 ,1, 宋福平
,1, 宋福平 ,2
,2The functions of yhcZ gene during Bacillus thuringiensis growth
Linda Jia1,2, Tantan Gao2, Qi Peng2, Jing Lv2, Jie Zhang2, Min Chen ,1, Fuping Song
,1, Fuping Song ,2
,2通讯作者:
编委: 何群
收稿日期:2018-03-7修回日期:2018-04-12网络出版日期:2018-05-20
| 基金资助: |
Editorial board:
Received:2018-03-7Revised:2018-04-12Online:2018-05-20
| Fund supported: |
作者简介 About authors
家琳达,硕士研究生,专业方向:森林保护(昆虫方向)E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1277KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
家琳达, 高坦坦, 彭琦, 吕静, 张杰, 陈敏, 宋福平. yhcZ基因在苏云金芽胞杆菌生长中的功能研究. 遗传[J], 2018, 40(5): 415-424 doi:10.16288/j.yczz.18-061
Linda Jia, Tantan Gao, Qi Peng, Jing Lv, Jie Zhang, Min Chen, Fuping Song.
细菌通过细胞内的双组分系统(two-component system, TCS)感应外界环境信号,进而调控基因表达,及时应答环境和生理的变化。典型的TCS由组氨酸激酶(histidine kinase, HK)和应答调控蛋白(response regulator, RR)两部分组成[1]。HK包含跨膜结构,分为膜外信号感应结构域和膜内催化结构域。RR的N-端结构域用于接收HK传递的磷酸基团,C-端结构域包含螺旋-转角-螺旋(HTH)序列,可与靶DNA或RNA特异性结合,通过激活或抑制靶基因的转录,进而调控相关基因的表达[2,3]。TCS广泛存在于细菌中,调控细菌的生长繁殖、营养代谢、趋化性、运动性、生物膜形成[4]、芽胞形成[5]、毒力表达及耐药性[6,7]等生理过程。
广义的蜡样芽胞杆菌组(B. cereus group)包含6种芽胞杆菌,除了狭义的3种,即苏云金芽胞杆菌(B. thuringiensis)、炭疽芽胞杆菌(B. anthracis)及蜡样芽胞杆菌(B. cereus)外,还包括蕈状芽胞杆菌(B. mycoides)、假蕈状芽胞杆菌(B. pseudomycoides)和韦氏芽胞杆菌(B. weihenstephanensis)[8]。在B. cereus组中,大部分对TCS的研究集中于细胞压力应答、毒力表达调控和芽胞的形成起始等方面。例如,目前已经证实CasKR参与B. cereus菌株低温适应性,通过提高脂肪酸脱氢酶DesA的活性适应低温环境,维持细胞生长和存活[9,10];ResDE响应细胞内的氧化还原变化,进而通过激活溶血素基因hbl和肠毒素基因nde的转录调控B. cereus的毒性[11];YvfTU参与毒力调控因子PlcR的表达,主要调控B. cereus菌株的毒性[12];WalRK(YycFC)响应温度和抗生素压力,调控B. anthracis的细胞分离及芽胞形成等生理过程[13];LytSR调控B. thuringiensis中spoIIP基因的表达,参与芽胞的形成过程[14]。然而,在B. cereus组细菌中仍存在许多参与细胞壁压力应答、药物抵抗性、生长调控的TCSs有待深入研究,如PhoRP、YycGF、YufLM、LiaSR、YbdKJ、YvcQP、YxdKJ等[15]。
苏云金芽胞杆菌(B. thuringiensis, Bt)在芽胞形成过程中,细胞内能够产生伴胞晶体,其主要成分是杀虫晶体蛋白。这些晶体蛋白对鳞翅目、鞘翅目、双翅目、半翅目、等翅目、直翅目、缨翅目等昆虫具有特异杀虫活性[16]。Bt产生的晶体和芽胞混合物作为生物杀虫剂也已成功应用于害虫的生物防治,并且成为目前农业上应用最广和产量最大的微生物杀虫剂[17]。在B. subtilis 168菌株中,YhcZ和上游YhcY组成双组分系统参与磷酸转移酶系统(phosphotransferase system, PTS)通路,调控菌株对葡萄糖的利用[18];在B. cereus模式菌株ATCC 14579中,仅推测YhcYZ双组分系统参与细胞壁压力响应[15]。然而在Bt菌株中,尚无yhcZ相关基因的功能报道。本研究通过比较基因在基因组上的排列模式,结合氨基酸序列比对和保守结构域分析,证明在Bt HD73基因组中存在yhcZ基因,对应的基因编号为HD73_5824。然而YhcZ在Bt菌株中对生长发挥的调控作用仍不清楚。通过测定Bt菌株的生长曲线及对不同碳源的利用情况,明确该基因对Bt菌株的生长代谢有促进作用。该研究为阐明YhcZ在Bt菌株中的生物学功能奠定基础。
1 材料和方法
1.1 菌株与质粒
本研究所用的菌株和质粒如表1所示。Table 1
表1
表1 本研究所用的菌株与质粒
Table 1
| 菌株/质粒 | 特性 | 来源 |
|---|---|---|
| SCS110 | 大肠杆菌菌株,用于对质粒进行去甲基化修饰 | 本实验室 |
| HD73 | 苏云金芽胞杆菌野生型菌株,产生Cry1Ac晶体蛋白 | 本实验室 |
| HD (ΔyhcZ) | 苏云金芽胞杆菌野生型菌株HD73中敲除yhcZ基因, Kanr | 本研究 |
| HD (ΔyhcZ::yhcZ) | HD (ΔyhcZ)菌株中转入互补质粒pHTHFyhcZ, Ermr | 本研究 |
| pMAD | 温敏型质粒,用于在芽胞杆菌中进行基因敲除, 9.7 kb, Ampr, Ermr | 本实验室 |
| pRN5105ΔcwlC | 同源重组质粒,用于在HD73菌株中敲除cwlC基因, Ampr, Ermr | 本实验室 |
| pMADΔyhcZ | 同源重组质粒,用于在HD73菌株中敲除yhcZ基因, Ampr, Ermr | 本研究 |
| pHT315 | 大肠杆菌和芽胞杆菌穿梭载体,6.5 kb, Ampr, Ermr, | 本实验室 |
| pHTHFyhcZ | 互补质粒,在pHT315中插入yhcZ基因的启动子和开放阅读框, Ermr | 本研究 |
新窗口打开|下载CSV
1.2 培养基及配比
LB培养基:1% Tryptone,1% NaCl,0.5% Yeast extract;SSM培养基:营养肉汤8 g,0.12% MgSO4,0.1% KCl,0.01% NaOH,121℃高温灭菌20 min后加入经0.22 μm滤膜过滤除菌的0.01 mmoL/L MnCl2,1 mmoL/L Ca(NO3)2,0.001 mmoL/L FeSO4;M9基础培养基:33.9 g/L Na2HPO4, 15 g/L KH2PO4, 5 g/L NH4Cl, 2.5 g/L NaCl,使用之前补充0.4%的葡萄糖。1.3 酶及试剂
Taq DNA聚合酶购自于北京博迈德生物技术公司;PCR产物回收试剂盒购自北京AXYGEN公司;Prime STAR HS DNA 聚合酶、相关的内切酶及T4 DNA连接酶均购自大连生物工程(TaKaRa)公司。Biolog GenⅢ 微孔板和IF-B接种液均购自北京华粤企业集团有限公司。其他生化试剂和抗生素均为进口分析纯级试剂(Sigma, 美国)。1.4 引物合成及序列测定
根据Bt HD73菌株的基因组序列设计引物,具体名称及序列见表2。引物合成和序列测定由上海生工生物工程股份有限公司完成。Table 2
表2
表2 本研究所用引物
Table 2
| 引物名称 | 序列(5′→3′) | 酶切位点 |
|---|---|---|
| yhcZ-A-F | GCTCGAATTCTTAATGTACTACCTGAAAAA | EcoRⅠ |
| yhcZ-A-R | TGAAATGGTTCGCTGAACTAACAATACTTTTATTT | |
| yhcZ-B-F | GCCTACGAGGAATTTGCAGTGAAAAATGGAATTGT | |
| yhcZ-B-R | TATCGGATCCTCATCTTTTATATGTATTAA | BamHⅠ |
| yhcZ-Kan-F | AAAGTATTGTTAGTTCAGCGAACCATTTGAGGTGA | |
| yhcZ-Kan-R | TCCATTTTTCACTGCAAATTCCTCGTAGGCGCTCG | |
| 5823-F | ATGGGAAGCCGAAAAC | |
| 5825-R | TTGTGCGAATGGAAGA | |
| yhcZHF-F | CGGAATTCGCCAAGAGGGCGTTAGAACAAGAT | EcoRⅠ |
| yhcZHF-R | GCGTCGACTTCCTCCTAGTACTCACTCTTATG | SalⅠ |
新窗口打开|下载CSV
1.5 培养条件
苏云金芽胞杆菌常规培养温度为30℃,摇床转速为220 r/min;筛选突变体过程中苏云金芽胞杆菌的培养温度为38℃,摇床转速为150 r/min;大肠杆菌(Escherichia coli)的培养温度为37℃,摇床转速为220 r/ min;必要时加入相应抗生素:苏云金芽胞杆菌中,红霉素工作浓度为5 μg/mL,卡那霉素工作浓度为100 μg/mL;大肠杆菌中,氨苄青霉素工作浓度为100 μg/mL。1.6 遗传操作
大肠杆菌质粒提取及DNA片段纯化按照试剂盒说明书进行;大肠杆菌转化方法参考分子克隆实验指南;Bt基因组DNA提取和电击转化参考之前发表的实验方法[19,20]。1.7 yhcZ基因生物信息学分析
根据 B. subtilis 168 (NCBI accession No. NZ_ CP010052.1)和B. cereus ATCC 14579 (NCBI accession No. NC_004722.1)菌株中yhcZ基因序列,在Bt HD73菌株的基因组(NCBI accession No. NC_ 020238.1)中进行BLAST比对,鉴定yhcZ同源基因;利用DNAMAN比对相应蛋白氨基酸序列的相似性;利用NCBI(https://www.ncbi.nlm.nih.gov/cdd/)进行YhcZ蛋白保守结构域的分析。1.8 yhcZ基因缺失菌株和互补菌株的构建
以野生型HD73的基因组为模板,yhcZ-A-F/ yhcZ-A-R和yhcZ-B-F/yhcZ-B-R为引物,PCR分别扩增出yhcZ基因的上游片段(yhcZ-A, 1024 bp)和下游片段(yhcZ-B, 1024 bp)。以pRN5105ΔcwlC质粒为模板,yhcZ-Kan-F/yhcZ-Kan-R为引物扩增出卡那霉素抗性基因片段(Kan, 1476 bp)。以yhcZ-A、Kan和yhcZ-B为模板,yhcZ-A-F/yhcZ-B-R为引物,PCR扩增出同源重组长片段yhcZ-A-Kan-yhcZ-B (3524 bp)。利用EcoRⅠ和BamHⅠ对该片段进行双酶切,胶回收后插入pMAD载体的相应酶切位点,获得同源重组质粒,命名为pMADΔyhcZ。该质粒经电击转入HD73菌株中,在红霉素和卡那霉素双抗性平板上筛选阳性转化子。经38℃高温诱导,筛选出对红霉素敏感但对卡那霉素抵抗的菌落。利用重组片段外侧引物5823-F和5825-R进行PCR鉴定,并对PCR片段进行测序确认,获得yhcZ基因缺失突变菌株HD (ΔyhcZ)。以HD73的基因组为模板,yhcZHF-F和yhcZHF-R为引物,PCR扩增得到yhcZ基因全序列片段(包含yhcZ启动子和ORF, 1687 bp),利用EcoRⅠ和SalⅠ酶切并连接到相同酶切处理的穿梭载体pHT315上,热激转化到大肠杆菌SCS110中,获得互补质粒pHTHFyhcZ,并进行测序确认序列正确性。进一步将重组质粒经电击转化到HD (ΔyhcZ)菌株中,得到互补菌株HD (ΔyhcZ::yhcZ)。
1.9 生长曲线的测定
挑取Bt单菌落接种于5 mL新鲜LB培养基中,30℃、220 r/min振荡过夜培养。母液按照1%的接种量转接于100 mL新鲜LB液体培养基中,30℃、220 r/min振荡培养,每间隔1 h进行取样,测定其在600 nm波长处的吸光值(OD600),并绘制各菌株的生长曲线,每组数据独立重复3次。1.10 Bt菌株对葡萄糖的吸收利用测定
挑取Bt单菌落接种于5 mL新鲜LB培养基中,30℃、220 r/min振荡过夜培养。母液以1%的接种量转接于100 mL新鲜M9液体培养基(加入0.4%葡萄糖作为唯一碳源)中,30℃、220 r/min振荡培养,每间隔1 h进行取样,测定其OD600,并绘制各菌株的生长曲线。每组数据独立重复3次。1.11 Biolog实验方法及数据分析
将Bt菌株接种至LB固体培养基上,置于30℃过夜培养;使用Inoculatorz棉签从平板上挑取长势良好的单菌落,接种于Inoculating Fluid B接种液中,并使用浊度仪调整接种液初始浓度,使其细胞浓度处于90%~98% T,形成均匀的菌悬液;将菌悬液倒入V型上样槽中,使用多孔道移液器将菌悬液加入Biolog GenⅢ微孔板的96孔中,每孔100 μL;将微孔板置于30℃培养箱中培养;根据菌株特性,选取特定的时间点(本实验选取22 h和32 h)将微孔板置于Plate Reader中进行读数;Biolog实验独立重复3次。Biolog GenⅢ微孔板的单孔颜色平均值(average well color development, AWCD)计算方法参考文献[21],即AWCD=∑(Ci-R)/95。其中,Ci表示95个实验孔在590 nm处的吸光值,R表示对照孔在590 nm处的吸光值,95为Biolog GenⅢ微孔板包含的碳源和化学物质的种类。若Ci-R数值小于0,则按照0计算。数值取3次独立实验中数据的平均值。
Biolog GenⅢ微孔板中,所有必要的营养物质以及生化试剂都预先填充、干化在96孔中。在微生物可以生长的孔中,增强的呼吸作用导致四唑氧化还原染料被还原,变成紫色;而在阴性孔中,由于无可供微生物利用的碳源,因此保持呈现无色。孵育后,通过显紫色孔所产生的表型指纹同Biolog数据库中的数据进行比较。
2 结果与分析
2.1 苏云金芽胞杆菌HD73中yhcZ基因的鉴定
根据基因在基因组上的排列,结合yhcZ基因的核苷酸序列比对,在Bt HD73菌株中发现一个编号为HD73_5824的基因,编码应答调控蛋白,其上游基因HD73_5825编码组氨酸激酶,下游基因HD73_5823编码偶氮还原酶, 三者排列模式与菌株B. subtilis 168和B. cereus ATCC 14579中的基因排列模式相同(图1)。氨基酸比对结果显示,HD73_5824与B. subtilis 168和B. cereus ATCC 14579菌株中调控蛋白YhcZ相似性分别为51.2%和97.8%;HD73_5825与B. subtilis 168和B. cereus ATCC 14579菌株中组氨酸激酶YhcY相似性分别为34.7%和98.3% (图1)。NCBI在线分析HD73_5824的保守结构域表明:HD73_5824蛋白的N-端包含Response_reg结构域,用于接收其同源组氨酸激酶传递的磷酸信号;C-端为HTH结构域,可结合目的基因DNA序列。以上分析表明在B. thuringiensis HD73基因组中,包含B. subtilis和B. cereus菌株中的yhcZ和上游yhcY的同源基因,对应基因编号分别为HD73_5824和HD73_5825,推测两者共同组成双组分信号系统YhcY-YhcZ,参与细菌细胞生长。图1
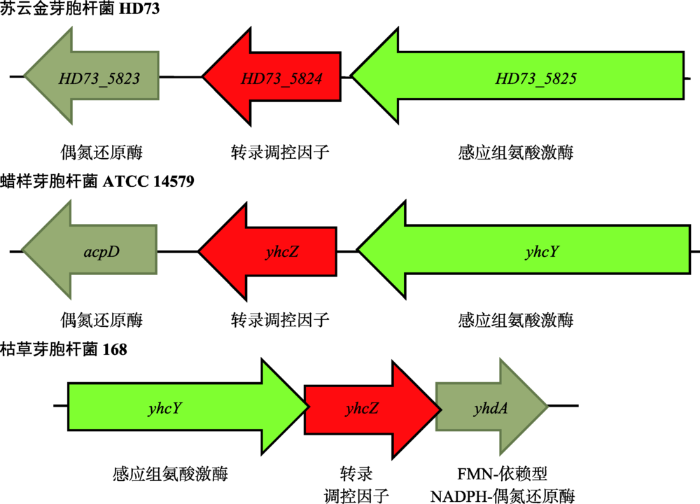 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同芽胞杆菌中yhcZ基因分析
Fig. 1Analysis of yhcZ gene from different Bacillus strains
2.2 yhcZ基因缺失突变体及互补菌株的构建
为研究yhcZ基因的生物学功能,首先构建yhcZ基因缺失突变体及互补菌株。将同源重组质粒pMADΔyhcZ (图2A)电击转入HD73菌株中,经过诱导突变筛选,得到卡那霉素抗性单菌落。利用重组片段外侧引物5823-F和5825-R进行PCR鉴定,结果如图2C所示。以HD73基因组为模板扩增片段大小为1.7 kb,突变株基因组为模板扩增片段大小为2.5 kb。通过对PCR片段进行测序,测序结果与设计序列一致,证明yhcZ基因缺失突变株构建正确,命名为HD (ΔyhcZ)。图2
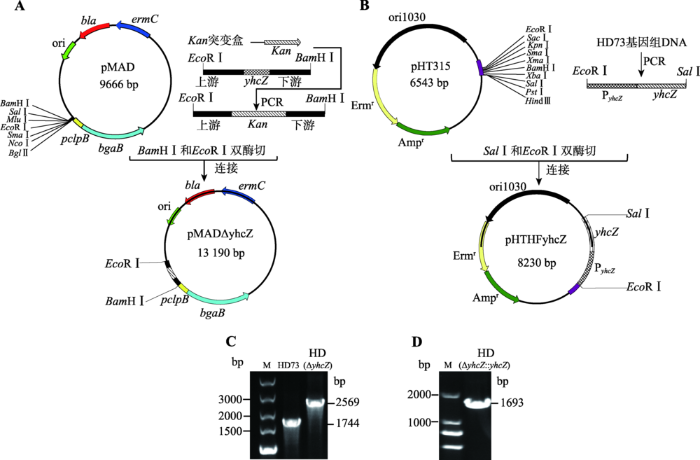 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2质粒构建与菌株鉴定
A:同源重组质粒pMADΔyhcZ的构建;B:yhcZ基因互补质粒pHTHFyhcZ的构建;C:缺失突变菌株HD (ΔyhcZ)的PCR鉴定;D:yhcZ互补菌株HD (ΔyhcZ::yhcZ)的PCR鉴定。
Fig. 2Construction of plasmids and identification of stains by PCR
同样将yhcZ基因互补质粒pHTHFyhcZ (图2B)电击转入HD (ΔyhcZ)菌株中,经过抗性筛选得到阳性转化子。提取单菌落的基因组,进行PCR鉴定,其结果如图2D所示,扩增片段大小约为1.7 kb,该结果与所设计的序列大小一致,表明互补菌株构建成功,命名为HD(ΔyhcZ::yhcZ)。
2.3 yhcZ基因缺失对菌株生长的影响
从0时开始每隔1 h测定OD600值最后绘制生长曲线。无论在丰富培养基LB中(图3A),或是在贫瘠营养诱导产胞培养基SSM中(图3B),缺失突变体HD (ΔyhcZ)生长均明显慢于野生型HD73,且互补菌株HD (ΔyhcZ::yhcZ)生长均得到了部分恢复。结果表明yhcZ基因的缺失影响了菌株的生长,证明yhcZ基因在Bt菌株营养利用及生长代谢过程中发挥重要的促进作用。图3
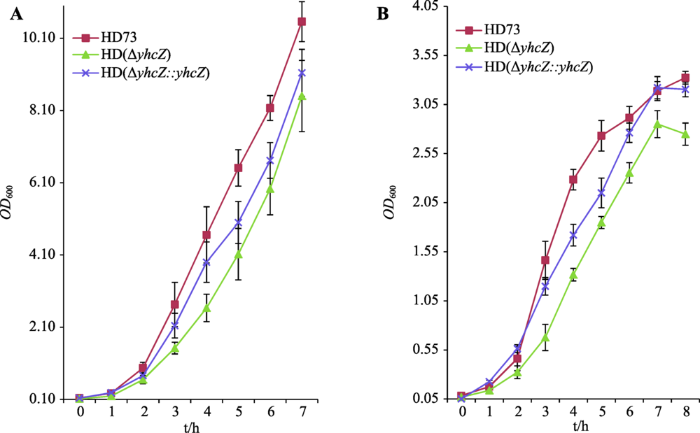 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3野生菌株、yhcZ缺失菌株及yhcZ互补菌株在不同培养基中的生长曲线
A:LB培养基;B:SSM培养基。
Fig. 3Growth curves of wild-type stain, yhcZ deletion mutant, and yhcZ-complementation strain in different mediums
2.4 yhcZ基因缺失对葡萄糖利用的影响
为评价Bt菌株对葡萄糖的利用效率,选用M9基础培养基并添加0.4%葡萄糖作为唯一碳源测定Bt菌株的生长曲线。在整个生长过程中,突变体HD (ΔyhcZ)的生长速度大于野生型HD73,表明yhcZ基因缺失后,菌株可以提高对葡萄糖的利用效率(图4)。菌株在M9培养基中,前期进行对数生长至最大值,后期开始下降。推测可能的原因是前期培养基中营养丰富,满足菌株快速生长;后期随着培养基中营养的消耗,Bt菌株生长速度下降,而且培养基中积累的有毒代谢产物造成细胞的裂解,使得OD600快速下降。本实验结果表明yhcZ基因在一定程度上负调控Bt菌株对葡萄糖的利用。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4yhcZ基因的缺失提高Bt菌株对葡萄糖的利用
Fig. 4Deletion of the yhcZ gene promotes glucose utilization of Bt strain
2.5 Biolog分析Bt菌株的碳源利用率和化学敏感性
在Biolog评价系统中,单孔颜色变化率(AWCD)反映出微生物对单一碳源的利用能力,以此评价微生物的代谢活性。突变菌株HD (ΔyhcZ)的AWCD值明显小于野生型HD73的AWCD值(图5),说明yhcZ基因的缺失导致Bt菌株利用碳源的能力降低。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5 yhcZ基因缺失影响Bt菌株对碳源的利用
Fig. 5The effect of yhcZ deletion on carbon utilization
Biolog GenⅢ微孔板可以对微生物进行94种表型测试,其中包括71种碳源利用测试(图6,1~9列)以及23种化学敏感性测试(图6,10~12列)。由于Biolog系统在检测微生物对碳源的利用中可能存在较大的波动性,为最大可能地排除实验误差,本研究中的Biolog实验进行了6次独立的生物学重复,选取了3次实验结果中野生型菌株HD73和yhcZ缺失突变体之间差异相同的样孔表型指纹图谱分析(图6)。两个菌株均可利用糊精(A2)、D-麦芽糖(A3)、D-海藻糖(A4)、β-甲酰-D-葡糖苷(B4)、N-乙酰-D-葡糖胺(B6)、α-D-葡糖(C1)、D-果糖(C3)、肌苷(C9)、甘油(D5)、D-葡糖-6-磷酸(D6)、D-果糖-6-磷酸(D7)、明胶(E1)、丙酮酸甲酯(G2)、L-乳酸(G4)、L-苹果酸(G8),且对这些碳源利用能力较强,对应碳源孔呈现紫色。HD73和HD(ΔyhcZ)同样均可利用D-纤维二糖(A5)、D-水杨苷(B5)、N-乙酰-β-D-甘露糖胺(B7)、L-岩藻糖(C7)、肌醇(D4)、e氨基乙酰-L-脯氨酸(E2)、L-丙氨酸(E3)、L-精氨酸(E4)、L-组胺(E7)、L-天冬氨酸(E5)、L-谷氨酸(E6)、L-焦谷氨酸(E8)、D-半乳糖醛酸(F2)、D-葡糖醛酸(F5)、葡糖醛酰胺(F6)、粘酸(F7)、糖质酸(F9)、吐温40(H1)、α-酮-戊二酸(G6)、溴-丁二酸(G9)、乙酰乙酸(H6)、乙酸(H8),但对这些碳源利用能力相对较弱,对应碳源孔呈现蓝色。HD73和HD(ΔyhcZ)均不可利用蔗糖(A7)、D-松二糖(A8)、水苏糖(A9)、蜜三糖(B1)、α-D-乳糖(B2)、蜜二糖(B3)、N-乙酰-D-半乳糖胺(B6)、N-乙酰神经氨酸(B9)、D-甘露糖(C2)、D-岩藻糖(C6)、L-鼠李糖(C8)、D-山梨醇(D1)、D-甘露醇(D2)、D-阿拉伯醇(D3)、D-天冬氨酸(D8)、果胶(F1)、L-半乳糖醛酸内酯(F3)、奎宁酸(F8)、p-羟基-苯乙酸(G1)、D-苹果酸(G7)、γ-氨基-丁酸(H2)、α-羟基-丁酸(H3)、β-羟基-D,L丁酸(H4)、α-酮-丁酸(H5),对应碳源孔表现出无色,同阴性对照孔(A1)。
图6
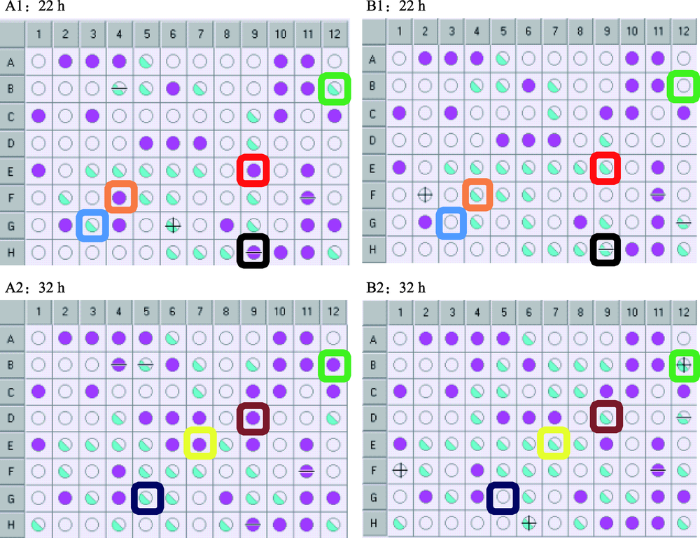 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6HD73和HD (ΔyhcZ)菌株表型指纹图谱
A1表示HD73菌株培养22 h,A2表示HD73菌株培养32 h;B1表示HD (ΔyhcZ)菌株培养22 h,B2表示HD (ΔyhcZ)菌株培养32 h。紫色和蓝色表示细菌能够生长的样品孔,紫色表示长势强,蓝色表示长势弱,无色表示细菌无法生长的样品孔;方框表示HD73与HD (ΔyhcZ)存在差异的样品孔;Biolog GenⅢ微孔板中的A1孔为碳源利用测试的阴性对照孔,A10孔为化学敏感性测试的阳性对照孔。
Fig. 6Phenotypic microarray analysis of HD73 and HD (ΔyhcZ) strains
培养22 h后发现,HD73 (图6,A1)对L-丝氨酸(E9)、甲酸(H9)、D-葡糖酸(F4)的利用能力及对8% NaCl(B12)的耐受性强于HD(ΔyhcZ) (图6,B1),且HD73可利用D-乳酸甲酯(G3),HD(ΔyhcZ)则无法利用;继续培养32 h后发现,HD73 (图6,A2)利用L-组胺(E7)和D-丝氨酸(D9)的能力强于HD(ΔyhcZ) (图6,B2),且HD73可以利用柠檬酸(G5),而HD (ΔyhcZ)则无法利用。
3 讨 论
通过BLAST搜寻、基因排列模式比较、氨基酸序列比对以及保守结构域分析明确了Bt菌株中的yhcZ的基因,推测其与上游基因yhcY共同组成双组份信号系统,调控细菌的生理过程。在B. cereus组细菌中,YhcYZ功能尚不明确。本研究在实验室条件下测定菌株的生长曲线,发现yhcZ缺失后显著影响Bt菌株的生长(图3),证明YhcZ对Bt菌株的生长具有促进作用。前期研究报道,在模式菌株枯草芽胞杆菌中,yhcZ抑制yjdD的表达[18]。yjdD基因编码PTS酶同系物,通过调控多种糖的运输与磷酸化,参与脂多糖和细胞膜核心成分的合成[22]。在Bt菌株中,YhcZ参与葡萄糖的吸收与利用,yhcZ缺失后,Bt菌株对葡萄糖的利用能力提高(图4),推测Bt中yhcZ基因的功能可能与B. subtilis中相似,抑制PTS相关基因的表达,进而调控细胞对葡萄糖的利用。采用Biolog系统分析Bt HD73菌株对碳源的利用率及化学物的敏感性,发现yhcZ基因缺失后,利用L-丝氨酸、D-葡糖酸、L-组胺、D-丝氨酸、D-乳酸甲酯、柠檬酸、甲酸的能力明显降低,耐受NaCl的能力显著低于野生型HD73 (图6,B12样品孔)。结果表明yhcZ基因不仅参与Bt菌株对不同碳源的利用,同时参与调控细胞膜应激反应,应答盐胁迫。在前期报道中,仅推测YhcYZ参与B. cereus对细胞壁压力的应答和杀菌剂的抵抗活性[15]。推测在高盐条件下,细胞壁的完整性受到改变,激活YhcYZ系统,YhcZ从而调控相关基因的表达。在B. cereus组细菌中,其他的TCSs,如YbdKJ、YvcQP、YxdKJ及LiaSR也具有响应细胞壁压力的功能[15],因此推测面对外界刺激,细菌启动多个TCSs共同调控细胞的生长及代谢。
分析Biolog结果,发现yhcZ基因缺失后,影响Bt菌株对多种碳源的利用效率。LB和SSM均为营养成分复杂的培养基,推测由于HD(ΔyhcZ)对多种碳源的利用效率降低,以及对化学物质敏感性增强,导致细胞生长减慢。YhcZ影响Bt菌株对碳源的利用及对外界压力的抵抗能力,由此推测Bt菌株在适应复杂多变的自然环境过程中,YhcZ可能发挥不可缺少的作用。然而YhcY感应到葡萄糖及盐压力信号后,YhcZ如何发挥调控作用的具体分子机制仍待深入研究。本研究重点证明了YhcZ在Bt菌株生长过程中发挥显著的促进作用,研究结果对提高菌株的发酵密度和发酵水平具有重要意义。根据生物信息学分析,推测在Bt HD73菌株中YhcY和YhcZ组成双组分系统,后续研究将会涉及遗传学和表型实验以验证YhcYZ是否为双组分系统。同时,根据Biolog结果分析验证yhcZ的调控途径亦是今后的重点研究方向。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:1233412 [本文引用: 1]

Two-component signal transduction systems, comprised of histidine kinases and their response regulator substrates, are the predominant means by which bacteria sense and respond to extracellular signals. These systems allow cells to adapt to prevailing conditions by modifying cellular physiology, including initiating programs of gene expression, catalyzing reactions, or modifying protein rotein interactions. These signaling pathways have also been demonstrated to play a role in coordinating bacterial cell cycle progression and development. Here we report a system-level investigation of two-component pathways in the model organismCaulobacter crescentus. First, by a comprehensive deletion analysis we show that at least 39 of the 106 two-component genes are required for cell cycle progression, growth, or morphogenesis. These include nine genes essential for growth or viability of the organism. We then use a systematic biochemical approach, called phosphotransfer profiling, to map the connectivity of histidine kinases and response regulators. Combining these genetic and biochemical approaches, we identify a new, highly conserved essential signaling pathway from the histidine kinase CenK to the response regulator CenR, which plays a critical role in controlling cell envelope biogenesis and structure. Depletion of eithercenKorcenRleads to an unusual, severe blebbing of cell envelope material, whereas constitutive activation of the pathway compromises cell envelope integrity, resulting in cell lysis and death. We propose that the CenK enR pathway may be a suitable target for new antibiotic development, given previous successes in targeting the bacterial cell wall. Finally, the ability of our in vitro phosphotransfer profiling method to identify signaling pathways that operate in vivo takes advantage of an observation that histidine kinases are endowed with a global kinetic preference for their cognate response regulators. We propose that this system-wide selectivity insulates two-component pathways from one another, preventing unwanted cross-talk. Histidine kinases and their (sensory) response regulators are screened for inC. crescentus.Follow-up experiments determine several essential components, including one pair critical for cell envelope biogenesis and structure.
[本文引用: 1]
[本文引用: 1]
URLPMID:20825354 [本文引用: 1]

Bacteria sense and respond to a wide range of physical and chemical signals. Central to sensing and responding to these signals are two-component systems, which have a sensor histidine kinase (SK) and a response regulator (RR) as basic components. Here we review the different molecular mechanisms by which these signals are integrated and modulate the phosphorylation state of SKs. Apart from the basic mechanism, which consists of signal recognition by the SK that leads to an alteration of its autokinase activity and subsequently a change in the RR phosphorylation state, a variety of alternative modes have evolved. The biochemical data available on SKs, particularly their molecular interactions with signals, nucleotides, and their cognate RRs, are also reviewed.
URLPMID:151940 [本文引用: 1]

The abilities of Streptococcus mutans to form biofilms and to survive acidic pH are regarded as two important virulence determinants in the pathogenesis of dental caries. Environmental stimuli are thought to regulate the expression of several genes associated with virulence factors through the activity of two-component signal transduction systems. Yet, little is known of the involvement of these systems in the physiology and pathogenicity of S. mutans. In this study, we describe a two-component regulatory system and its involvement in biofilm formation and acid resistance in S. mutans. By searching the S. mutans genome database with tblastn with the HK03 and RR03 protein sequences from S. pneumoniae as queries, we identified two genes, designated hk11 and rr11, that encode a putative histidine kinase and its cognate response regulator. To gain insight into their function, a PCR-mediated allelic-exchange mutagenesis strategy was used to create the hk11 (Em(r)) and rr11 (Em(r)) deletion mutants from S. mutans wild-type NG8 named SMHK11 and SMRR11, respectively. The mutants were examined for their growth rates, genetic competence, ability to form biofilms, and resistance to low-pH challenge. The results showed that deletion of hk11 or rr11 resulted in defects in biofilm formation and resistance to acidic pH. Both mutants formed biofilms with reduced biomass (50 to 70% of the density of the parent strain). Scanning electron microscopy revealed that the biofilms formed by the mutants had sponge-like architecture with what appeared to be large gaps that resembled water channel-like structures. The mutant biofilms were composed of longer chains of cells than those of the parent biofilm. Deletion of hk11 also resulted in greatly diminished resistance to low pH, although we did not observe the same effect when rr11 was deleted. Genetic competence was not affected in either mutant. The results suggested that the gene product of hk11 in S. mutans might act as a pH sensor that could cross talk with one or more response regulators. We conclude that the two-component signal transduction system encoded by hk11 and rr11 represents a new regulatory system involved in biofilm formation and acid resistance in S. mutans.
[本文引用: 1]
URLPMID:16481212 [本文引用: 1]

The use of hydraulic power transmission to transport offshore wind energy from deep offshore sites to shore may present a more feasible option for integrating wind farms with land-based hydro-energy storage systems. Yet the incurred losses resulting from fluid friction are significantly larger than those encountered in electrical power cables. This study investigates the possibility of compensating for such losses by exploiting cold deep-seawater (DSW) from below thermoclines. A numerical study simulating a single large-scale offshore wind turbine-driven pump supplying DSW to shore across a pipeline in the Central Mediterranean is presented. Seawater leaving the grid-connected hydroelectric power plant is allowed to flow through a centralised district air-conditioning unit operating on a vapour compression cycle. Any shortfall in DSW supply due to lack of wind is compensated for by sea surface water to maintain a constant flow rate. The analysis is repeated for seawater pipelines having different sizes. It is shown that the deep seawater supply from the offshore wind turbine, though being intermittent, reduces the energy consumption of the air-conditioning system considerably. The resulting savings are found to compensate for a significant proportion of the losses encountered in the hydraulic transmission pipeline.
URLPMID:23859207 [本文引用: 1]

Abstract Infections caused by multidrug-resistant bacteria are a considerable and increasing global problem. The development of new antibiotics is not keeping pace with the rapid evolution of resistance to almost all clinically available drugs, and novel strategies are required to fight bacterial infections. One such strategy is the control of pathogenic behaviors, as opposed to simply killing bacteria. Bacterial two-component system (TCS) signal transduction pathways control many pathogenic bacterial behaviors, such as virulence, biofilm formation and antibiotic resistance and are, therefore, an attractive target for the development of new drugs. This review presents an overview of TCS that are potential targets for such a strategy, describes small-molecules inhibitors of TCS identified to date and discusses assays for the identification of novel inhibitors. The future perspective for the identification and use of inhibitors of TCS to potentially provide new therapeutic options for the treatment of drug-resistant bacterial infections is discussed.
[本文引用: 1]
URLPMID:3993181 [本文引用: 1]

The different strains of Bacillus cereus can grow at temperatures covering a very diverse range. Some B. cereus strains can grow in chilled food and consequently cause food poisoning. We have identified a new sensor/regulator mechanism involved in low-temperature B. cereus growth. Construction of a mutant of this two-component system enabled us to show that this system, called CasKR, is required for growth at the minimal temperature (Tmin). CasKR was also involved in optimal cold growth above Tmin and in cell survival below Tmin. Microscopic observation showed that CasKR plays a key role in cell shape during cold growth. Introducing the casKR genes in a 螖casKR mutant restored its ability to grow at Tmin. Although it was first identified in the ATCC 14579 model strain, this mechanism has been conserved in most strains of the B. cereus group. We show that the role of CasKR in cold growth is similar in other B. cereus sensu lato strains with different growth temperature ranges, including psychrotolerant strains.
URLPMID:27435329 [本文引用: 1]

Abstract Two-component systems (TCS) allow a cell to elaborate a variety of adaptive responses to environment changes. The recently discovered CasK/R TCS plays a role in the optimal unsaturation of fatty acids necessary for cold adaptation of the foodborne-pathogen Bacillus cereus Here, we showed that the promoter activity of the operon encoding this TCS was repressed during growth at low temperature in the stationary phase in the parental strain when compared to the casK/R mutant, suggesting that CasR negatively regulates the activity of its own promoter in these conditions. The promoter activity of the desA gene encoding the 02005 fatty acid desaturase, providing unsaturated fatty acids (UFAs) required for low temperature adaptation, was repressed in the casK/R mutant grown at 1200°C versus 3700°C. This result suggests that CasK/R activates desA expression during B. cereus growth at low temperature, allowing an optimal unsaturation of the fatty acids. In contrast, desA expression was repressed during the lag phase at low temperature in presence of UFAs, in a CasK/R-independent manner. Our findings confirm that the involvement of this major TCS in B. cereus cold adaptation is linked to the upregulation of a fatty acid desaturase. 0008 FEMS 2016. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
URLPMID:2698483 [本文引用: 1]

Abstract In the food-borne pathogen Bacillus cereus F4430/73, the production of major virulence factors hemolysin BL (Hbl) and nonhemolytic enterotoxin (Nhe) is regulated through complex mechanisms. The two-component regulatory system ResDE is involved in the activation of hbl and nhe transcription. Here, the response regulator ResD and the sensor kinase ResE were overexpressed and purified, and autophosphorylation of ResE and transphosphorylation of ResD by ResE were demonstrated in vitro. ResD is mainly monomeric in solution, regardless of its phosphorylation state. ResD was shown to interact directly with promoter regions (p) of the enterotoxin regulator genes resDE, fnr, and plcR and the enterotoxin structural genes nhe and hbl, but with different affinities. Binding of ResD to pplcR, pnhe, and phbl was not dependent on the ResD phosphorylation status. In contrast, ResD phosphorylation significantly increased interactions between ResD and presDE and pfnr. Taken together, these results showed that phosphorylation of ResD results in a different target expression pattern. Furthermore, ResD and the redox activator Fnr were found to physically interact and simultaneously bind their target DNAs. We propose that unphosphorylated ResD acts as an antiactivator of Fnr, while phosphorylated ResD acts as a coactivator of Fnr. Finally, our findings represent the first molecular evidence of the role of ResDE as a sentinel system capable of sensing redox changes and coordinating a response that modulates B. cereus virulence.
[本文引用: 1]
URLPMID:3907690 [本文引用: 1]

Two-component signal transduction systems (TCS), consisting of a sensor histidine protein kinase and its cognate response regulator, are an important mode of environmental sensing in bacteria. Additionally, they have been found to regulate virulence determinants in several pathogens. Bacillus anthracis, the causative agent of anthrax and a bioterrorism agent, harbours 41 pairs of TCS. However, their role in its pathogenicity has remained largely unexplored. Here, we show that WalRK of B. anthracis forms a functional TCS which exhibits some species-specific functions. Biochemical studies showed that domain variants of WalK, the histidine kinase, exhibit classical properties of autophosphorylation and phosphotransfer to its cognate response regulator WalR. Interestingly, these domain variants also show phosphatase activity towards phosphorylated WalR, thereby making WalK a bifunctional histidine kinase/phosphatase. An in silico regulon determination approach, using a consensus binding sequence from Bacillus subtilis, provided a list of 30 genes that could form a putative WalR regulon in B. anthracis. Further, electrophoretic mobility shift assay was used to show direct binding of purified WalR to the upstream regions of three putative regulon candidates, an S-layer protein EA1, a cell division ABC transporter FtsE and a sporulation histidine kinase KinB3. Our work lends insight into the species-specific functions and mode of action of B. anthracis WalRK.
[本文引用: 1]
URL [本文引用: 4]
URLPMID:24575310 [本文引用: 1]

The bacteriumBacillus thuringiensis(Bt) produces delta-endotoxins that possess toxic properties and can be used as biopesticides, as well as a source of genes for the construction of transgenic plants resistant to insects. In Brazil, the introduction ofBtsoybean with insecticidal properties to the velvetbean caterpillar, the main insect pest of soybean, has been seen a promising tool in the management of these agroecosystems. However, the increase in stink bug populations in this culture, in various regions of the country, which are not susceptible to the existing genetically modified plants, requires application of chemicals that damage the environment. Little is known about the actual toxicity ofBtto Hemiptera, since these insects present sucking mouthparts, which hamper toxicity assays with artificial diets containing toxins of this bacterium. In recent studies of cytotoxicity with the gut of different hemipterans, susceptibility in the mechanism of action of delta-endotoxins has been demonstrated, which can generate promising subsidies for the control of these insect pests in soybean. This paper aims to review the studies related to the selection, application and mode of action ofBtin the biological control of the major pest of soybean,Anticarsia gemmatalis, and an analysis of advances in research on the use ofBtfor control hemipterans.
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:11447120 [本文引用: 1]

Abstract Top of page Abstract Introduction Results and discussion Materials and methods Acknowledgements References The transcriptional antiterminator protein LicT regulates the expression of Bacillus subtilis operons involved in 尾-glucoside metabolism. It belongs to a newly characterized family of bacterial regulators whose activity is controlled by the phosphoenolpyruvate:sugar phosphotransferase system (PTS). LicT contains an N-terminal RNA-binding domain (56 residues), and a PTS regulation domain (PRD, 221 residues) that is phosphorylated on conserved histidines in response to substrate availability. Replacement of both His207 and His269 with a negatively charged residue (aspartic acid) led to a highly active LicT variant that no longer responds to either induction or catabolite repression signals from the PTS. In contrast to wild type, the activated mutant form of the LicT regulatory domain crystallized easily and provided the first structure of a PRD, determined at 1.55 resolution. The structure is a homodimer, each monomer containing two analogous 伪-helical domains. The phosphorylation sites are totally buried at the dimer interface and hence inaccessible to phosphorylating partners. The structure suggests important tertiary and quaternary rearrangements upon LicT activation, which could be communicated from the protein C-terminal end up to the RNA-binding domain.
