were studied in stably transfected human embryonic kidney 293 cells. Tyramine reduced forskolin-induced cyclic AMP production in a dose-dependent manner with an EC50 of approximately 130 nM. A similar effect of tyramine was observed in membrane homogenates of honeybee brains. Octopamine also reduced cyclic AMP production in the transfected cell line but was both less potent (EC50 of approximately 3 microM) and less efficacious than tyramine. Receptor-encoding mRNA has a wide-spread distribution in the brain and subesophageal ganglion of the honeybee, suggesting that this tyramine receptor is involved in sensory signal processing as well as in higher-order brain functions.
[本文引用: 1]
[本文引用: 1]
Magsci [本文引用: 1]

本试验旨在研究饲粮蛋白质水平对意大利蜜蜂归巢能力及记忆相关基因表达的影响。选择9群群势相当的本地意大利蜜蜂,随机分为3组,每组3群。Ⅰ组、Ⅱ组、Ⅲ组分别饲喂蛋白质水平为15%、25%、35%的试验饲粮。饲喂45 d后,测定采集蜂分别在1 000、2 000 m处的回归率及不同日龄(刚出房、10日龄、20日龄)工蜂3个记忆相关基因[谷氨酸受体A型基因(<em>GluRA</em>)、N-甲基-<em>D</em>-天冬氨酸受体1型基因(<em>Nmdar</em>1)、酪胺受体1型基因(<em>Tyr</em>1)]的表达情况。结果表明:Ⅱ组和Ⅲ组蜜蜂在1 000、2 000 m处的回归率均显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);Ⅲ组10和20日龄工蜂的<em>GluRA</em>相对表达量显著高于Ⅱ组和Ⅰ组(<em>P</em><0.05),且Ⅱ组工蜂的<em>GluRA</em>相对表达量也显著高于Ⅰ组(<em>P</em><0.05);Ⅱ组和Ⅲ组10和20日龄工蜂的<em>Nmdar</em>1相对表达量显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);Ⅱ组和Ⅲ组10日龄工蜂的<em>Tyr</em>1相对表达量显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);各试验组刚出房工蜂的<em>GluRA</em>、<em>Nmdar</em>1、<em>Tyr</em>1以及20日龄工蜂的<em>Tyr</em>1相对表达量差异均不显著(<em>P</em>>0.05)。由此得出,饲粮蛋白质水平过低会影响意大利蜜蜂的归巢能力及记忆相关基因的表达。
Magsci [本文引用: 1]

本试验旨在研究饲粮蛋白质水平对意大利蜜蜂归巢能力及记忆相关基因表达的影响。选择9群群势相当的本地意大利蜜蜂,随机分为3组,每组3群。Ⅰ组、Ⅱ组、Ⅲ组分别饲喂蛋白质水平为15%、25%、35%的试验饲粮。饲喂45 d后,测定采集蜂分别在1 000、2 000 m处的回归率及不同日龄(刚出房、10日龄、20日龄)工蜂3个记忆相关基因[谷氨酸受体A型基因(<em>GluRA</em>)、N-甲基-<em>D</em>-天冬氨酸受体1型基因(<em>Nmdar</em>1)、酪胺受体1型基因(<em>Tyr</em>1)]的表达情况。结果表明:Ⅱ组和Ⅲ组蜜蜂在1 000、2 000 m处的回归率均显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);Ⅲ组10和20日龄工蜂的<em>GluRA</em>相对表达量显著高于Ⅱ组和Ⅰ组(<em>P</em><0.05),且Ⅱ组工蜂的<em>GluRA</em>相对表达量也显著高于Ⅰ组(<em>P</em><0.05);Ⅱ组和Ⅲ组10和20日龄工蜂的<em>Nmdar</em>1相对表达量显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);Ⅱ组和Ⅲ组10日龄工蜂的<em>Tyr</em>1相对表达量显著高于Ⅰ组(<em>P</em><0.05),但Ⅱ组与Ⅲ组之间差异不显著(<em>P</em>>0.05);各试验组刚出房工蜂的<em>GluRA</em>、<em>Nmdar</em>1、<em>Tyr</em>1以及20日龄工蜂的<em>Tyr</em>1相对表达量差异均不显著(<em>P</em>>0.05)。由此得出,饲粮蛋白质水平过低会影响意大利蜜蜂的归巢能力及记忆相关基因的表达。
URL [本文引用: 1]

【目的】本试验旨在研究不同发育温度对意大利蜜蜂Apis mellifera ligustica工蜂归巢能力及学习记忆相关基因表达的影响。【方法】选择3群群势一致的意大利蜜蜂,控制蜂王在一张经工蜂清理的工蜂巢脾中产卵48 h,之后移至孵化区。子脾即将封盖时,平均分成3块,分别放入33℃(Ⅰ组)、35℃(Ⅱ组)、37℃(Ⅲ组)的恒温恒湿箱中继续发育(相对湿度为75%±5%),每群为1个重复。工蜂羽化出房时,用不同颜色标记不同发育温度下羽化的工蜂,并放回原蜂王产卵群,测定其采集时期在1000 m、2000 m的回归率及不同日龄(刚出房,10日龄,20日龄)工蜂3个记忆相关基因[谷氨酸受体A型基因(Glu-RA)、N-甲基-D-天冬氨酸受体1型基因(Nmdar1)、酪氨酸受体1型基因(tyr1)]的表达情况。【结果】结果表明:试验Ⅱ组和试验Ⅲ组发育的采集蜂在1000 m和2000 m处的回归率均显著高于Ⅰ组,但试验Ⅱ组与试验Ⅲ组之间差异不显著;试验Ⅱ组和Ⅲ组刚出房工蜂、10日龄工蜂和20日龄工蜂的GluRA和Nmdar1基因相对表达量显著高于试验Ⅰ组,但试验Ⅱ组与Ⅲ组之间差异不显著;试验Ⅱ组刚出房工蜂的 Tyr1基因相对表达量显著高于试验Ⅰ组与Ⅲ组,但试验Ⅰ组与试验Ⅲ组之间差异不显著,另外,各试验组10日龄和20日龄工蜂Tyr1基因相对表达量差异均不显著。【结论】过低发育温度会影响蜜蜂的归巢能力和记忆相关基因的表达。
URL [本文引用: 1]

【目的】本试验旨在研究不同发育温度对意大利蜜蜂Apis mellifera ligustica工蜂归巢能力及学习记忆相关基因表达的影响。【方法】选择3群群势一致的意大利蜜蜂,控制蜂王在一张经工蜂清理的工蜂巢脾中产卵48 h,之后移至孵化区。子脾即将封盖时,平均分成3块,分别放入33℃(Ⅰ组)、35℃(Ⅱ组)、37℃(Ⅲ组)的恒温恒湿箱中继续发育(相对湿度为75%±5%),每群为1个重复。工蜂羽化出房时,用不同颜色标记不同发育温度下羽化的工蜂,并放回原蜂王产卵群,测定其采集时期在1000 m、2000 m的回归率及不同日龄(刚出房,10日龄,20日龄)工蜂3个记忆相关基因[谷氨酸受体A型基因(Glu-RA)、N-甲基-D-天冬氨酸受体1型基因(Nmdar1)、酪氨酸受体1型基因(tyr1)]的表达情况。【结果】结果表明:试验Ⅱ组和试验Ⅲ组发育的采集蜂在1000 m和2000 m处的回归率均显著高于Ⅰ组,但试验Ⅱ组与试验Ⅲ组之间差异不显著;试验Ⅱ组和Ⅲ组刚出房工蜂、10日龄工蜂和20日龄工蜂的GluRA和Nmdar1基因相对表达量显著高于试验Ⅰ组,但试验Ⅱ组与Ⅲ组之间差异不显著;试验Ⅱ组刚出房工蜂的 Tyr1基因相对表达量显著高于试验Ⅰ组与Ⅲ组,但试验Ⅰ组与试验Ⅲ组之间差异不显著,另外,各试验组10日龄和20日龄工蜂Tyr1基因相对表达量差异均不显著。【结论】过低发育温度会影响蜜蜂的归巢能力和记忆相关基因的表达。
URLPMID:28167800 [本文引用: 1]

Abstract Honeybees are well known for their complex division of labor. Each bee sequentially performs a series of social tasks during its life. The changes in social task performance are linked to gross differences in behavior and physiology. We here tested whether honeybees performing different social tasks (nursing vs. foraging) would differ in their gustatory responsiveness and associative learning behavior in addition to their daily tasks in the colony. Further, we investigated the role of the biogenic amine tyramine and its receptors in the behavior of nurse bees and foragers. Tyramine is an important insect neurotransmitter, which has long been neglected in behavioral studies since it was believed to only act as the metabolic precursor of the better-known amine octopamine. With the increasing number of characterized tyramine receptors in diverse insects, we need to understand the functions of tyramine on its own account.Our findings suggest an important role for tyramine and its two receptors in regulating honeybee gustatory responsiveness, social organization and learning behavior. Foragers, which were more responsive to gustatory stimuli than nurse bees and performed better in appetitive learning, also differed from nurse bees in their tyramine brain titers and in the mRNA expression of a tyramine receptor in the brain. Pharmacological activation of tyramine receptors increased gustatory responsiveness of nurse bees and foragers and improved appetitive learning in nurse bees. These data suggest that a large part of behavioral differences between honeybees may be directly linked to tyramine signaling in the brain.
URL [本文引用: 1]

Biodiversity Conservation and Agricultural Production
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:2715894 [本文引用: 1]

Abstract BACKGROUND: Over the last two winters, there have been large-scale, unexplained losses of managed honey bee (Apis mellifera L.) colonies in the United States. In the absence of a known cause, this syndrome was named Colony Collapse Disorder (CCD) because the main trait was a rapid loss of adult worker bees. We initiated a descriptive epizootiological study in order to better characterize CCD and compare risk factor exposure between populations afflicted by and not afflicted by CCD. METHODS AND PRINCIPAL FINDINGS: Of 61 quantified variables (including adult bee physiology, pathogen loads, and pesticide levels), no single measure emerged as a most-likely cause of CCD. Bees in CCD colonies had higher pathogen loads and were co-infected with a greater number of pathogens than control populations, suggesting either an increased exposure to pathogens or a reduced resistance of bees toward pathogens. Levels of the synthetic acaricide coumaphos (used by beekeepers to control the parasitic mite Varroa destructor) were higher in control colonies than CCD-affected colonies. CONCLUSIONS/SIGNIFICANCE: This is the first comprehensive survey of CCD-affected bee populations that suggests CCD involves an interaction between pathogens and other stress factors. We present evidence that this condition is contagious or the result of exposure to a common risk factor. Potentially important areas for future hypothesis-driven research, including the possible legacy effect of mite parasitism and the role of honey bee resistance to pesticides, are highlighted.
URLPMID:3214027 [本文引用: 1]

Three dopamine receptor genes have been identified that are highly conserved among arthropod species. One of these genes, referred to in honey bees as Amdop2, shows a close phylogenetic relationship to the a-adrenergic-like octopamine receptor family. In this study we examined in parallel the functional and pharmacological properties of AmDOP2 and the honey bee octopamine receptor, AmOA1. For comparison, pharmacological properties of the honey bee dopamine receptors AmDOP1 and AmDOP3, and the tyramine receptor AmTYR1, were also examined.Using HEK293 cells heterologously expressing honey bee biogenic amine receptors, we found that activation of AmDOP2 receptors, like AmOA1 receptors, initiates a rapid increase in intracellular calcium levels. We found no evidence of calcium signaling via AmDOP1, AmDOP3 or AmTYR1 receptors. AmDOP2- and AmOA1-mediated increases in intracellular calcium were inhibited by 10 碌M edelfosine indicating a requirement for phospholipase C-尾 activity in this signaling pathway. Edelfosine treatment had no effect on AmDOP2- or AmOA1-mediated increases in intracellular cAMP. The synthetic compounds mianserin and epinastine, like cis-(Z)-flupentixol and spiperone, were found to have significant antagonist activity on AmDOP2 receptors. All 4 compounds were effective antagonists also on AmOA1 receptors. Analysis of putative ligand binding sites offers a possible explanation for why epinastine acts as an antagonist at AmDOP2 receptors, but fails to block responses mediated via AmDOP1.Our results indicate that AmDOP2, like AmOA1, is coupled not only to cAMP, but also to calcium-signalling and moreover, that the two signalling pathways are independent upstream of phospholipase C-尾 activity. The striking similarity between the pharmacological properties of these 2 receptors suggests an underlying conservation of structural properties related to receptor function. Taken together, these results strongly support phylogenetic analyses indicating that the AmDOP2 and AmOA1 receptor genes are immediate paralogs.
URLPMID:25743690 [本文引用: 1]

The monoamines octopamine and tyramine, which are the invertebrate counterparts of epinephrine and norepinephrine, transmit their action through sets of G protein-coupled receptors. Four different oct
[本文引用: 1]
URLPMID:12717701 [本文引用: 1]

Abstract We have isolated a cDNA clone from the honeybee brain encoding a dopamine receptor, Am Dop2, which is positively coupled to adenylyl cyclase. The transmembrane domains of this receptor are 88% identical to the orthologous Drosophila D2 dopamine receptor, Dm Dop2, though phylogenetic analysis and sequence homology both indicate that invertebrate and vertebrate D2 receptors are quite distinct. In situ hybridization to mRNA in whole-mount preparations of honeybee brains reveals gene expression in the mushroom bodies, a primary site of associative learning. Furthermore, two anatomically distinct cell types in the mushroom bodies exhibit differential regulation of Am Dop2 expression. In all nonreproductive females (worker caste) and reproductive males (drones) the receptor gene is strongly and constitutively expressed in all mushroom body interneurons with small cell bodies. In contrast, the large cell-bodied interneurons exhibit dramatic plasticity of Am Dop2 gene expression. In newly emerged worker bees (cell-cleaning specialists) and newly emerged drones, no Am Dop2 transcript is observed in the large interneurons whereas this transcript is abundant in these cells in the oldest worker bees (resource foragers) and older drones. Differentiation of the mushroom body interneurons into two distinct classes (i.e., plastic or nonplastic with respect to Am Dop2 gene expression) indicates that this receptor contributes to the differential regulation of distinct neural circuits. Moreover, the plasticity of expression observed in the large cells implicates this receptor in the behavioral maturation of the bee.
URLPMID:15944083 [本文引用: 1]

Abstract Dopamine is an important neurotransmitter in vertebrate and invertebrate nervous systems and is widely distributed in the brain of the honey bee, Apis mellifera. We report here the functional characterization and cellular localization of the putative dopamine receptor gene, Amdop3, a cDNA clone isolated and identified in previous studies as AmBAR3 (Apis mellifera Biogenic Amine Receptor 3). The Amdop3 cDNA encodes a 694 amino acid protein, AmDOP3. Comparison of AmDOP3 to Drosophila melanogaster sequences indicates that it is orthologous to the D2-like dopamine receptor, DD2R. Using AmDOP3 receptors expressed in HEK293 cells we show that of the endogenous biogenic amines, dopamine is the most potent AmDOP3 agonist, and that activation of AmDOP3 receptors results in down regulation of intracellular levels of cAMP, a property characteristic of D2-like dopamine receptors. In situ hybridization reveals that Amdop3 is widely expressed in the brain but shows a pattern of expression that differs from that of either Amdop1 or Amdop2, both of which encode D1-like dopamine receptors. Nonetheless, overlaps in the distribution of cells expressing Amdop1, Amdop2 and Amdop3 mRNAs suggest the likelihood of D1:D2 receptor interactions in some cells, including subpopulations of mushroom body neurons.
[本文引用: 1]
URLPMID:25904854 [本文引用: 1]

Long-term behavioral changes related to learning and experience have been shown to be associated with structural remodeling in the brain. Leaf-cutting ants learn to avoid previously preferred plants after they have proved harmful for their symbiotic fungus, a process that involves long-term olfactory memory. We studied the dynamics of brain microarchitectural changes after long-term olfactory memory formation following avoidance learning in Acromyrmex ambiguus. After performing experiments to control for possible neuronal changes related to age and body size, we quantified synaptic complexes (microglomeruli, MG) in olfactory regions of the mushroom bodies (MBs) at different times after learning. Long-term avoidance memory formation was associated with a transient change in MG densities. Two days after learning, MG density was higher than before learning. At days 4 and 15 after learning-when ants still showed plant avoidance-MG densities had decreased to the initial state. The structural reorganization of MG triggered by long-term avoidance memory formation clearly differed from changes promoted by pure exposure to and collection of novel plants with distinct odors. Sensory exposure by the simultaneous collection of several, instead of one, non-harmful plant species resulted in a decrease in MG densities in the olfactory lip. We hypothesize that while sensory exposure leads to MG pruning in the MB olfactory lip, the formation of long-term avoidance memory involves an initial growth of new MG followed by subsequent pruning.
URLPMID:10454379 [本文引用: 1]

ABSTRACT In a classical conditioning procedure, honeybees associate an odor with sucrose resulting in the capacity of the odor to evoke an appetitive response, the extension of the proboscis (PER). Here, we study the effects of pairing an odor with injections of octopamine (OA) as a substitute for sucrose into three putative brain sites of odor/sucrose convergence. OA injected into the mushroom body (MB) calyces or the antennal lobe but not the lateral protocerebral lobe produces a lasting, pairing-specific enhancement of PER. During pairings, OA injected into the MB calyces results in an additional pairing-specific effect, because it does not lead to an acquisition but a consolidation after conditioning. These results suggest that the neuromodulator OA has the capacity of inducing associative learning in an insect brain. Moreover, they suggest the antennal lobes and the calyces as at least partially independent sites of associating odors that may contribute differently to learning and memory consolidation.
URLPMID:10454370 [本文引用: 1]

Mushroom bodies are prominent neuropils found in annelids and in all arthropod groups except . First explicitly identified in 1850, the mushroom bodies differ in size and complexity between taxa, as well as between different castes of a single species of social insect. These differences led some early biologists to suggest that the mushroom bodies endow an arthropod with intelligence or the ability to execute voluntary actions, as opposed to innate . Recent physiological studies and mutant analyses have led to divergent interpretations. One interpretation is that the mushroom bodies conditionally relay to higher protocerebral centers information about sensory stimuli and the context in which they occur. Another interpretation is that they play a central role in and . Anatomical studies suggest that arthropod mushroom bodies are predominately associated with olfactory pathways except in phylogenetically basal . The prominent olfactory input to the mushroom body calyces in more recent insect orders is an acquired character. An overview of the history of research on the mushroom bodies, as well as comparative and evolutionary considerations, provides a conceptual framework for discussing the roles of these neuropils.
URLPMID:22684942 [本文引用: 1]

The mushroom bodies are prominent lobed centers in the forebrain, or protocerebrum, of most insects. Previous studies on mushroom bodies have focused on higher olfactory processing, including olfactory-based learning and memory. Anatomical studies provide strong support that in terrestrial insects with mushroom bodies, the primary input region, or calyces, are predominantly supplied by olfactory projection neurons from the antennal lobe glomeruli. In aquatic species that generally lack antennal lobes, the calyces are vestigial or absent. Here we report an exception to this in the whirligig beetle Dineutus sublineatus (Coleoptera: Gyrinidae). This aquatic species lives on water and is equipped with two separate pairs of compound eyes, one pair viewing above and one viewing below the water surface. As in other aquatic insects, the whirligig beetle lacks antennal lobes, but unlike other aquatic insects its mushroom bodies possess robust calyces. Golgi impregnations and fluorescent tracer injections revealed that the calyces are exclusively supplied by visual neurons from the medulla of the dorsal eye optic lobes. No other sensory inputs reach the calyces, thereby showing a complete switch of calyx modality from olfaction to vision. Potential functions of the mushroom bodies of D. sublineatus are discussed in the context of the behavioral ecology of whirligig beetles.
[本文引用: 1]
URLPMID:12859685 [本文引用: 1]

Abstract Biogenic amines and their receptors regulate and modulate many physiological and behavioural processes in animals. In vertebrates, octopamine is only found in trace amounts and its function as a true neurotransmitter is unclear. In protostomes, however, octopamine can act as neurotransmitter, neuromodulator and neurohormone. In the honeybee, octopamine acts as a neuromodulator and is involved in learning and memory formation. The identification of potential octopamine receptors is decisive for an understanding of the cellular pathways involved in mediating the effects of octopamine. Here we report the cloning and functional characterization of the first octopamine receptor from the honeybee, Apis mellifera. The gene was isolated from a brain-specific cDNA library. It encodes a protein most closely related to octopamine receptors from Drosophila melanogaster and Lymnea stagnalis. Signalling properties of the cloned receptor were studied in transiently transfected human embryonic kidney (HEK) 293 cells. Nanomolar to micromolar concentrations of octopamine induced oscillatory increases in the intracellular Ca2+ concentration. In contrast to octopamine, tyramine only elicited Ca2+ responses at micromolar concentrations. The gene is abundantly expressed in many somata of the honeybee brain, suggesting that this octopamine receptor is involved in the processing of sensory inputs, antennal motor outputs and higher-order brain functions.
URLPMID:12679144 [本文引用: 1]

Histamine, a major neurotransmitter both in vertebrates and invertebrates, transmits its actions through a set of well-known receptors. In vertebrates, these receptors belong to the family of G-protein-coupled receptors. In invertebrates, the few well-characterized actions of histamine are transmitted through ionotropic histamine receptors. To evaluate if metabotropic histamine receptors are part of the invertebrate histaminergic signaling cascade, I identified the complete set of metabotropic bioamine receptors from two invertebrates, whose entire genome has been sequenced: the fruitfly Drosophila melanogaster and the soil nematode Caenorhabditis elegans . A comparison with representatives of all groups of metabotropic bioamine receptors from vertebrates and invertebrates showed that none of these receptors clusters together with any of the four groups of vertebrate histamine receptors. This implies that no direct homologues to vertebrate metabotropic histamine receptor are present in invertebrates. Therefore, it is reasonable to account that the histaminergic neurotransmission in invertebrates is exclusively transmitted through ionotropic histamine receptors and that metabotropic histamine receptors evolved after the split between vertebrates and invertebrates.
[本文引用: 1]
