 ,中山大学生命科学学院,有害生物控制与资源利用国家重点实验室,广州 510275
,中山大学生命科学学院,有害生物控制与资源利用国家重点实验室,广州 510275CRISPR/Cas systems in genome engineering of bacteriophages
Caijiao Liang, Fanmei Meng, Yuncan Ai ,State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China
,State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China通讯作者:
编委: 张天宇
收稿日期:2017-12-25修回日期:2018-02-6网络出版日期:2018-05-20
| 基金资助: |
Editorial board:
Received:2017-12-25Revised:2018-02-6Online:2018-05-20
| Fund supported: |
作者简介 About authors
梁彩娇,硕士研究生,研究方向:微生物学E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (804KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
梁彩娇, 孟繁梅, 艾云灿. 基于CRISPR/Cas系统的噬菌体基因组编辑. 遗传[J], 2018, 40(5): 378-389 doi:10.16288/j.yczz.17-419
Caijiao Liang, Fanmei Meng, Yuncan Ai.
噬菌体(bacteriophage)是地球上独特的生物 体[1,2,3,4]。目前GenBank数据库中有2293条噬菌体基因组序列,但是大部分开放阅读框(open reading frame, ORF)功能未知[5]。受限于技术等因素,对噬菌体功能基因组学研究的速率滞后于噬菌体基因组测序速率。
CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated genes)系统主要存在于原核生物中[6],但是在霍乱弧菌(Vibrio cholerae)噬菌体中也发现了有活性的CRISPR/Cas系统[7]。根据cas位点构造及标签Cas蛋白类别特征,CRISPR/Cas系统被划分为Ⅰ~Ⅵ类和22种亚型[8,9]。本文涉及其中4种亚型,表1简单归纳了其相应的免疫机制。它们的免疫过程大致可以分为3步:(1) 适应:Cas1和Cas2等利用外来核酸(DNA或RNA)形成CRISPR阵列中的新间隔(spacer);(2) 表达:CRISPR阵列的转录产物pre-crRNA (precursor CRISPR RNA)在特定Cas蛋白及某些细胞因子加工下形成crRNAs (CRISPR RNAs)[10,11];(3) 干涉:crRNA与特定Cas蛋白组装形成的crRNP (CRISPR ribonucleoprotein)复合物,在外来核酸原间隔(protospacer)处切割并降解外来核酸。因此,基于CRISPR/Cas系统的基因组编辑技术主要是利用了该系统干涉过程中的两个特性:(1) crRNAs与原间隔的碱基互补配对定位靶标序列;(2) 特定Cas蛋白的核酸酶活性切割、降解外来核酸。
Table 1
表1
表1 CRISPR/Cas系统中4种亚型的免疫机制
Table 1
| 类型 | 代表 | 适应 | pre-crRNA加工 | crRNP复合物 | 干涉 | 参考文献 |
|---|---|---|---|---|---|---|
| Ⅰ-E | E. coli K12 Ⅰ-E | RecBCD和Cas1-2参与,由Cas1识别PAM (CTT) | Cas6特异加工 | Cascade: CasA1B2C6D1E1:61 nt crRNA1 (PDB: 4U7U) | Cse1 (CasA)识别PAM,Cas3切割dsDNA | [12~17] |
| Ⅰ-F | P. aeruginosa UCBPP PA14 Ⅰ-F | Cas1-3与Csy1-4参与 | Cas6特异加工 | 缺乏研究 | Cas2/3切割dsDNA | [18~20] |
| Ⅱ-A | S. pyogenes SF370 Ⅱ-A | tracrRNA-Cas9-Cas1- Cas2-Csn2参与,由Cas9识别PAM (NGG) | tracrRNA、RNaseⅢ和Cas9参与 | SpyCas9:RNA (tracrRNA:crRNA) (EMD-5859) | Cas9识别PAM,并切割dsDNA | [10, 21, 22] |
| Ⅲ-A | S. epidermidis RP62a Ⅲ-A | 缺乏研究 | PNPase参与,Cas6特异加工 | 缺乏研究* | 非PAM依赖,Cas10切割dsDNA有义链,Csm3切割RNA | [11, 23~25] |
新窗口打开|下载CSV
CRISPR阵列中的间隔序列,大部分与噬菌体的核酸片段同源[26]。这表明CRISPR/Cas系统免疫的主要对象是噬菌体,又暗示任意CRISPR/Cas系统都具有编辑噬菌体基因组的可行性。发展CRISPR/Cas基因组编辑技术,有望提供新手段,解决噬菌体功能基因组学研究滞后的问题,促进发展噬菌体基因组工程,发现分子生物学新工具,拓展合成生物学范畴。本文主要综述了目前基于CRISPR/Cas系统的噬菌体基因组编辑的相关研究,拓展了该系统与噬菌体重组系统在噬菌体基因组编辑中的联合应用,并讨论了联合应用中可能存在的问题。
1 基于CRISPR/Cas系统的噬菌体基因组编辑
1.1 天然含CRISPR/Cas系统宿主细菌中噬菌体的基因组编辑
CRISPR/Cas系统存在于45%细菌和87%古生菌中[6],这意味着在应用CRISPR/Cas系统进行噬菌体基因组编辑时会存在一定便利:既无需额外设计、购买和构建由CRISPR/Cas系统衍生的基因编辑质粒,也无需担心质粒在宿主细菌中是否复制和表达及质粒对细胞的毒害作用等问题。1.1.1 基于Ⅱ-A类CRISPR/Cas系统的噬菌体基因组编辑
Martel和Moineau[27]在天然含Ⅱ-A类CRISPR/ Cas系统嗜热链球菌DGCC7710 (Streptococcus thermophilus DGCC7710)中开展了编辑2972烈性噬菌体基因组的研究(图1A)。编辑前,首先解析嗜热链球菌DGCC7710 CRISPR1位点及其原间隔邻近基序(protospacer adjacent motif, PAM) NNAGAAW[28];其次用2972噬菌体侵染DGCC7710,使CRISPR1位点获取系列新间隔从而得到系列噬菌体不敏感突变株;随后,开展两类噬菌体基因组编辑[27],简述如下:
图1
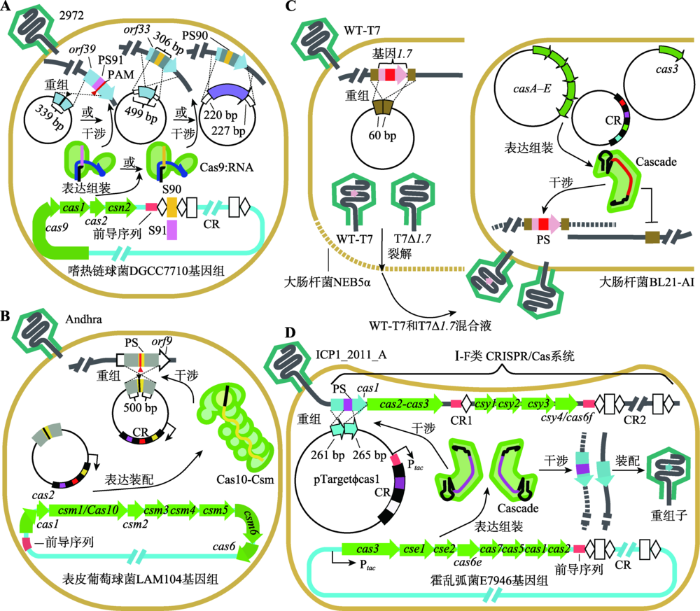 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1基于CRISPR/Cas系统的噬菌体基因组编辑
A:采用Ⅱ-A类CRISPR/Cas系统编辑2972噬菌体基因组。从左到右模板质粒的作用:在原间隔PS91旁侧PAM处引入无义突变(GAA→TAA,红色三角形→黑色三角形)、使非必需基因orf33被删除306 bp长的DNA片段和使orf33被基因LlaDCHIA (紫色)替换。B:采用Ⅲ-A类CRISPR/Cas系统编辑Andhra噬菌体orf9。三角形标示处为编辑点(沉默突变:ATT→ATA,红色三角形→黑色三角形)。C:采用Ⅰ-E类CRISPR/Cas系统编辑T7噬菌体基因1.7。D:采用Ⅰ-E类CRISPR/Cas系统(位于霍乱弧菌E7946基因组上)编辑ICP1_2011_A噬菌体(本身含有Ⅰ-F类CRISPR/Cas系统)基因cas1。S:间隔;PS:原间隔;CR:CRISPR阵列;WT-T7:未发生重组的野生型(Wild type) T7噬菌体;T7Δ1.7:基因1.7被删除的T7噬菌体;csn2为Nmeni亚类cas;csm1-6为Mtube亚类cas;csy1-4为Ypest亚类cas;cse1-2为Ecoli亚类cas。Cas9:RNA、Cascade和Cas10-Csm见
Fig. 1CRISPR/Cas systems in bacteriophage genome editing
第一类:CRISPR/Cas系统对随机突变的选择作用。在Ⅱ-A类CRISPR/Cas系统中,crRNP复合物对外来核酸免疫的前提是:Cas9对PAM的特异性识别[22]以及crRNAs与原间隔之间的碱基互补配对。当外来核酸发生的随机突变为原间隔种子区(seed region)单个或两个碱基的突变、PAM关键碱基的特定突变、包含种子区或PAM在内的序列删除时,往往导致外来核酸对该免疫系统的逃脱[27,29,30]。由此,采用2972噬菌体感染系列噬菌体不敏感突变株,以1%~22%比例筛选系列逃逸CRISPR噬菌体随机突变株,其中随机突变包括同义突变、非同义突变、PAM中GAA到TAA的无义突变和orf33中306 bp长DNA片段的删除[27]。通过大量分析系列逃逸CRISPR噬菌体突变株的原间隔及其PAM碱基的变化情况,既可以确定原间隔的种子区,又可以确定PAM中对靶向噬菌体关键的碱基(AGAA)[27]。
第二类:CRISPR/Cas系统联合同源重组介导噬菌体基因组的点突变、移码突变、基因删除与替换。同源重组在噬菌体基因组编辑中的应用受限于缺乏选择压力,而CRISPR/Cas系统的定点切割作用恰使它成为一个选择工具。该工具切割野生型噬菌体基因组,保留目的同源重组子,从而提高目的重组子的噬菌斑占总噬菌斑的比例。采用高拷贝数载体pNZ123构建系列用于同源重组的模板质粒,获取噬菌体系列重组子:非必需基因orf39中原间隔PS91旁侧PAM处的无义突变(GAA→TAA)和移码突变(ACAAGAAT→AC---AAT)、非必需基因orf33中306 bp长DNA片段的删除、orf33与基因LlaDCHIA的交换[27](图1A)。该实验结果表明,若菌株内含有模板质粒,则相应的噬菌体成斑率(efficiencies of plaquing, EOP)与对照相比,点突变和基因片段的删除都提高2~4个数量级[27]。成斑率提高的原因是:同源重组产生的目的重组子可逃脱CRISPR/Cas系统的靶向[27];Cas9在噬菌体基因组靶标处造成的DNA双链断裂(double strand break, DSB)可以诱发同源重组,从而间接提高同源重组的效率[28]。
然而,此类噬菌体基因组编辑的缺点是:模板质粒作为外来元件,有可能被宿主细菌中天然存在的完整的CRISPR/Cas系统捕获间隔,从而使得新导入的质粒成为CRISPR/Cas系统免疫的对象,最终导致质粒丢失。
1.1.2 基于Ⅲ-A类CRISPR/Cas系统的噬菌体基因组编辑
Bari等[31]在天然含Ⅲ-A类CRISPR/Cas系统表皮葡萄球菌LAM104 (Staphylococcus epidermidis LAM104,即S. epidermidis RP62a?crispr)中开展了编辑Andhra和ISP烈性噬菌体基因组的研究[32](图1B)。其中,菌株RP62a CRISPR阵列的删除,可使新导入的质粒不易被丢失。与Ⅱ-A类CRISPR/Cas系统相比,当利用Ⅲ-A类CRISPR/Cas系统作为选择压力时,双层平板中具有随机突变的噬菌斑的比例会降低[27,31]。分析其原因主要是:其一,该类系统不具备PAM[23];其二,该类系统既能够以转录依赖的方式靶向DNA[25],也能够靶向RNA,且靶标RNA不存在种子区[33]。
Bari等[31]构建了质粒pcrispr/spc?-donor,该质粒不仅能够通过同源重组途径在Andhra和ISP噬菌体的基因组中引入沉默突变,还可以表达特定crRNA,使形成的Cas10-Csm复合物靶向非目的重组子,保留目的重组子(图1B)。此外,采用Ⅲ-A类CRISPR/ Cas系统作为选择压力时,crRNA需要同时满足两个条件[31]:(1) crRNA互补于ORF的有义链[25];(2) 5°末端的标签序列不可与原间隔有义链下游的反标签序列互补,以防Cas10不切割DNA[25,34]。
Martel和Moineau[27]及Bari等[31]为了靶向噬菌体的特定基因,构建加载CRISPR位点的质粒pRS91R与pcrispr/spc?。但是,Hynes等[35]创建了一种在天然CRISPR阵列中引入特定噬菌体源的间隔的方法:首先,将噬菌体源的特定原间隔及其PAM加载于高拷贝数载体pNZ123,得pNZCR1或pNZCR3;其次,将质粒导入嗜热链球菌DGCC7710中;再次,用2972噬菌体侵染已经拥有pNZCR1或pNZCR3的DGCC7710;最后,确定在平板上生长的单菌落,大多数对应CRISPR阵列内含有特定间隔,并且无质粒的菌株。
1.2 无CRISPR/Cas系统宿主细菌中噬菌体的基因组编辑
该类噬菌体基因组编辑的基本思路是:为了编辑靶基因,以质粒的方式导入同源重组模板;为了富集噬菌体重组子,将CRISPR位点和cas位点转到无CRISPR/Cas系统的宿主细菌中。这类研究尚不多,本文仅对4例情形介绍如下。Kiro等[37]在无CRISPR/Cas系统大肠杆菌(Escherichia coli) NEB5α和BL21-AI中对T7烈性噬菌体基因组进行了编辑(图1C)。大肠杆菌NEB5α含同源重组模板质粒(pBAD-1.760或pUC19-4.360),而BL21-AI含3种质粒:pAnti-1.7/4.3具有靶向相应基因(基因1.7或4.3)的间隔,pWUR400具有casA/B/C/ D/E系列基因,pWUR397编码Cas3[37]。首先采用T7噬菌体感染含模板质粒的大肠杆菌NEB5α,获得同时具有重组子和野生型噬菌体的混合裂解液;然后采用该混合噬菌体感染BL21-AI[37]。在BL21-AI中,crRNP复合物首先利用CasA亚基识别噬菌体基因组中的PAM[16],然后利用crRNAs与原间隔的碱基互补配对定位在基因1.7或4.3中的靶标序列,再召集Cas3酶以切割靶标序列[17,38,39],从而降解野生型噬菌体。应用这种方法编辑基因1.7得到的成斑率约为对照的10 000倍[37]。
Box等[40]在无CRISPR/Cas系统霍乱弧菌E7946中编辑ICP1_2011_A烈性噬菌体基因组(图1D)。首先,采用自然转化(natural transformation),将霍乱弧菌O395的基因组岛GI-24 (含Ⅰ-E类CRISPR/Cas系统)转入霍乱弧菌E7946中,使得E7946具备CRISPR/Cas系统;其次,设计系列质粒,如pTarget?cas1 (图1D,左下角),使它们既可用于同源重组,又可编码靶向ICP1_2011_A噬菌体Ⅰ-F类CRISPR/Cas系统基因cas1或cas2-3 (图1D,顶部)的crRNAs[7];最后,实施对各个基因的编辑[40]。其中,对cas1中原间隔的删除实验获得理想的实验结果:成斑率是对照的10倍[40]。但是,Box等[40]的方法(图1D)与Martel和Moineau [27]的(图1A)都存在相同的缺点:导入的外来质粒容易被CRISPR/Cas系统所免疫。
Kiro等[37]方法(图1C)和Box等[40]方法(图1D)的最大不同点在于同源重组模板、CRISPR位点和cas位点三者之间的搭配方式。Kiro等[37]将三者分别加载于不同的质粒载体;Box等[40]则将同源重组模板和CRISPR位点加载于同一质粒载体,利用霍乱弧菌自然转化的特性将cas位点整合到宿主细菌染色体中。显然,无CRISPR/Cas系统宿主细菌中噬菌体的基因组编辑方案会因菌种不同而有所改变。
酿脓链球菌(Streptococcus pyogenes)Ⅱ-A类CRISPR/Cas系统衍生出来的基因编辑工具的成份简单、应用成熟,在无CRISPR/Cas系统的宿主细菌中编辑噬菌体基因组时,可优先考虑利用该系统作为选择压力。Lemay等[41]和Tao等[30]采用电转化方式将质粒(加载酿脓链球菌Ⅱ-A类CRISPR/Cas系统)导入待编辑噬菌体的特定宿主细菌中。其中,cas9、tracrRNA (trans-activating CRISPR RNA)和CRISPR位点共同加载于一种质粒载体(如pTRK2或cloDF13-aadA)上,同源重组模板则单独加载于另一种质粒载体(如pNZ123或pET28b)上[30,41]。Lemay等[41]利用这类方法,对乳酸乳球菌MG163 (Lactococcus lactis MG163)的p2烈性噬菌体基因组实施基因删除、点突变和His6序列插入系列操作。Tao等[30]采用该方案,对大肠杆菌野生型T4或T4(C) (此类T4噬菌体的胞嘧啶在大肠杆菌B834中不被修饰)烈性噬菌体基因组实施无义突变、沉默突变和基因片段删除系列操作。
2 CRISPR/Cas系统在噬菌体基因组编辑中的拓展应用
2.1 联合使用CRISPR/Cas系统与噬菌体重组系统
前述的噬菌体基因组编辑实例都有一个共同点:宿主细菌本身具有的重组系统(如RecA/RecBCD)介导了质粒模板与靶基因之间的重组。然而,细菌的重组系统利用寡核苷酸来编辑自身基因组中的靶基因的能力不足,而这种不足可由宿主细菌特异的噬菌体重组系统来弥补[42,43]。例如,利用寡核苷酸在大肠杆菌gal基因中引入点突变时,选择菌株SIMD (含bet基因)作为编辑对象要比HME75 (无Red与Rac系统)效果好,前者得到的重组子是后者的6 × 104倍[42];大肠杆菌MG1655天然重组系统介导寡核苷酸与靶基因之间的重组效率要低于HME63 (含缺陷λ原噬菌体)[43]。λ噬菌体的Red系统和Rac原噬菌体的RecE/T系统,具有相近的重组原理,且重组过程都独立于RecA[44,45]。利用这两个系统实施重组的优点是:以PCR扩增所得的dsDNA及寡核苷酸为模板时,要比以质粒为模板更容易设计。Red和RecE/T系统不仅可以应用于其他物种的基因组编辑,还可以用于噬菌体本身的基因组编辑。比如,Oppenheim等[46]采用Red系统编辑裂解性λ噬菌体基因组;Marinelli等[47]采用分枝杆菌噬菌体Che9c (Mycobacteriophage Che9c) RecE/T类蛋白gp60-61编辑分枝杆菌噬菌体基因组。下面以Oppenheim等[46]、Marinelli等[47]及Jiang等[43]研究为参考,对联合使用CRISPR/Cas系统及Red系统或BRED (Bacteriophage Recombineering of Electroporated DNA)策略编辑噬菌体基因组的实验方案做介绍。
第一类:联合使用CRISPR/Cas系统及Red系统在大肠杆菌内编辑裂解性λ噬菌体的基因组[46]。Oppenheim等[46]所用系列大肠杆菌的染色体上整合有缺陷λ原噬菌体,原噬菌体上的cⅠ857温敏抑制子用来调节red操纵子PL启动子的开与关(图2)。该实验的操作过程简化为:首先,用噬菌体感染宿主细菌;其次,42 ℃热击细胞,致使cⅠ857表达的温敏阻遏物失效、red操纵子表达;再次,将PCR扩增所得的dsDNA或寡核苷酸模板电转化进入细胞内,使模板和噬菌体靶基因可以在Exo外切酶、Beta重组酶和Gam蛋白的作用下发生重组;最后,根据噬菌斑形态特征、测序结果、裂解液重侵染所得溶源菌的抗生素抗性情况和PCR来判定重组子。该方法虽然可以对λ噬菌体的基因组进行基因的无义突变、删除和替换系列操作(图2),但是重组子的噬菌斑占总噬菌斑的百分比并不高,仅为1%~13%。为了提高重组子比例,可以利用CRISPR/Cas系统制造选择压力,留下已发生重组的噬菌体,淘汰未发生重组的噬菌体。若选择酿脓链球菌Ⅱ-A类CRISPR/ Cas系统作为选择压力,则具体操作是:首先,将加载了cas9和tracrRNA的质粒pCas9-1、加载了CRISPR位点的质粒pRSnR导入宿主细菌(染色体上整合缺陷λ原噬菌体);其次,诱导质粒表达Cas9、tracrRNA和CRISPR阵列,诱导时间根据具体情况而定;最后,后续实验保持和前述一致,即噬菌体感染宿主细菌、red操纵子诱导表达等(图2)。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2CRISPR/Cas系统联合Red系统对λ噬菌体基因组的编辑
S1、S2和S3分别为间隔1、间隔2和间隔3;PS1、PS2和PS3则分别为原间隔1、原间隔2和原间隔3;CR:CRISPR阵列;SpyCas9:RNA见
Fig. 2Editing of the bacteriophage λ genome with CRISPR/Cas and Red systems
因为大肠杆菌天然存在的CRISPR/Cas系统可能都为Ⅰ类,所以从CRISPR/Cas系统与细胞契合度高低的角度看,也许选择Ⅰ类系统作为选择压力比Ⅱ类更佳。目前尚缺少专门比较这两类系统在提高噬菌体重组子比例方面的研究。此外,当有意选择具有Ⅰ-E类CRISPR/Cas系统的细菌作为宿主时,虽然该系统可以直接作为选择压力,但是为了防止Cas1和Cas2形成的复合物从噬菌体和导入的质粒中获取新间隔来免疫外来元件[12,13,14],应当采用同源重组等方法将“cas1和cas2”或CRISPR位点敲除。
第二类:联合使用CRISPR/Cas系统及BRED在耻垢分枝杆菌(Mycobacterium smegmatis)内编辑分枝杆菌烈性噬菌体的基因组[47,48]。其中,BRED操作分3步:第一,诱导耻垢分枝杆菌mc2155:pJV53,使得质粒pJV53表达外切酶gp60和重组酶gp61之后,制备感受态细胞[48],并将噬菌体DNA与PCR扩增所得的dsDNA底物同时电转化进入细胞,铺平板;第二,挑选平板上多个噬菌斑,采用引物P1/2或P2/3开展常规PCR或选择性PCR(图3),电泳(若电泳条带不单一,则相应的噬菌斑是重组子与非重组子混合的噬菌斑);第三,将混合噬菌斑的裂解液混合,实施双层平板实验,经过挑斑与PCR扩增后,电泳检测纯合重组子[47]。这样,与CRISPR/Cas系统和Red系统联合类似,将CRISPR/Cas系统与BRED联合使用,有望提高第二步混合斑和第三步重组子斑的比例。
图3
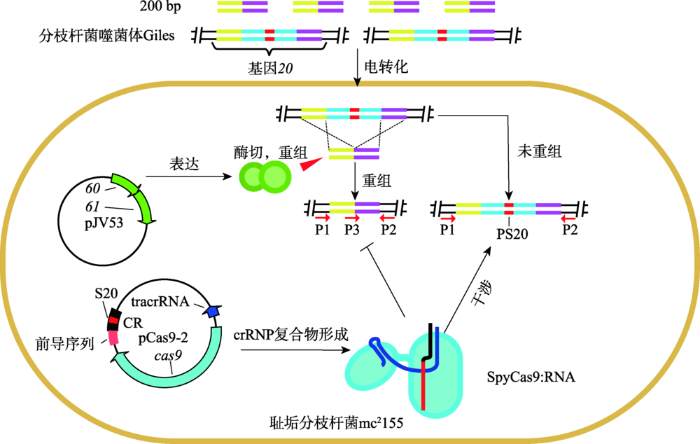 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3CRISPR/Cas系统联合BRED对分枝杆菌噬菌体基因的编辑
S20:间隔20;PS20;原间隔20;CR:CRISPR阵列;P1、P2和P3分别为引物1 (primer1)、引物2和引物3;SpyCas9:RNA见
Fig. 3Editing of the mycobacteriophage gene with CRISPR/Cas systems and BRED
联合使用CRISPR/Cas系统与噬菌体重组系统实施噬菌体基因组编辑时,可以将两类系统独立地转入到宿主细菌内(在第一类中,Red系统存在于缺陷λ原噬菌体上,而CRISPR/Cas系统加载于质粒 中[46];在第二类中,表达外切酶gp60和重组酶gp61的基因60-61与CRISPR/Cas系统加载于不同的质粒 中[47,48]),也可以将两类系统进行搭配。第一类可以采用同源重组使cas9和tracrRNA整合于缺陷λ原噬菌体上[49];第二类可以考虑利用宿主细菌已知的温和噬菌体,构建包含cas9和tracrRNA、基因60-61、与温和噬菌体包装相关基因的噬粒[50]。
2.2 拓展应用中的潜在问题
联合CRISPR/Cas系统与噬菌体重组系统,构建裂解性噬菌体重组子,需要注意以下几个问题。第一,噬菌体重组系统的选择问题。当利用寡核苷酸在耻垢分枝杆菌基因组中引入点突变时,重组酶来源不同,它在该菌中所体现的活性亦不同,活性大小分别为Che9c gp61>Rac RecT>Halo gp43> Giles gp53≈λ Beta[51]。显然,分枝杆菌噬菌体Che9c来源的重组酶活性最高。反之,当利用寡核苷酸在大肠杆菌基因组中引入突变时,不宜使用Che9c gp61,但是可以用大肠杆菌λ噬菌体的Beta重组酶[42]。这些意味着噬菌体重组酶在其专一性寄生的宿主细菌中才可能达到较佳活性状态,从而有利于发生重组(这可能是宿主细菌的细胞内液体环境更有利于自身噬菌体的重组酶基因的表达、酶活性的稳定或维持[51],还可能与宿主细菌的基因序列或者GC含量等特征有关)。为此,在编辑噬菌体基因组时,应该选择来自该噬菌体(或近缘的噬菌体)的重组系统。
第二,CRISPR/Cas系统类型的选择问题。正如噬菌体重组酶活性在其专一性寄生的宿主细菌(及其近缘的细菌)中表现较佳,选择该噬菌体的宿主细菌(或与其近缘的菌株)所具有CRISPR/Cas系统,实施噬菌体基因组编辑,可能更为恰当。Kiro等[37]就采用了大肠杆菌K12所具有的Ⅰ-E类CRISPR/Cas系统在BL21-AI中进行T7噬菌体基因的删除实验;类似地,Box等[40]则采用了霍乱弧菌O395 Ⅰ-E类CRISPR/Cas系统在E7946中进行ICP1_2011_A噬菌体基因组的编辑。
第三,质粒载体选择问题。首先,考虑质粒拷贝数高低,质粒必须在特定宿主细菌中复制,并表达Cas蛋白及CRISPR阵列等。该质粒一般来源于相应宿主细菌。Lemay等[41]研究发现高拷贝数质粒pCas9[43]不能在乳酸乳球菌MG1363中克隆,高拷贝数质粒pNZCas9不能转入乳酸乳球菌MG1363中。Lemay等[41]推测造成这一现象的原因是:高拷贝数质粒过量表达的Cas9,毒害了宿主细胞。其次,考虑不同质粒具有不同复制系统(避免质粒不相容性)及不同抗性标记基因。Kiro等[37]采用3种质粒pWUR397、pWUR400和pAnti-1.7/pAnti-4.3,分别具备卡那霉素、链霉素和氯霉素抗性,质粒载体则分别为pRSF-1b、pCDF-1b和pACYCDuet-1[15]。此外,还应该考虑质粒不能含原间隔、质粒不能含与噬菌体或细菌基因组发生重组的序列等问题。
第四,模板底物问题。应考虑底物单双链及底物同源臂的合适长度等问题。Marinelli等[47]对Giles基因20实施片段删除的实验中,发现dsDNA底物比ssDNA好,而200 bp dsDNA又比100 bp dsDNA重组效率高。此外,应用“Red系列蛋白或重组酶gp61”和ssDNA,在宿主细菌基因组中引入点突变时,基因后随链的互补ssDNA比前导链的互补ssDNA更容易获得高的重组效率[42,44,51]。因此,在应用类似的方法对噬菌体的基因组进行编辑时,应该考虑ssDNA与靶基因单链的互补性问题。
第五,宿主细菌的防御与噬菌体的反防御问题。由于Ⅲ-A类CRISPR/Cas系统以转录依赖的方式切割DNA有义链,使得这类系统不易免疫原噬菌 体[24,25]。但是,Ⅰ-E类CRISPR/Cas系统不同,只要原噬菌体上存在原间隔及其PAM,就会发生免 疫[52]。据推测,Ⅱ类CRISPR/Cas系统与Ⅰ-E类CRISPR/Cas系统具有相似的机制免疫原噬菌体[53]。如同某些噬菌体可以通过限制性内切酶酶切位点修饰等策略来避免宿主细菌的限制—修饰系统[54],原噬菌体中存在着类似机制:采用抗CRISPR蛋白来阻止CRISPR/Cas系统对于原噬菌体的免疫防御。例如,铜绿假单胞菌PA14 (Pseudomonas aeruginosa PA14) (含有Ⅰ-F类CRISPR/Cas系统)的溶源性噬菌体JBD30等,可编码抗CRISPR蛋白。该类蛋白通过与特定Cas蛋白互作来抑制宿主细菌Ⅰ-F类CRISPR/Cas系统的免疫活性,从而维持JBD30等的溶源性[55,56]。又例如,单核细胞李斯特菌1043s (Listeria monocytogenes 1043s) (含有Ⅱ-A类CRISPR/Cas系统)的原噬菌体?J0161a,可编码抗CRISPR蛋白AcrⅡA1和AcrⅡA2。AcrⅡA1和AcrⅡA2蛋白可以使得含有靶标序列的质粒pT成功转化过量表达Cas9的单核细胞李斯特菌1043s::?J0161a[57]。此外,Hynes等[58]发现,嗜热链球菌DGCC7854的烈性噬菌体D4276可表达抗CRISPR蛋白AcrⅡA5;Seed等[7]在霍乱弧菌ICP1_2011_A噬菌体中发现具免疫活性的Ⅰ-F类CRISPR/Cas系统;Tao等[30]发现,T4噬菌体基因组修饰对于Cas9活性的抑制强度因为原间隔序列的不同而不同。因此,分析噬菌体基因组中是否存在抗CRISPR基因、有活性的CRIPSR/ Cas系统及碱基修饰等,对于选择CRISPR/Cas系统开展噬菌体基因组编辑是十分重要的环节。
第六,重组子的筛选问题。除利用互补实验以外,必需基因被敲除的纯重组子难以获得,因为这类重组子可能不形成噬菌斑,或者本就无法产生病毒颗粒。即使假设有噬菌斑的产生,也尚缺少一个从众多斑中准确挑取重组子噬菌斑的方法。此外,噬菌体基因组编辑中存在着选择标记缺乏的问题,而CRISPR/Cas系统仅在一定程度上弥补了这个问题:作为选择压力来富集重组子并间接提高重组效率。无疑,重组子筛选与选择标记缺乏是噬菌体研究领域需要攻克的问题。这些问题的解决有助于利用CRISPR/Cas系统将噬菌体基因组的基因按生命周期关键程度划分等级,从而更有利于噬菌体功能基因组学的研究与利用。
3 结语与展望
噬菌体入侵,使细菌进化出CRISPR/Cas获得性免疫系统。细菌的Ⅰ、Ⅱ、Ⅲ和Ⅵ类CRISPR/Cas系统作为防御武器可降解入侵的噬菌体[15,23,59,60]。随着揭示CRISPR/Cas系统作用机制及其各亚型用于基因组编辑案例的积累,各类系统(尤其是占60%的Ⅰ类[8])都有望用于编辑噬菌体基因组。CRISPR/Cas系统与噬菌体重组系统(如Red系统及RecE/T系统)的联合使用,可能促进编辑噬菌体基因组。对噬菌体近百年来的研究,奠定了其在分子遗传学、合成生物学、纳米生物技术(如噬菌体展示技术[61])、细菌性疾病致病机制和食品安全等领域不可替代的基础作用[62]。下一个百年研究有望带来更多惊喜,噬菌体基因组编辑技术可能成为剖析及利用噬菌体资源的有力工具。例如,基于CRISPR/Cas系统编辑噬菌体基因组,从头合成噬菌体基因组、在酵母体内进行噬菌体DNA片段组装等[63],还可以考虑将某种噬菌体基因组DNA构建成噬粒,统一在大肠杆菌中进行修饰、编辑后,将噬菌体突变株导入相应宿主细菌中,分析相应基因功能等,由此可促进噬菌体基因组编辑在合成生物学中的潜在应用。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:2755508 [本文引用: 1]

Abstract The concentration of bacteriophages in natural unpolluted waters is in general believed to be low, and they have therefore been considered ecologically unimportant. Using a new method for quantitative enumeration, we have found up to 2.5 x 10(8) virus particles per millilitre in natural waters. These concentrations indicate that virus infection may be an important factor in the ecological control of planktonic micro-organisms, and that viruses might mediate genetic exchange among bacteria in natural aquatic environments.
URLPMID:11229937 [本文引用: 1]

In this study, we optimized procedures to enumerate viruses from marine sediments by epifluorescence microscopy using SYBR Green I as a stain. The highest virus yields from the bulk of the sediments were obtained by utilizing pyrophosphate and 3 min of sonication. The efficiency of extraction benthic viruses by pyrophosphate-ultrasound treatment was about 60% of the extractable virus particles. Samples treated with nucleases had increased virus counts, suggesting a masking effect of extracellular DNA. No significant differences were observed between virus counts obtained by epifluorescence microscopy and transmission electron microscopy. Both formaldehyde and glutaraldehyde gave significant reductions of virus counts after only 24 h of sediment storage, but no further loss occurred after 7 days.
URLPMID:15933010 [本文引用: 1]

The importance of viruses in marine microbial ecology has been established over the past decade. Specifically, viruses influence bacterial abundance and community composition through lysis and alter bacterial genetic diversity through transduction and lysogenic conversion. By contrast, the abundance and distribution of viruses in soils are almost completely unknown. This study describes the abundance and diversity of autochthonous viruses in six Delaware soils: two agricultural soils, two coastal plain forest soils, and two piedmont forest soils. Viral abundance was measured using epifluorescence microscopy, while viral diversity was assessed from morphological data obtained through transmission electron microscopy. Extracted soil virus communities were dominated by bacteriophages that demonstrated a wide range of capsid diameters (20 nm to 160 nm) and morphologies, including filamentous forms and phages with elongated capsids. The reciprocal Simpson's index suggests that forest soils harbor more diverse assemblages of viruses, particularly in terms of morphological distribution. Repeated extractions of virus-like particles (VLPs) from soils indicated that the initial round of extraction removes approximately 70% of extractable viruses. Higher VLP abundances were observed in forest soils (1.31 x 10(9) to 4.17 x 10(9) g(-1) dry weight) than in agricultural soils (8.7 x 10(8) to 1.1 x 10(9) g(-1) dry weight). Soil VLP abundance was significantly correlated to moisture content (r = 0.988) but not to soil texture. Land use (agricultural or forested) was significantly correlated to both bacterial (r = 0.885) and viral (r = 0.812) abundances, as were soil organic matter and water content. Thus, land use is a significant factor influencing viral abundance and diversity in soils.
URL [本文引用: 1]
URLPMID:25919952 [本文引用: 1]

Abstract The bacteriophage population is large, dynamic, ancient, and genetically diverse. Limited genomic information shows that phage genomes are mosaic, and the genetic architecture of phage populations remains ill-defined. To understand the population structure of phages infecting a single host strain, we isolated, sequenced, and compared 627 phages of Mycobacterium smegmatis. Their genetic diversity is considerable, and there are 28 distinct genomic types (clusters) with related nucleotide sequences. However, amino acid sequence comparisons show pervasive genomic mosaicism, and quantification of inter-cluster and intra-cluster relatedness reveals a continuum of genetic diversity, albeit with uneven representation of different phages. Furthermore, rarefaction analysis shows that the mycobacteriophage population is not closed, and there is a constant influx of genes from other sources. Phage isolation and analysis was performed by a large consortium of academic institutions, illustrating the substantial benefits of a disseminated, structured program involving large numbers of freshman undergraduates in scientific discovery.
URLPMID:17521438 [本文引用: 2]

Background In Archeae and Bacteria, the repeated elements called CRISPRs for "clustered regularly interspaced short palindromic repeats" are believed to participate in the defence against viruses....
URLPMID:23446421 [本文引用: 3]

Bacteriophages (or phages) are the most abundant biological entities on earth, and are estimated to outnumber their bacterial prey by tenfold(1). The constant threat of phage predation has led to the evolution of a broad range of bacterial immunity mechanisms that in turn result in the evolution of diverse phage immune evasion strategies, leading to a dynamic co-evolutionary arms race(2,3). Although bacterial innate immune mechanisms against phage abound, the only documented bacterial adaptive immune system is the CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins) system, which provides sequence-specific protection from invading nudeic acids, including phage(4-11). Here we show a remarkable turn of events, in which a phage-encoded CRISPR/Cas system is used to counteract a phage inhibitory chromosomal island of the bacterial host. A successful lytic infection by the phage is dependent on sequence identity between CRISPR spacers and the target chromosomal island. In the absence of such targeting, the phage-encoded CRISPR/Cas system can acquire new spacers to evolve rapidly and ensure effective targeting of the chromosomal island to restore phage replication.
URLPMID:5426118 [本文引用: 2]

Abstract The evolution of CRISPR-cas loci, which encode adaptive immune systems in archaea and bacteria, involves rapid changes, in particular numerous rearrangements of the locus architecture and horizontal transfer of complete loci or individual modules. These dynamics complicate straightforward phylogenetic classification, but here we present an approach combining the analysis of signature protein families and features of the architecture of cas loci that unambiguously partitions most CRISPR-cas loci into distinct classes, types and subtypes. The new classification retains the overall structure of the previous version but is expanded to now encompass two classes, five types and 16 subtypes. The relative stability of the classification suggests that the most prevalent variants of CRISPR-Cas systems are already known. However, the existence of rare, currently unclassifiable variants implies that additional types and subtypes remain to be characterized.
URLPMID:28111461 [本文引用: 1]

Class 2 CRISPR-Cas systems are characterized by effector modules that consist of a single multidomain protein, such as Cas9 or Cpf1. We designed a computational pipeline for the discovery of novel class 2 variants and used it to identify six new CRISPR-Cas subtypes. The diverse properties of these new systems provide potential for the development of versatile tools for genome editing and regulation. In this Analysis article, we present a comprehensive census of class 2 types and class 2 subtypes in complete and draft bacterial and archaeal genomes, outline evolutionary scenarios for the independent origin of different class 2 CRISPR-Cas systems from mobile genetic elements, and propose an amended classification and nomenclature of CRISPR-Cas.
[本文引用: 1]
URLPMID:27701071 [本文引用: 1]

Abstract CRISPR-Cas (Clustered regularly interspaced short palindromic repeats-CRISPR-associated proteins) is a prokaryotic immune system that destroys foreign nucleic acids in a sequence-specific manner using Cas nucleases guided by short RNAs (crRNAs). Staphylococcus epidermidis harbours a Type III-A CRISPR-Cas system that encodes the Cas10-Csm interference complex and crRNAs that are subjected to multiple processing steps. The final step, called maturation, involves a concerted effort between Csm3, a ruler protein in Cas10-Csm that measures six-nucleotide increments, and the activity of a nuclease(s) that remains unknown. Here, we elucidate the contributions of the Cas10-Csm complex toward maturation and explore roles of non-Cas nucleases in this process. Using genetic and biochemical approaches, we show that charged residues in Csm3 facilitate its self-assembly and dictate the extent of maturation cleavage. Additionally, acidic residues in Csm5 are required for efficient maturation, but recombinant Csm5 fails to cleave crRNAs in vitro However, we detected cellular nucleases that co-purify with Cas10-Csm, and show that Csm5 regulates their activities through distinct mechanisms. Altogether, our results support roles for non-Cas nuclease(s) during crRNA maturation and establish a link between Type III-A CRISPR-Cas immunity and central nucleic acid metabolism. The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
URLPMID:25874675 [本文引用: 1]

CRISPR-Cas (clustered, regularly interspaced short palindromic repeats coupled with CRISPR-associated proteins) is a bacterial immunity system that protects against invading phages or plasmids. In the process of CRISPR adaptation, short pieces of DNA ('spacers') are acquired from foreign elements and integrated into the CRISPR array. So far, it has remained a mystery how spacers are preferentially acquired from the foreign DNA while the self chromosome is avoided. Here we show that spacer acquisition is replication-dependent, and that DNA breaks formed at stalled replication forks promote spacer acquisition. Chromosomal hotspots of spacer acquisition were confined by Chi sites, which are sequence octamers highly enriched on the bacterial chromosome, suggesting that these sites limit spacer acquisition from self DNA. We further show that the avoidance of self is mediated by the RecBCD double-stranded DNA break repair complex. Our results suggest that, in Escherichia coli, acquisition of new spacers largely depends on RecBCD-mediated processing of double-stranded DNA breaks occurring primarily at replication forks, and that the preference for foreign DNA is achieved through the higher density of Chi sites on the self chromosome, in combination with the higher number of forks on the foreign DNA. This model explains the strong preference to acquire spacers both from high copy plasmids and from phages.
URLPMID:26478180 [本文引用: 1]

Abstract Bacteria acquire memory of viral invaders by incorporating invasive DNA sequence elements into the host CRISPR locus, generating a new spacer within the CRISPR array. We report on the structures of Cas1-Cas2-dual-forked DNA complexes in an effort toward understanding how the protospacer is sampled prior to insertion into the CRISPR locus. Our study reveals a protospacer DNA comprising a 23-bp duplex bracketed by tyrosine residues, together with anchored flanking 3' overhang segments. The PAM-complementary sequence in the 3' overhang is recognized by the Cas1a catalytic subunits in a base-specific manner, and subsequent cleavage at positions 5 nt from the duplex boundary generates a 33-nt DNA intermediate that is incorporated into the CRISPR array via a cut-and-paste mechanism. Upon protospacer binding, Cas1-Cas2 undergoes a significant conformational change, generating a flat surface conducive to proper protospacer recognition. Here, our study provides important structure-based mechanistic insights into PAM-dependent spacer acquisition. Copyright 2015 Elsevier Inc. All rights reserved.
URLPMID:25707795 [本文引用: 1]

Abstract Bacteria and archaea insert spacer sequences acquired from foreign DNAs into CRISPR loci to generate immunological memory. The Escherichia coli Cas1-Cas2 complex mediates spacer acquisition in vivo, but the molecular mechanism of this process is unknown. Here we show that the purified Cas1-Cas2 complex integrates oligonucleotide DNA substrates into acceptor DNA to yield products similar to those generated by retroviral integrases and transposases. Cas1 is the catalytic subunit and Cas2 substantially increases integration activity. Protospacer DNA with free 3'-OH ends and supercoiled target DNA are required, and integration occurs preferentially at the ends of CRISPR repeats and at sequences adjacent to cruciform structures abutting AT-rich regions, similar to the CRISPR leader sequence. Our results demonstrate the Cas1-Cas2 complex to be the minimal machinery that catalyses spacer DNA acquisition and explain the significance of CRISPR repeats in providing sequence and structural specificity for Cas1-Cas2-mediated adaptive immunity.
URLPMID:18703739 [本文引用: 2]

Abstract Prokaryotes acquire virus resistance by integrating short fragments of viral nucleic acid into clusters of regularly interspaced short palindromic repeats (CRISPRs). Here we show how virus-derived sequences contained in CRISPRs are used by CRISPR-associated (Cas) proteins from the host to mediate an antiviral response that counteracts infection. After transcription of the CRISPR, a complex of Cas proteins termed Cascade cleaves a CRISPR RNA precursor in each repeat and retains the cleavage products containing the virus-derived sequence. Assisted by the helicase Cas3, these mature CRISPR RNAs then serve as small guide RNAs that enable Cascade to interfere with virus proliferation. Our results demonstrate that the formation of mature guide RNAs by the CRISPR RNA endonuclease subunit of Cascade is a mechanistic requirement for antiviral defense.
URLPMID:26863189 [本文引用: 1]

Abstract Clustered regularly interspaced short palindromic repeats (CRISPRs) and the cas (CRISPR-associated) operon form an RNA-based adaptive immune system against foreign genetic elements in prokaryotes. Type I accounts for 95% of CRISPR systems, and has been used to control gene expression and cell fate. During CRISPR RNA (crRNA)-guided interference, Cascade (CRISPR-associated complex for antiviral defence) facilitates the crRNA-guided invasion of double-stranded DNA for complementary base-pairing with the target DNA strand while displacing the non-target strand, forming an R-loop. Cas3, which has nuclease and helicase activities, is subsequently recruited to degrade two DNA strands. A protospacer adjacent motif (PAM) sequence flanking target DNA is crucial for self versus foreign discrimination. Here we present the 2.450900090105 crystal structure of Escherichia coli Cascade bound to a foreign double-stranded DNA target. The 5'-ATG PAM is recognized in duplex form, from the minor groove side, by three structural features in the Cascade Cse1 subunit. The promiscuity inherent to minor groove DNA recognition rationalizes the observation that a single Cascade complex can respond to several distinct PAM sequences. Optimal PAM recognition coincides with wedge insertion, initiating directional target DNA strand unwinding to allow segmented base-pairing with crRNA. The non-target strand is guided along a parallel path 250900090105 apart, and the R-loop structure is further stabilized by locking this strand behind the Cse2 dimer. These observations provide the structural basis for understanding the PAM-dependent directional R-loop formation process.
URLPMID:24748111 [本文引用: 1]

In bacteria, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated (Cas) DNA-targeting complex Cascade (CRISPR-associated complex for antiviral defense) uses CRISPR RNA (crRNA) guides to bind complementary DNA targets at sites adjacent to a trinucleotide signature sequence called the protospacer adjacent motif (PAM). The Cascade complex then recruits Cas3, a nuclease-helicase that catalyzes unwinding and cleavage of foreign double-stranded DNA (dsDNA) bearing a sequence matching that of the crRNA. Cascade comprises the CasA-E proteins and one crRNA, forming a structure that binds and unwinds dsDNA to form an R loop in which the target strand of the DNA base pairs with the 32-nt RNA guide sequence. Single-particle electron microscopy reconstructions of dsDNA-bound Cascade with and without Cas3 reveal that Cascade positions the PAM-proximal end of the DNA duplex at the CasA subunit and near the site of Cas3 association. The finding that the DNA target and Cas3 colocalize with CasA implicates this subunit in a key target-validation step during DNA interference. We show biochemically that base pairing of the PAM region is unnecessary for target binding but critical for Cas3-mediated degradation. In addition, the L1 loop of CasA, previously implicated in PAM recognition, is essential for Cas3 activation following target binding by Cascade. Together, these data show that the CasA subunit of Cascade functions as an essential partner of Cas3 by recognizing DNA target sites and positioning Cas3 adjacent to the PAM to ensure cleavage.
URLPMID:4678832

CRISPR immunity depends on acquisition of fragments of foreign DNA into CRISPR arrays. For type I-E CRISPR–Cas systems two modes of spacer acquisition, na07ve and primed adaptation, were described. Na07ve adaptation requires just two most conserved Cas1 and Cas2 proteins; it leads to spacer acquisition from both foreign and bacterial DNA and results in multiple spacers incapable of immune response. Primed adaptation requires all Cas proteins and a CRISPR RNA recognizing a partially matching target. It leads to selective acquisition of spacers from DNA molecules recognized by priming CRISPR RNA, with most spacers capable of protecting the host. Here, we studied spacer acquisition by a type I-F CRISPR–Cas system. We observe both na07ve and primed adaptation. Both processes require not just Cas1 and Cas2, but also intact Csy complex and CRISPR RNA. Primed adaptation shows a gradient of acquisition efficiency as a function of distance from the priming site and a strand bias that is consistent with existence of single-stranded adaption intermediates. The results provide new insights into the mechanism of spacer acquisition and illustrate surprising mechanistic diversity of related CRISPR–Cas systems.
URLPMID:3133607

Abstract Many bacteria and archaea contain clustered regularly interspaced short palindromic repeats (CRISPRs) that confer resistance to invasive genetic elements. Central to this immune system is the production of CRISPR-derived RNAs (crRNAs) after transcription of the CRISPR locus. Here, we identify the endoribonuclease (Csy4) responsible for CRISPR transcript (pre-crRNA) processing in Pseudomonas aeruginosa. A 1.8 angstrom crystal structure of Csy4 bound to its cognate RNA reveals that Csy4 makes sequence-specific interactions in the major groove of the crRNA repeat stem-loop. Together with electrostatic contacts to the phosphate backbone, these enable Csy4 to bind selectively and cleave pre-crRNAs using phylogenetically conserved serine and histidine residues in the active site. The RNA recognition mechanism identified here explains sequence- and structure-specific processing by a large family of CRISPR-specific endoribonucleases.
URLPMID:28465439

Fluorination represents an important strategy in developing high-performance conjugated polymers for photovoltaic applications. Here, we use regioregular poly(3-ethylhexylthiophene) (P3EHT) and poly(3-ethylhexyl-4-fluorothiophene) (F-P3EHT) as simplified model materials, using single-molecule/aggregate spectroscopy and molecular dynamic simulations, to elucidate the impacts of backbone fluorination on morphology and excitonic coupling on the molecular scale. Despite its high regioregularity, regioregular P3EHT exhibits a rather broad distribution in polymer chain conformation due to the strong steric hindrance of bulky ethylhexyl side chains. This conformational variability results in disordered interchain morphology even between a few chains, prohibiting long-range effective interchain coupling. In stark contrast, the experimental and molecular dynamic calculations reveal that backbone fluorination of F-P3EHT leads to an extended rod-like single-chain conformation and hence highly ordered interchain packing in aggregates. Surprisingly, the ordered and close interchain packing in F-P3EHT does not lead to strong excitonic coupling between the chains but rather to dominant intrachain excitonic coupling that greatly reduces the molecular energetic heterogeneity.
URLPMID:25707807

Clustered regularly interspaced short palindromic repeat (CRISPR) loci and their associated (Cas) proteins provide adaptive immunity against viral infection in prokaryotes. Upon infection, short phage sequences known as spacers integrate between CRISPR repeats and are transcribed into small RNA molecules that guide the Cas9 nuclease to the viral targets (protospacers). Streptococcus pyogenes Cas9 cleavage of the viral genome requires the presence of a 5'-NGG-3' protospacer adjacent motif (PAM) sequence immediately downstream of the viral target. It is not known whether and how viral sequences flanked by the correct PAM are chosen as new spacers. Here we show that Cas9 selects functional spacers by recognizing their PAM during spacer acquisition. The replacement of cas9 with alleles that lack the PAM recognition motif or recognize an NGGNG PAM eliminated or changed PAM specificity during spacer acquisition, respectively. Cas9 associates with other proteins of the acquisition machinery (Cas1, Cas2 and Csn2), presumably to provide PAM-specificity to this process. These results establish a new function for Cas9 in the genesis of prokaryotic immunological memory.
URL [本文引用: 1]
[本文引用: 2]
URLPMID:4214910 [本文引用: 1]

A fundamental feature of immune systems is the ability to distinguish pathogenic from self and commensal elements, and to attack the former but tolerate the latter. Prokaryotic CRISPR-Cas immune systems defend against phage infection by using Cas nucleases and small RNA guides that specify one or more target sites for cleavage of the viral genome. Temperate phages include viruses that can integrate into the bacterial chromosome, and they can carry genes that provide a fitness advantage to the lysogenic host. However, CRISPR-Cas targeting that relies strictly on DNA sequence recognition provides indiscriminate immunity both to lytic and lysogenic infection by temperate phages-compromising the genetic stability of these potentially beneficial elements altogether. Here we show that the Staphylococcus epidermidis CRISPR-Cas system can prevent lytic infection but tolerate lysogenization by temperate phages. Conditional tolerance is achieved through transcription-dependent DNA targeting, and ensures that targeting is resumed upon induction of the prophage lytic cycle. Our results provide evidence for the functional divergence of CRISPR-Cas systems and highlight the importance of targeting mechanism diversity. In addition, they extend the concept of 'tolerance to non-self' to the prokaryotic branch of adaptive immunity.
URLPMID:25959775 [本文引用: 4]

Immune systems must recognize and destroy different pathogens that threaten the host. CRISPR-Cas immune systems protect prokaryotes from viral and plasmid infection utilizing small CRISPR RNAs that are complementary to the invader鈥檚 genome and specify the targets of RNA-guided Cas nucleases. Type III CRISPR-Cas immunity requires target transcription, and whereas genetic studies demonstrated DNA targeting, invitro data have shown crRNA-guided RNA cleavage. The molecular mechanism behind these disparate activities is not known. Here, we show that transcription across the targetsofthe Staphylococcus epidermidis type III-A CRISPR-Cas system results in the cleavage of the target DNA and its transcripts, mediated by independent active sites within the Cas10-Csm ribonucleoprotein effector complex. Immunity against plasmidsand DNA viruses requires DNA, but not RNA, cleavage activity. Our studies reveal a highly versatilemechanism of CRISPR immunity that can defend microorganisms against diverse DNA and RNA invaders.
URLPMID:15791728 [本文引用: 1]

Prokaryotes contain short DNA repeats known as CRISPR, recognizable by the regular spacing existing between the recurring units. They represent the most widely distributed family of repeats among prokaryotic genomes, suggesting a biological function. The origin of the intervening sequences, at present unknown, could provide clues about their biological activities. Here we show that CRISPR spacers derive from preexisting sequences, either chromosomal or within transmissible genetic elements such as bacteriophages and conjugative plasmids. Remarkably, these extrachromosomal elements fail to infect the specific spacer-carrier strain, implying a relationship between CRISPR and immunity against targeted DNA. Bacteriophages and conjugative plasmids are involved in prokaryotic population control, evolution, and pathogenicity. All these biological traits could be influenced by the presence of specific spacers. CRISPR loci can be visualized as mosaics of a repeated unit, separated by sequences at some time present elsewhere in the cell.
URLPMID:25063295 [本文引用: 11]

Abstract Bacteriophages are now widely recognized as major players in a wide variety of ecosystems. Novel genes are often identified in newly isolated phages as well as in environmental metavirome studies. Most of these novel viral genes have unknown functions but appear to be coding for small, non-structural proteins. To understand their biological role, very efficient genetic tools are required to modify them, especially in the genome of virulent phages. We first show that specific point mutations and large deletions can be engineered in the genome of the virulent phage 2972 using the Streptococcus thermophilus CRISPR-Cas Type II-A system as a selective pressure to increase recombination efficiencies. Of significance, all the plaques tested contained recombinant phages with the desired mutation. Furthermore, we show that the CRISPR-Cas engineering system can be used to efficiently introduce a functional methyltransferase gene into a virulent phage genome. Finally, synthetic CRISPR bacteriophage insensitive mutants were constructed by cloning a spacer-repeat unit in a low-copy vector illustrating the possibility to target multiple regions of the phage genome. Taken together, this data shows that the CRISPR-Cas system is an efficient and adaptable tool for editing the otherwise intractable genomes of virulent phages and to better understand phage-host interactions. The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
URLPMID:21048762 [本文引用: 2]

Bacteria and Archaea have developed several defence strategies against foreign nucleic acids such as viral genomes and plasmids. Among them, clustered regularly interspaced short palindromic repeats (CRISPR) loci together with cas (CRISPR-associated) genes form the CRISPR/Cas immune system, which involves partially palindromic repeats separated by short stretches of DNA called spacers, acquired from extrachromosomal elements. It was recently demonstrated that these variable loci can incorporate spacers from infecting bacteriophages and then provide immunity against subsequent bacteriophage infections in a sequence-specific manner. Here we show that the Streptococcus thermophilus CRISPR1/Cas system can also naturally acquire spacers from a self-replicating plasmid containing an antibiotic-resistance gene, leading to plasmid loss. Acquired spacers that match antibiotic-resistance genes provide a novel means to naturally select bacteria that cannot uptake and disseminate such genes. We also provide in vivo evidence that the CRISPR1/Cas system specifically cleaves plasmid and bacteriophage double-stranded DNA within the proto-spacer, at specific sites. Our data show that the CRISPR/Cas immune system is remarkably adapted to cleave invading DNA rapidly and has the potential for exploitation to generate safer microbial strains.
URLPMID:22745249 [本文引用: 1]

A prokaryotic RNA–directed targeting system can be designed to cleave any DNA sequence.
URLPMID:28657724 [本文引用: 5]

Abstract Bacteriophages likely constitute the largest biomass on Earth. However, very few phage genomes have been well-characterized, the tailed phage T4 genome being one of them. Even in T4, much of the genome remained uncharacterized. The classical genetic strategies are tedious, compounded by genome modifications such as cytosine hydroxylmethylation and glucosylation which makes T4 DNA resistant to most restriction endonucleases. Here, using the type-II CRISPR-Cas9 system, we report the editing of both modified (ghm-Cytosine) and unmodified (Cytosine) T4 genomes. The modified genome, however, is less susceptible to Cas9 nuclease attack when compared to the unmodified genome. The efficiency of restriction of modified phage infection varied greatly in a spacer-dependent manner, which explains some of the previous contradictory results. We developed a genome editing strategy by codelivering into E. coli a CRISPR-Cas9 plasmid and a donor plasmid containing the desired mutation(s). Single and multiple point mutations, insertions and deletions were introduced into both modified and unmodified genomes. As short as 50-bp homologous flanking arms were sufficient to generate recombinants that can be selected under the pressure of CRISPR-Cas9 nuclease. A 294-bp deletion in RNA ligase gene rnlB produced viable plaques, demonstrating the usefulness of this editing strategy to determine the essentiality of a given gene. These results provide the first demonstration of phage T4 genome editing that might be extended to other phage genomes in nature to create useful recombinants for phage therapy applications.
URLPMID:28885820 [本文引用: 5]

react-text: 116 Drug-resistant staphylococci, particularly Staphylococcus aureus and Staphylococcus epidermidis , are leading causes of hospital-acquired infections. Bacteriophages and their peptidoglycan hydrolytic enzymes (lysins) are currently being explored as alternatives to conventional antibiotics; however, only a limited diversity of staphylococcal phages and their lysins has yet been characterized.... /react-text react-text: 117 /react-text [Show full abstract]
URL [本文引用: 1]
[本文引用: 1]
URLPMID:2813891 [本文引用: 1]

All immune systems must distinguish self from non-self to repel invaders without inducing autoimmunity. Clustered, regularly interspaced, short palindromic repeat (CRISPR) loci protect bacteria and archaea from invasion by phage and plasmid DNA through a genetic interference pathway. CRISPR loci are present in approximately 40% and approximately 90% of sequenced bacterial and archaeal genomes, respectively, and evolve rapidly, acquiring new spacer sequences to adapt to highly dynamic viral populations. Immunity requires a sequence match between the invasive DNA and the spacers that lie between CRISPR repeats. Each cluster is genetically linked to a subset of the cas (CRISPR-associated) genes that collectively encode >40 families of proteins involved in adaptation and interference. CRISPR loci encode small CRISPR RNAs (crRNAs) that contain a full spacer flanked by partial repeat sequences. CrRNA spacers are thought to identify targets by direct Watson-Crick pairing with invasive 'protospacer' DNA, but how they avoid targeting the spacer DNA within the encoding CRISPR locus itself is unknown. Here we have defined the mechanism of CRISPR self/non-self discrimination. In Staphylococcus epidermidis, target/crRNA mismatches at specific positions outside of the spacer sequence license foreign DNA for interference, whereas extended pairing between crRNA and CRISPR DNA repeats prevents autoimmunity. Hence, this CRISPR system uses the base-pairing potential of crRNAs not only to specify a target, but also to spare the bacterial chromosome from interference. Differential complementarity outside of the spacer sequence is a built-in feature of all CRISPR systems, indicating that this mechanism is a broadly applicable solution to the self/non-self dilemma that confronts all immune pathways.
URLPMID:27143383 [本文引用: 1]

The adaptive immune system of prokaryotes, called CRISPR-Cas (clustered regularly interspaced short palindromic repeats and CRISPR-associated genes), results in specific cleavage of invading nucleic acid sequences recognized by the cell’s “memory” of past encounters. Here, we exploited the properties of native CRISPR-Cas systems to program the natural “memorization” process, efficiently generating immunity not only to a bacteriophage or plasmid but to any specifically chosen DNA sequence. CRISPR-Cas systems have entered the public consciousness as genome editing tools due to their readily programmable nature. In industrial settings, natural CRISPR-Cas immunity is already exploited to generate strains resistant to potentially disruptive viruses. However, the natural process by which bacteria acquire new target specificities (adaptation) is difficult to study and manipulate. The target against which immunity is conferred is selected stochastically. By biasing the immunization process, we offer a means to generate customized immunity, as well as provide a new tool to study adaptation.
URLPMID:27773522

For a long time the mechanism of immunity provided by the Type III CRISPR-Cas systems appeared to be inconsistent: the Type III-A Csm complex ofStaphylocccus epidermidiswas first reported to target DNA while Type III-B Cmr complexes were shown to target RNA. This long-standing conundrum has now been resolved by finding that the Type III CRISPR-Cas systems are both RNases and target RNA-activated DNA nucleases. The immunity is achieved by coupling binding and cleavage of RNA transcripts to the degradation of invading DNA. The base-pairing potential between the target RNA and the CRISPR RNA (crRNA) 5鈥-handle seems to play an important role in discriminating self and non-self nucleic acids; however, the detailed mechanism remains to be uncovered.
URLPMID:24457913 [本文引用: 8]

The clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) system has recently been used to engineer genomes of various organisms, but surprisingly, not those of bacteriophages (phages). Here we present a method to genetically engineer the Escherichia coli phage T7 using the type I-E CRISPR-Cas system. T7 phage genome is edited by homologous recombination with a DNA sequence flanked by sequences homologous to the desired location. Non-edited genomes are targeted by the CRISPR-Cas system, thus enabling isolation of the desired recombinant phages. This method broadens CRISPR Cas-based editing to phages and uses a CRISPR-Cas type other than type II. The method may be adjusted to genetically engineer any bacteriophage genome.
URLPMID:25132177 [本文引用: 1]

Abstract CRISPR drives prokaryotic adaptation to invasive nucleic acids such as phages and plasmids, using an RNA-mediated interference mechanism. Interference in type I CRISPR-Cas systems requires a targeting Cascade complex and a degradation machine, Cas3, which contains both nuclease and helicase activities. Here we report the crystal structures of Thermobifida fusca Cas3 bound to single-stranded (ss) DNA substrate and show that it is an obligate 3'-to-5' ssDNase that preferentially accepts substrate directly from the helicase moiety. Conserved residues in the HD-type nuclease coordinate two irons for ssDNA cleavage. We demonstrate ATP coordination and conformational flexibility of the SF2-type helicase domain. Cas3 is specifically guided toward Cascade-bound target DNA by a PAM sequence, through physical interactions with both the nontarget substrate strand and the CasA protein. The sequence of recognition events ensures well-controlled DNA targeting and degradation of foreign DNA by Cascade and Cas3.
URLPMID:22521689 [本文引用: 1]

Abstract The prokaryotic CRISPR/Cas immune system is based on genomic loci that contain incorporated sequence tags from viruses and plasmids. Using small guide RNA molecules, these sequences act as a memory to reject returning invaders. Both the Cascade ribonucleoprotein complex and the Cas3 nuclease/helicase are required for CRISPR interference in Escherichia coli, but it is unknown how natural target DNA molecules are recognized and neutralized by their combined action. Here we show that Cascade efficiently locates target sequences in negatively supercoiled DNA, but only if these are flanked by a protospacer-adjacent motif (PAM). PAM recognition by Cascade exclusively involves the crRNA-complementary DNA strand. After Cascade-mediated R loop formation, the Cse1 subunit recruits Cas3, which catalyzes nicking of target DNA through its HD-nuclease domain. The target is0002then progressively unwound and cleaved by the joint ATP-dependent helicase activity and Mg(2+)-dependent HD-nuclease activity of Cas3, leading to complete target DNA degradation and invader neutralization. Copyright 0008 2012 Elsevier Inc. All rights reserved.
[本文引用: 7]
URLPMID:28324650 [本文引用: 5]

Abstract Phages are biological entities found in every ecosystem. Although much has been learned about them in past decades, significant knowledge gaps remain. Manipulating virulent phage genomes is challenging. To date, no efficient gene-editing tools exist for engineering virulent lactococcal phages. Lactococcus lactis is a bacterium extensively used as a starter culture in various milk fermentation processes, and its phage sensitivity poses a constant risk to the cheese industry. The lactococcal phage p2 is one of the best-studied models for these virulent phages. Despite its importance, almost half of its genes have no functional assignment. CRISPR-Cas9 genome editing technology, which is derived from a natural prokaryotic defense mechanism, offers new strategies for phage research. Here, the well-known Streptococcus pyogenes CRISPR-Cas9 was used in a heterologous host to modify the genome of a strictly lytic phage. Implementation of our adapted CRISPR-Cas9 tool in the prototype phage-sensitive host L. lactis MG1363 allowed us to modify the genome of phage p2. A simple, reproducible technique to generate precise mutations that allow the study of lytic phage genes and their encoded proteins in vivo is described.
URLPMID:18230724 [本文引用: 4]

We report the identification and functional analysis of nine genes from Gram-positive and Gram-negative bacteria and their phages that are similar to lambda (位) bet or Escherichia coli recT. Beta and RecT are single-strand DNA annealing proteins, referred to here as recombinases. Each of the nine other genes when expressed in E. coli carries out oligonucleotide-mediated recombination. To our knowledge, this is the first study showing single-strand recombinase activity from diverse bacteria. Similar to bet and recT, most of these other recombinases were found to be associated with putative exonuclease genes. Beta and RecT in conjunction with their cognate exonucleases carry out recombination of linear double-strand DNA. Among four of these foreign recombinase/exonuclease pairs tested for recombination with double-strand DNA, three had activity, albeit barely detectable. Thus, although these recombinases can function in E. coli to catalyze oligonucleotide recombination, the double-strand DNA recombination activities with their exonuclease partners were inefficient. This study also demonstrated that Gam, by inhibiting host RecBCD nuclease activity, helps to improve the efficiency of 位 Red-mediated recombination with linear double-strand DNA, but Gam is not absolutely essential. Thus, in other bacterial species where Gam analogs have not been identified, double-strand DNA recombination may still work in the absence of a Gam-like function. We anticipate that at least some of the recombineering systems studied here will potentiate oligonucleotide and double-strand DNA-mediated recombineering in their native or related bacteria.
URLPMID:23360965 [本文引用: 4]

Abstract Here we use the clustered, regularly interspaced, short palindromic repeats (CRISPR)-associated Cas9 endonuclease complexed with dual-RNAs to introduce precise mutations in the genomes of Streptococcus pneumoniae and Escherichia coli. The approach relies on dual-RNA:Cas9-directed cleavage at the targeted genomic site to kill unmutated cells and circumvents the need for selectable markers or counter-selection systems. We reprogram dual-RNA:Cas9 specificity by changing the sequence of short CRISPR RNA (crRNA) to make single- and multinucleotide changes carried on editing templates. Simultaneous use of two crRNAs enables multiplex mutagenesis. In S. pneumoniae, nearly 100% of cells that were recovered using our approach contained the desired mutation, and in E. coli, 65% that were recovered contained the mutation, when the approach was used in combination with recombineering. We exhaustively analyze dual-RNA:Cas9 target requirements to define the range of targetable sequences and show strategies for editing sites that do not meet these requirements, suggesting the versatility of this technique for bacterial genome engineering.
URLPMID:14673109 [本文引用: 2]

Homologous recombination can be used to generate recombinants on episomes or directly on the Escherichia coli chromosome with PCR products or synthetic single-stranded DNA (ssDNA) oligonucleotides (oligos). Such recombination is possible because bacteriophage 位-encoded functions, called Red, efficiently recombine linear DNA with homologies as short as 20-70 bases. This technology, termed recombineering, provides ways to modify genes and segments of the chromosome as well as to study homologous recombination mechanisms. The Red Beta function, which binds and anneals ssDNA to complementary ssDNA, is able to recombine 70-base oligos with the chromosome. In E. coli, methyl-directed mismatch repair (MMR) can affect these ssDNA recombination events by eliminating the recombinant allele and restoring the original sequence. In so doing, MMR can reduce the apparent recombination frequency by >100-fold. In the absence of MMR, Red-mediated oligo recombination can incorporate a single base change into the chromosome in an unprecedented 25% of cells surviving electroporation. Our results show that Beta is the only bacteriophage function required for this level of recombination and suggest that Beta directs the ssDNA to the replication fork as it passes the target sequence.
URLPMID:10921910 [本文引用: 1]

Abstract The initial steps of double-stranded break (DSB) repair by homologous recombination mediated by the 5'-3' exonuclease/annealing protein pairs, RecE/RecT and Redalpha/Redbeta, were analyzed. Recombination was RecA-independent and required the expression of both components of an orthologous pair, even when the need for exonuclease activity was removed by use of preresected substrates. The required orthologous function correlated with a specific protein-protein interaction, and recombination was favored by overexpression of the annealing protein with respect to the exonuclease. The need for both components of an orthologous pair was observed regardless of whether recombination proceeded via a single-strand annealing or a putative strand invasion mechanism. The DSB repair reactions studied here are reminiscent of the RecBCD/RecA reaction and suggest a general mechanism that is likely to be relevant to other systems, including RAD52 mediated recombination.
URL [本文引用: 5]
URLPMID:19088849 [本文引用: 6]

Advances in DNA sequencing technology have facilitated the determination of hundreds of complete genome sequences both for bacteria and their bacteriophages. Some of these bacteria have well-developed and facile genetic systems for constructing mutants to determine gene function, and recombineering is a particularly effective tool. However, generally applicable methods for constructing defined mutants of bacteriophages are poorly developed, in part because of the inability to use selectable markers such as drug resistance genes during viral lytic growth. Here we describe a method for simple and effective directed mutagenesis of bacteriophage genomes using Bacteriophage Recombineering of Electroporated DNA (BRED), in which a highly efficient recombineering system is utilized directly on electroporated phage DNA; no selection is required and mutants can be readily detected by PCR. We describe the use of BRED to construct unmarked gene deletions, in-frame internal deletions, base substitutions, precise gene replacements, and the addition of gene tags.
URLPMID:3503148 [本文引用: 3]

Bacteriophages are central components in the development of molecular tools for microbial genetics. Mycobacteriophages have proven to be a rich resource for tuberculosis genetics, and the recent development of a mycobacterial recombineering system based on mycobacteriophage Che9c-encoded proteins offers new approaches to mycobacterial mutagenesis. Expression of the phage exonuclease and recombinase substantially enhances recombination frequencies in both fast- and slow-growing mycobacteria, thereby facilitating construction of both gene knockout and point mutants; it also provides a simple and efficient method for constructing mycobacteriophage mutants. Exploitation of host-specific phages thus provides a general strategy for recombineering and mutagenesis in genetically naive systems.
URLPMID:28322317 [本文引用: 1]

Discovery of clustered, regularly interspaced, short palindromic repeats and the Cas9 RNA-guided nuclease (CRISPR/Cas9) system provides a new opportunity to create programmable gene-specific antimicrobials that are far less likely to drive resistance than conventional antibiotics. However, the practical therapeutic use of CRISPR/Cas9 is still questionable due to current shortcomings in phage-based delivery systems such as inefficient delivery, narrow host range, and potential transfer of virulence genes by generalized transduction. In this study, we demonstrate genetic engineering strategies to overcome these shortcomings by integrating CRISPR/Cas9 system into a temperate phage genome, removing major virulence genes from the host chromosome, and expanding host specificity of the phage by complementing tail fiber protein. This significantly improved the efficacy and safety of CRISPR/Cas9 antimicrobials to therapeutic levels in bothin vitroandin vivoassays. The genetic engineering tools and resources established in this study are expected to provide an efficacious and safe CRISPR/Cas9 antimicrobial, broadly applicable toStaphylococcus aureus.
URLPMID:25282355 [本文引用: 1]

Antibiotics target conserved bacterial cellular pathways or growth functions and therefore cannot selectively kill specific members of a complex microbial population. Here, we develop programmable, sequence-specific antimicrobials using the RNA-guided nuclease Cas9 (refs.1,2) delivered by a bacteriophage. We show that Cas9, reprogrammed to target virulence genes, kills virulent, but not avirulent, Staphylococcus aureus. Reprogramming the nuclease to target antibiotic resistance genes destroys staphylococcal plasmids that harbor antibiotic resistance genes and immunizes avirulent staphylococci to prevent the spread of plasmid-borne resistance genes. We also show that CRISPR-Cas9 antimicrobials function in vivo to kill S. aureus in a mouse skin colonization model. This technology creates opportunities to manipulate complex bacterial populations in a sequence-specific manner.
URLPMID:18221264 [本文引用: 3]

Summary Top of page Summary Introduction Results Discussion Experimental procedures Acknowledgements References Supporting Information Construction of genetically isogenic strains of mycobacteria is complicated by poor recombination rates and the lack of generalized transducing phages for Mycobacterium tuberculosis . We report here a powerful method for introducing single point mutations into mycobacterial genomes using oligonucleotide-derived single-stranded DNA recombineering and mycobacteriophage-encoded proteins. Phage Che9c gp61-mediated recombination is sufficiently efficient that single base changes can be introduced without requirement for direct selection, with isogenic mutant strains identified simply by PCR. Efficient recombination requires only short (50 nucleotide) oligonucleotides, but there is an unusually strong strand bias and an oligonucleotide targeting lagging strand DNA synthesis can recombine more than 10000-fold efficiently than its complementary oligonucleotide. This ssDNA recombineering provides a simple assay for comparing the activities of related phage recombinases, and we find that both Escherichia coli RecET and phage 位 Red recombination proteins function inefficiently in mycobacteria, illustrating the utility of developing recombineering in new bacterial systems using host-specific bacteriophage recombinases. ssDNA mycobacterial recombineering provides a simple approach to characterizing antimycobacterial drug targets, and we have constructed and characterized single point mutations that confer resistance to isoniazid, rifampicin, ofloxacin and streptomycin.
[本文引用: 1]
[本文引用: 1]
URLPMID:23979432 [本文引用: 1]

Bacteria and their viral predators (bacteriophages) are locked in a constant battle. In order to proliferate in phage-rich environments, bacteria have an impressive arsenal of defence mechanisms, and in response, phages have evolved counter-strategies to evade these antiviral systems. In this Review, we describe the various tactics that are used by phages to overcome bacterial resistance mechanisms, including adsorption inhibition, restriction-modification, CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR-associated proteins) systems and abortive infection. Furthermore, we consider how these observations have enhanced our knowledge of phage biology, evolution and phage-host interactions.
URLPMID:23242138 [本文引用: 1]

A widespread system used by bacteria for protection against potentially dangerous foreign DNA molecules consists of the clustered regularly interspaced short palindromic repeats (CRISPR) coupled with cas (CRISPR-associated) genes(1). Similar to RNA interference in eukaryotes(2), these CRISPR/Cas systems use small RNAs for sequence-specific detection and neutralization of invading genomes(3). Here we describe the first examples of genes that mediate the inhibition of a CRISPR/Cas system. Five distinct 'anti-CRISPR' genes were found in the genomes of bacteriophages infecting Pseudomonas aeruginosa. Mutation of the anti-CRISPR gene of a phage rendered it unable to infect bacteria with a functional CRISPR/Cas system, and the addition of the same gene to the genome of a CRISPR/Cas-targeted phage allowed it to evade the CRISPR/Cas system. Phage-encoded anti-CRISPR genes may represent a widespread mechanism for phages to overcome the highly prevalent CRISPR/Cas systems. The existence of anti-CRISPR genes presents new avenues for the elucidation of CRISPR/Cas functional mechanisms and provides new insight into the co-evolution of phages and bacteria.
URLPMID:26416740 [本文引用: 1]

Abstract The battle for survival between bacteria and the viruses that infect them (phages) has led to the evolution of many bacterial defence systems and phage-encoded antagonists of these systems. Clustered regularly interspaced short palindromic repeats (CRISPR) and the CRISPR-associated (cas) genes comprise an adaptive immune system that is one of the most widespread means by which bacteria defend themselves against phages. We identified the first examples of proteins produced by phages that inhibit a CRISPR-Cas system. Here we performed biochemical and in vivo investigations of three of these anti-CRISPR proteins, and show that each inhibits CRISPR-Cas activity through a distinct mechanism. Two block the DNA-binding activity of the CRISPR-Cas complex, yet do this by interacting with different protein subunits, and using steric or non-steric modes of inhibition. The third anti-CRISPR protein operates by binding to the Cas3 helicase-nuclease and preventing its recruitment to the DNA-bound CRISPR-Cas complex. In vivo, this anti-CRISPR can convert the CRISPR-Cas system into a transcriptional repressor, providing the first example-to our knowledge-of modulation of CRISPR-Cas activity by a protein interactor. The diverse sequences and mechanisms of action of these anti-CRISPR proteins imply an independent evolution, and foreshadow the existence of other means by which proteins may alter CRISPR-Cas function.
158.
URLPMID:28041849 [本文引用: 1]

Bacterial CRISPR-Cas systems utilize sequence-specific RNA-guided nucleases to defend against bacteriophage infection. As a countermeasure, numerous phages are known that produce proteins to block the function of class 1 CRISPR-Cas systems. However, currently no proteins are known to inhibit the widely used class 2 CRISPR-Cas9 system. To find these inhibitors, we searched cas9-containing bacterial genomes for the co-existence of a CRISPR spacer and its target, a potential indicator for CRISPR inhibition. This analysis led to the discovery of four unique type II-A CRISPR-Cas9 inhibitor proteins encoded by Listeria monocytogenes prophages. More than half of L. monocytogenes strains with cas9 contain at least one prophage-encoded inhibitor, suggesting widespread CRISPR-Cas9 inactivation. Two of these inhibitors also blocked the widely used Streptococcus pyogenes Cas9 when assayed in Escherichia coli and human cells. These natural Cas9-specific "anti-CRISPRs" present tools that can be used to regulate the genome engineering activities of CRISPR-Cas9.
[本文引用: 1]
URLPMID:17379808 [本文引用: 1]

Clustered regularly interspaced short palindromic repeats (CRISPR) are a distinctive feature of the genomes of most Bacteria and Archaea and are thought to be involved in resistance to bacteriophages. We found that, after viral challenge, bacteria integrated new spacers derived from phage genomic sequences. Removal or addition of particular spacers modified the phage-resistance phenotype of the cell. Thus, CRISPR, together with associated cas genes, provided resistance against phages, and resistance specificity is determined by spacer-phage sequence similarity.
URLPMID:27256883 [本文引用: 1]

The clustered regularly interspaced short palindromic repeat (CRISPR)鈥揅RISPR-associated genes (Cas) adaptive immune system defends microbes against foreign genetic elements via DNA or RNA-DNA interference. We characterize the class 2 type VI CRISPR-Cas effector C2c2 and demonstrate its RNA-guided ribonuclease function. C2c2 from the bacterium Leptotrichia shahii provides interference against RNA phage. In vitro biochemical analysis shows that C2c2 is guided by a single CRISPR RNA and can be programmed to cleave single-stranded RNA targets carrying complementary protospacers. In bacteria, C2c2 can be programmed to knock down specific mRNAs. Cleavage is mediated by catalytic residues in the two conserved Higher Eukaryotes and Prokaryotes Nucleotide-binding (HEPN) domains, mutations of which generate catalytically inactive RNA-binding proteins. These results broaden our understanding of CRISPR-Cas systems and suggest that C2c2 can be used to develop new RNA-targeting tools.
URLMagsci [本文引用: 1]

噬菌体展示技术(Phage display technology, PDT)是一种特殊的基因工程重组表达技术, 噬菌体展示技术系统(Phage display system, PDS)是指包括经过遗传改造后的系列噬菌体、辅助噬菌体、宿主细菌等集成平台(含试剂盒)。文章从噬菌体分子遗传学及其基因(基因组)遗传工程改良角度, 基于噬菌体M13、λ、T4和T7等4大类典型噬菌体展示技术系统的发展进展进行了综述。重点强调不同展示系统中的核心部件及其基因工程改造的分子遗传学原理、不同展示锚定位点的技术特征、相关试剂盒的研制状况及选择依据。
URLMagsci [本文引用: 1]

噬菌体展示技术(Phage display technology, PDT)是一种特殊的基因工程重组表达技术, 噬菌体展示技术系统(Phage display system, PDS)是指包括经过遗传改造后的系列噬菌体、辅助噬菌体、宿主细菌等集成平台(含试剂盒)。文章从噬菌体分子遗传学及其基因(基因组)遗传工程改良角度, 基于噬菌体M13、λ、T4和T7等4大类典型噬菌体展示技术系统的发展进展进行了综述。重点强调不同展示系统中的核心部件及其基因工程改造的分子遗传学原理、不同展示锚定位点的技术特征、相关试剂盒的研制状况及选择依据。
URLPMID:26548913 [本文引用: 1]

Viruses that infect bacteria (bacteriophages; also known as phages) were discovered 100 years ago. Since then, phage research has transformed fundamental and translational biosciences. For example, phages were crucial in establishing the central dogma of molecular biology - information is sequentially passed from DNA to RNA to proteins - and they have been shown to have major roles in ecosystems, and help drive bacterial evolution and virulence. Furthermore, phage research has provided many techniques and reagents that underpin modern biology - from sequencing and genome engineering to the recent discovery and exploitation of CRISPR-Cas phage resistance systems. In this Timeline, we discuss a century of phage research and its impact on basic and applied biology.
URLPMID:27250768 [本文引用: 1]

Soon after their discovery in the early 20th century, bacteriophages were recognized to have great potential as antimicrobial agents, a potential that has yet to be fully realized. The nascent field of phage therapy was adversely affected by inadequately controlled trials and the discovery of antibiotics. Although the study of phages as anti-infective agents slowed, phages played an important role in the development of molecular biology. In recent years, the increase in multidrug-resistant bacteria has renewed interest in the use of phages as antimicrobial agents. With the wide array of possibilities offered by genetic engineering, these bacterial viruses are being modified to precisely control and detect bacteria and to serve as new sources of antibacterials. In applications that go beyond their antimicrobial activity, phages are also being developed as vehicles for drug delivery and vaccines, as well as for the assembly of new materials. This review highlights advances in techniques used to engineer phages for all of these purposes and discusses existing challenges and opportunities for future work.
