 ,上海交通大学系统生物医学研究院比较生物医学研究中心,系统生物医学教育部重点实验室,上海 200240
,上海交通大学系统生物医学研究院比较生物医学研究中心,系统生物医学教育部重点实验室,上海 200240N-WASP regulates cortical neuron migration through its polyPro and VCA domains
Xiulian Shen, Yichao Lu, Zhilian Jia, Qiang Wu ,Key Laboratory of Systems Biomedicine (Ministry of Education), Center for Comparative Biomedicine, Institute of Systems Biomedicine, Shanghai Jiao Tong University, Shanghai 200240, China
,Key Laboratory of Systems Biomedicine (Ministry of Education), Center for Comparative Biomedicine, Institute of Systems Biomedicine, Shanghai Jiao Tong University, Shanghai 200240, China通讯作者:
编委: 许执恒
收稿日期:2018-03-15修回日期:2018-04-19网络出版日期:2018-05-20
| 基金资助: |
Received:2018-03-15Revised:2018-04-19Online:2018-05-20
| Fund supported: |
作者简介 About authors
沈秀莲,硕士研究生,专业方向:发育神经生物学E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (7955KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
沈秀莲, 逯宜超, 甲芝莲, 吴强. N-WASP通过polyPro和VCA结构域调控大脑皮层神经元迁移. 遗传[J], 2018, 40(5): 390-401 doi:10.16288/j.yczz.18-066
Xiulian Shen, Yichao Lu, Zhilian Jia, Qiang Wu.
人类大脑皮层是进化上最晚出现、结构上最为复杂的脑部结构[1],其在脑的高级认知或执行功能(cognition or executive function)中发挥着重要作用。大脑皮层的发育涉及一系列复杂的过程,包括神经元的增殖(proliferation)、迁移(migration)和分化(differentiation)等。大脑皮层是由出生于脑室区(ventricular zone, VZ)的兴奋性神经元前体和出生于皮质下区(subpallium)的抑制性神经元前体,分别通过放射状(radial migration)和切线状(tangential migration)并以先到内侧后到外侧(inside-out)模式迁移形成的板层结构(laminar structure)[2,3,4,5,6,7]。其中,兴奋性神经元前体放射状迁移模式是一个非常复杂的动态协同调控过程:首先,脑室区(ventricular zone, VZ)的神经祖细胞通过不对称分裂产生中间祖细胞(transit amplifying intermediate neuronal progenitor cells);然后,这些祖细胞在脑室下区(subventricular zone, SVZ)继续分裂1~3次后,再以多极状态在中间区(intermediate zone, IZ)中迁移;最后,祖细胞转变成双极状态沿着神经胶质细胞纤维以放射状迁移的方式到达皮质区(cortical plate, CP)[8,9,10]。该过程需要多种信号通路协同调控细胞骨架的动态平衡来完成[4],其正常进行对于后续神经元回路以及神经系统功能的正常发挥起着重要作用。而且,人们发现神经元迁移异常能够导致多种神经精神疾病,因此研究其分子调控机制对于了解精神疾病的发生原因有着重要意义[1]。
原钙粘蛋白介导的细胞粘连与神经元迁移和神经细胞多样性密切相关[11,12,13,14]。原钙粘蛋白家族在神经元树突和轴突发育中也起到关键作用[15,16,17,18,19,20]。最近的研究发现原钙粘蛋白可能与WASP-WAVE (Wiskott- Aldrich syndrome protein and WASP-family verprolin- homologous protein)信号通路有关[13,21]。WASP是Wiskott-Aldrich综合征(WAS)的致病基因[22],该疾病是一种包括湿疹(eczema)在内的性染色体连锁的隐性免疫缺陷疾病[23,24]。WASP蛋白家族共包含11个成员,该家族与肌动蛋白的动态调控有关[25],在各种生理和病理过程中对诸如胞吞(endocytosis)、胞吐(exocytosis)、丝状伪足(filopodia)、板状伪足(lamellipodia)、侵袭伪足(invadopodia)等细胞形态的调控起到关键作用[26]。细胞接收外界信息通过复杂的信号传递通路最终汇集到Arp2/3复合体(actin-related protein 2/3 complex)上,然后调控肌动蛋白丝状分支的动态组装,此动态组装对于神经元迁移以及神经细胞的形态发生也是非常重要的[27,28]。
N-WASP(neural-Wiskott-Aldrich syndrome protein)是WASP-WAVE家族成员之一[25],最初发现于牛(Bos taurus)的脑提取物中,因其与WASP氨基酸序列的高度相似性,故命名为N-WASP(神经性WASP)[29]。它是一个广泛表达的蛋白,不仅在大脑中有丰富的表达,而且在心脏、肺等其他组织中也有表达[29]。N-WASP蛋白主要含有4个功能性结构域:WH1 (WASP homology 1)、GBD (GTPase-binding domain)、polyPro (proline-rich SH3-adaptor-binding region)和VCA (verprolin and cofilin homology regions and the acidic domain)[30]。研究已证实,WH1结构域与N-WASP被招募到肌动蛋白组装的位点有关[31],而GBD、polyPro和VCA这3个结构域主要调控N-WASP蛋白的活性状态[25,30]。N-WASP主要通过构象转换(conformational change)释放出VCA结构域激活Arp2/3复合体来调控肌动蛋白的组装[32]。具体来讲,N-WASP有激活和未激活两种构象状态:在未激活构象状态下,N-WASP的GBD结构域和VCA结构域相互作用而使VCA结构域被掩藏,不能与肌动蛋白单体和Arp2/3复合体相互作用;在激活构象状态下,VCA结构域被暴露出来并与Arp2/3复合体相互作用,verprolin结构域上结合的肌动蛋白单体输送到肌动蛋白丝状分支,从而促进肌动蛋白的组装[30,33]。一些上游的信号分子可以激活N- WASP,使其释放出VCA结构域。例如,Cdc42 (cell division cycle 42)通过结合GBD结构域而释放出VCA结构域;Grb2 (growth factor receptor-bound protein 2),Nck(non-catalytic region of tyrosine kinase adaptor protein)等含有SH3(Src homology 3)结构域的接头蛋白(adaptor proteins)则通过与polyPro结构域相互作用,从而解除这种自抑制状态,释放VCA结构域[34,35,36,37,38]。最后,N-WASP通过形成二聚体或寡聚体进一步激活Arp2/3复合体,从而达到最大的活性[30,39]。
N-WASP敲除会导致小鼠(Mus musculus)胚胎致死并伴随神经管形成缺陷[40],在小鼠大脑中条件性敲除N-WASP会导致脑积水的症状[41],说明N-WASP在小鼠大脑的发育中起着重要作用。此外,之前有报道称mDab1 (mouse disabled homologue1)基因突变会导致小鼠大脑皮层发育异常,造成皮层的异位,与摇晃蛋白Reelin基因敲除小鼠表型一致[42]。而体外细胞实验显示mDab1可与N-WASP相互作用诱导丝状伪足(filopodia)的生成[43],说明N-WASP可能在神经元迁移中起重要作用,但至今未见有直接的证据。
本研究利用蛋白免疫印迹(Western blot, WB)检测发现N-WASP在小鼠大脑皮层的发育过程中持续表达并且呈现逐渐下降的模式,然后采用在体子宫内胚胎电转技术在E14.5的小鼠大脑皮层神经元中过表达与敲低N-WASP,实验结果均表明会造成神经元迁移障碍,而且过表达N-WASP所导致的神经元迁移障碍会比敲低N-WASP产生的影响更严重。此外,通过在体子宫内胚胎电转技术过表达显性负性突变蛋白,发现过表达ΔVCA,ΔpolyPro或者ΔWH1突变体,以及VCA或者polyPro结构域均会导致神经元迁移障碍,而过表达WH1,WH1-GBD结构域以及H208D突变体却对神经元迁移没有明显影响。这些实验结果表明N-WASP主要是通过polyPro和VCA结构域的协调作用来调控大脑皮层的神经元迁移。
1 材料和方法
1.1 材料
C57BL/6小鼠(南方模式动物中心);HEK293T细胞(上海细胞库);N-WASP抗体(Abcam公司,英国);β-actin抗体、抗鼠以及抗兔二抗(Proteintech公司,美国);DNA质粒中抽试剂盒Compactprep Plasmid Midi Kit(25)(Qiagen公司,德国);固绿FCF (Fast Green FCF)、戊巴比妥钠(Sigma公司,美国);Stbl3大肠杆菌菌株、脂质体LipofectamineTM3000以及ProlongTM Gold antifade reagent(Invitrogen公司,美国);逆转录试剂盒Reverse Transcription System (Promega公司,美国);电转仪(Bio-Rad,美国);自动震荡切片机(Leica,德国);共聚焦显微镜(Leica,德国);Western blot全套装置(Bio-Rad,美国);荧光显微镜(Zeiss,德国)。1.2 质粒构建
全长的N-WASP cDNA (GenBank No.AJ318416)是通过提取小鼠大脑总RNA再经过逆转录PCR (RT-PCR)获得,并被克隆到pCAG-Myc载体中,其突变体WH1, ΔWH1,WH1-GBD, H208D, ΔVCA, VCA, ΔpolyPro, polyPro通过设计突变引物由全长N-WASP突变得到。用于敲低实验的短发夹环RNA (shRNA)编码序列是克隆到pLKO.1载体(Sigma)上的。实验所用引物序列列于表1。Table 1
表1
表1 本研究使用的引物
Table 1
| 引物 | 序列(5°→3°) |
|---|---|
| N-WASP shRNA1-F | CCGGAAGACGAGATGCTCCAAATGGCTCGAGCCATTTGGAGCATCTCGTCTTTTTTTG |
| N-WASP shRNA1-R | AATTCAAAAAAAGACGAGATGCTCCAAATGGCTCGAGCCATTTGGAGCATCTCGTCTT |
| N-WASP shRNA2-F | CCGGCAGATACGACAGGGCATTCAACTCGAGTTGAATGCCCTGTCGTATCTGTTTTTG |
| N-WASP shRNA2-R | AATTCAAAAACAGATACGACAGGGCATTCAACTCGAGTTGAATGCCCTGTCGTATCTG |
| N-WASP_EcoRI_F | TGGAATTCGACACCATGAGCTCGGGCCAGCAG |
| N-WASP_HindIII_R | CCCAAGCTTGCGTCTTCCCACTCATCATC |
| N-WASP_H208D_F | TCCAGGACATTGGGCATGTTG |
| N-WASP_H208D_R | CCAATGTCCTGGAAATTACTTGG |
| N-WASP_ΔVCA_R | CCCAAGCTTGCGTCACCATCAGAAGGCAGGC |
| VCA_EcoRI_F | TGGAATTCGACACCATGGACCATCAAGTTCCAGC |
| WH1_HindIII_R | CCCAAGCTTGCTAGATTGGGACCATTTGGAGCAT |
| N-WASP_ΔWH1_F | TGGAATTCGACACCATGCTACCCATGGCTACAGTTG |
| N-WASP_ΔpolyPro_R | CTTGATGGTCTGGTGCTTGTCTTCGGAGTTC |
| N-WASP_ΔpolyPro_F | ACAAGCACCAGACCATCAAGTTCCAGC |
| polyPro_EcoRI_F | TGGAATTCGACACCATGAGACAAGCACCACCACCACCT |
| WH1_GBD_HindIII_R | CCCAAGCTTGCTGGTGCTTGTCTTCGGAGTTC |
| SCR_F | CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTTG |
| SCR_R | AATTCAAAAACAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTG |
新窗口打开|下载CSV
1.3 细胞培养、转染
HEK293T细胞的培养基是DMEM(10% FBS, 100 μg/mL青霉素/链霉素双抗),首先使用不含抗生素的培养基培养HEK293T细胞,待细胞密度达到70%~90% (大约24 h)即可使用LipofectamineTM 3000进行细胞转染,24 h后换液,48 h后裂解细胞收取蛋白,进行Western blot实验。1.4 蛋白免疫印迹
配制10%分离胶(10 mL):3.97 mL MilliQ水,3.33 mL 30%Arc-Bis,2.5 mL 1.5 mol/L Tris-HCl(pH = 8.8),100 μL 10%SDS,100 μL 10%过硫酸铵,4 μL四甲基乙二胺(TEMED);4%浓缩胶(4 mL):2.39 mL MilliQ水,0.53 mL 30%Arc-Bis,1 mL 0.5 mol/L Tris-HCl (pH = 6.8),40 μL 10% SDS,40 μL 10%过硫酸铵,4 μL四甲基乙二胺(TEMED);将样品与SDS-PAGE上样缓冲液按比例混合好,95℃变性5 min后加入上样孔中;电泳:80 V,30 min;110 V,120 min;电泳完毕后,根据蛋白Marker大小,将包含目的蛋白的PAGE胶切下:110 V,105 min将蛋白转至NC膜上;转膜完毕后,将膜放入适量的封闭液(5%的脱脂牛奶)中,室温孵育1 h;倒掉封闭液,加入一抗,4℃孵育过夜;将膜加入1×TBST溶液中,室温下洗膜5 min,重复4次;加入二抗,室温孵育1 h;将膜放入1×TBST溶液中,室温下洗膜5 min,重复4次;最后用Odyssey双色红外激光成像系统扫膜成像。1.5 小鼠饲养
C57BL/6J小鼠的饲养环境为23℃恒温,12 h (7:00~19:00)光照和12 h (19:00~7:00)黑暗。所有的小鼠实验均符合Institutional Animal Care and Use Committee的规定。取成年的雌性与雄性小鼠于下午16:00后进行合笼,第二天上午12:00前检查雌鼠有没有阴道栓,如果有阴道栓就将时间记为胚胎期(embryonic day 0.5: E0.5)。1.6 胚胎电转
E14.5时,向怀孕的母鼠腹腔注射0.6%的戊巴比妥钠进行麻醉,期间配制注射的质粒混合物[pCAG- Myc质粒(2.5 μg/μL)或者pLKO.1质粒(4 μg/μL)、eGFP质粒(0.5 μg/μL)以及0.3% Fast green]。将母鼠放在加热板上,用剪刀将母鼠腹部的毛剃干净,再用酒精与碘酒进行消毒。将母鼠腹部剪开一个口子,再将一块灭菌的纱布剪开一个口子并将其放于母鼠腹部开口处,将子宫取出放在纱布上,并加0.9%的NaCl溶液于胚胎上。然后向胚胎脑室注射配制好的质粒混合物,使用含有钳片电极的电转仪将质粒转到胚胎大脑皮层脑室区的神经祖细胞中。电转使用的程序为电压37 V,持续时间50 ms,间隔900 ms,5次。
将子宫放回母鼠腹腔中并加1 mL 0.9%的NaCl溶液,缝合好伤口后将母鼠放在恒温板上,待母鼠醒来后放回饲养笼盒,继续饲养。
1.7 取脑、切片、制片及拍片
E17.5时,将胎鼠的头部剪下,在显微镜下操作取出大脑,放入含有4% PFA的离心管中,放于摇床上室温孵育24 h进行固定。使用自动震荡切片机切出50 μm的脑片,用DAPI进行细胞核染色,再用1×PBS清洗3次,然后将脑片放在载玻片上,用封片剂封好。
使用共聚焦显微镜获取处理好的脑片图像。
1.8 数据分析
RNA-seq数据分析:数据下载于ENCODE (Encyclopedia of DNA Elements)数据库[44],经Cufflinks 2.1.1处理计算分析得到基因表达水平数值。文中使用的数据Gene Expression Omnibus (GEO)序列号:GEO:GSE82852,GEO:GSE78340,GEO:GSE78323,GEO:GSE78374。数据分析使用GraphPad Prism 5.0软件,各组间平均值差异程度的检验方法用Student’s t test或者One-way ANOVA进行检验分析。
2 结果与分析
2.1 N-WASP在小鼠胚胎期大脑皮层中呈现逐渐降低的表达模式
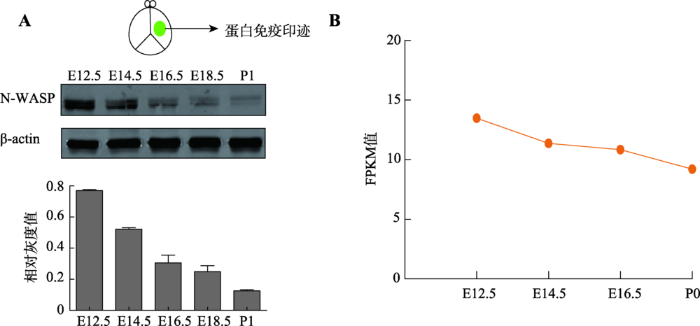
已有研究报道,N-WASP蛋白的表达在出生后的大鼠皮层中是逐渐增加的,并在出生后16周维持稳定[45],同时有文献报道其对于神经突起的延伸以及突触发生有重要作用[46,47,48],说明N-WASP在大脑发育中起着重要的作用,但N-WASP在小鼠胚胎期大脑皮层发育过程中的具体表达情况却未见相关报道。本研究首先提取了不同时期(E12.5、E14.5、E16.5、E18.5和P1)的小鼠大脑皮层组织进行免疫印迹实验检测蛋白表达水平,发现N-WASP蛋白在胚胎期皮层有表达,并随着胚胎发育呈现下降的模式(图1A)。然后从ENCODE数据库中下载了小鼠(C57BL/6)前脑(forebrain)在不同时期(E12.5、E14.5、E16.5和P0)的RNA-seq数据[44],使用Cufflinks 2.1.1对其进行分析,发现N-WASP在mRNA水平上有表达并且随着发育过程呈现下降的模式(图1B),该结果与蛋白免疫印迹实验一致。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1N-WASP在小鼠皮层中的表达
A:蛋白免疫印迹检测在胚胎发育过程中N-WASP蛋白的表达量,下方是对蛋白免疫印迹进行的定量分析;B:Cufflinks 2.1.1分析N-WASP在胚胎发育过程中mRNA水平。FPKM,fragments per kilobase of transcript per million mapped reads,每一百万个mapped reads中map到1kb转录本上的fragments个数。FPKM=cDNA fragments/(mapped reads(millions)× transcript length(kb))。
Fig. 1The expression profile of N-WASP in mouse cortex
2.2 N-WASP过表达或者敲低均会造成小鼠大脑皮层神经元迁移障碍
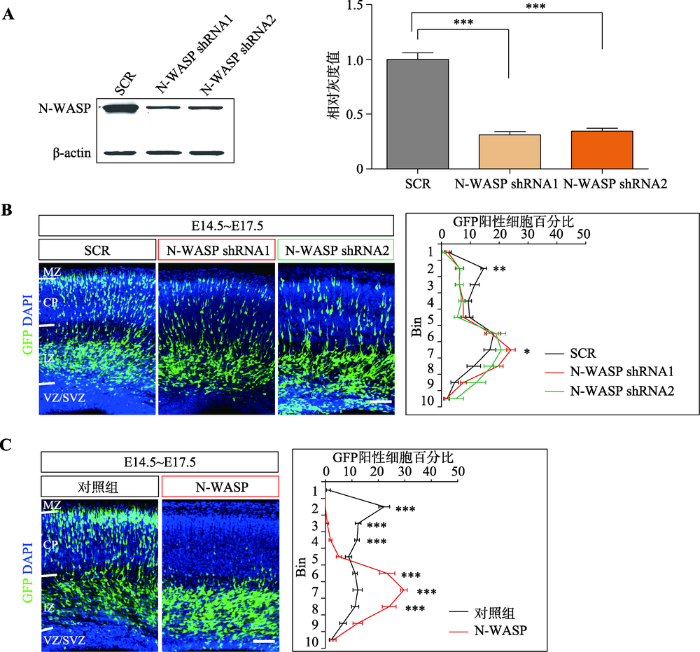
N-WASP蛋白在小鼠大脑皮层的表达从E12.5到P1呈现逐渐下降的模式,其表达量高的时期与大脑皮层神经元迁移的时间段(E11~E16)吻合,预示其可能参与调控神经元的迁移。由此,设计了2个不同的shRNA进行在体胚胎电转敲低实验。首先,通过WB检验了shRNA的效率,发现它们能有效敲低N-WASP蛋白的表达水平(图2A)。然后,利用子宫内胚胎电转技术,将shRNA表达质粒转入E14.5的胎鼠大脑皮层神经祖细胞中,在E17.5时取脑并切片观察。与对照组相比,发现N-WASP敲低会造成小鼠大脑皮层神经元的迁移缺陷(图2B)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2过表达和敲低N-WASP均会造成大脑皮层神经元迁移障碍
A:蛋白免疫印迹检测shRNA敲低效率,右图是对蛋白免疫印迹进行的定量分析;B:子宫内胚胎电转实验观察敲低N-WASP对神经元迁移的影响,右侧为每个区域中GFP阳性细胞所占总阳性细胞百分比的统计图,每组样本数量n≥3只小鼠胚胎,显著性表示的是N-WASP shRNA1或者N-WASP shRNA2与SCR之间的对比,SCR:scrambled shRNA,即不靶向任何基因;C:子宫内胚胎电转实验观察过表达N-WASP对神经元迁移的影响,右侧为每个区域中GFP阳性细胞所占总阳性细胞百分比统计图,每组样本数量n≥3只小鼠胚胎。数据结果以Mean±SEM表示,标尺:100 μm,*:P<0.05 表示有统计学差异,**:P<0.01表示有显著的统计学差异,***:P<0.001 表示有极其显著的统计学差异。MZ:marginal zone,边缘区;CP:cortical plate,皮质区;IZ:intermediate zone,中间区;SVZ:subventricular zone,室下区;VZ:ventricular zone,脑室区。
Fig. 2Both overexpression and knockdown of N-WASP impair cortical neuron migration
为进一步研究N-WASP调控神经元迁移的具体功能,构建了N-WASP真核过表达质粒,然后通过子宫内胚胎电转技术将该质粒转入E14.5的胎鼠大脑皮层神经祖细胞中,同样在E17.5时切片观察。与对照组相比,发现过表达N-WASP也会造成小鼠大脑皮层神经元的迁移缺陷(图2C)。并且,过表达N-WASP造成的神经元迁移障碍比敲低N-WASP所造成的神经元迁移障碍更加显著(图2:B,C)。
上述结果说明,N-WASP过表达或敲低均会造成神经元迁移障碍,表明其在神经元迁移中起着重要作用。
2.3 N-WASP主要通过polyPro和VCA结构域调控小鼠大脑皮层神经元迁移
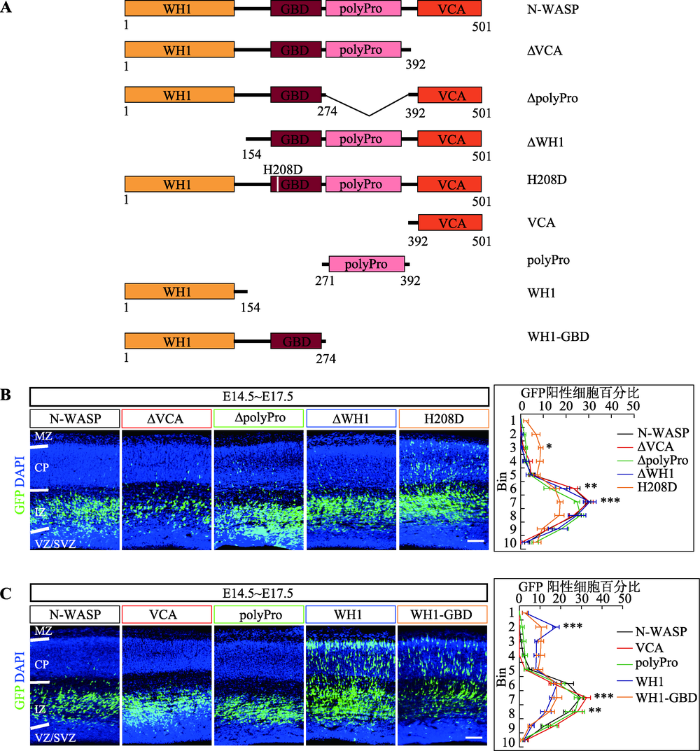
N-WASP主要通过VCA结构域结合活化的肌动蛋白以及Arp2/3复合体引起肌动蛋白组装[32],其在未激活的情况下处于自抑制的状态(GBD与VCA相互作用),需要上游信号Cdc42激活并打开这种自抑制状态,进而引起肌动蛋白的组装[25,33]。N-WASP除了通过上述经典的GTPase激活通路之外,还可以通过polyPro结构域与含有SH3结构域的接头蛋白结合进行激活[25],而它的WH1结构域主要与WIP (WASP-interacting protein)家族相互作用,该作用与N-WASP招募到肌动蛋白组装的地点有关[31]。为更加深入研究N-WASP调控神经元迁移的分子机制,首先构建了VCA结构域删除(ΔVCA)、polyPro结构域删除(ΔpolyPro)、WH1结构域删除(ΔWH1)以及破坏了Cdc42结合位点[34](H208D突变体)的N-WASP蛋白表达质粒(图3A)。然后通过子宫内胚胎电转技术,将这些质粒分别转到E14.5的大脑皮层中,在E17.5时切片观察。结果发现单独过表达ΔVCA、ΔpolyPro或者ΔWH1的N-WASP蛋白均会造成严重的神经元迁移障碍,而过表达H208D突变的N-WASP蛋白却没有造成明显的神经元迁移障碍(图3B)。最后,利用构建的VCA结构域过表达质粒(VCA)、polyPro结构域过表达质粒(polyPro)、WH1结构域过表达质粒(WH1)、WH1-GBD结构域(同时删除polyPro和VCA结构域)过表达质粒(WH1-GBD)(图3A),进行了类似的子宫内胚胎电转实验。结果表明单独过表达VCA蛋白或polyPro蛋白均会造成严重的神经元迁移障碍,但是过表达WH1蛋白或WH1-GBD蛋白对神经元迁移没有明显影响(图3C)。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3N-WASP主要通过polyPro和VCA结构域调控大脑皮层神经元迁移
A:实验所用质粒示意图;B:过表达删除或突变各结构域的N-WASP蛋白对神经元迁移的影响,右侧为每个区域中GFP阳性细胞所占总阳性细胞的百分比统计图,每组样本数量n≥3只小鼠胚胎,显著性表示的是H208D与N-WASP之间的对比;C:过表达N-WASP蛋白各结构域对神经元迁移的影响,右侧为每个区域中GFP阳性细胞所占总阳性细胞的百分比统计图,每组样本数量n≥3只小鼠胚胎,显著性表示的是WH1或WH1-GBD与N-WASP之间的对比。数据结果以Mean ± SEM表示,标尺:100 μm,*:P<0.05表示有统计学差异,**:P<0.01表示有显著的统计学差异,***:P<0.001表示有极其显著的统计学差异。MZ:marginal zone,边缘区;CP:cortical plate,皮质区;IZ:intermediate zone,中间区;SVZ:subventricular zone,室下区;VZ:ventricular zone,脑室区。
Fig. 3N-WASP regulates cortical neuron migration mainly through its polyPro and VCA domains
综上所述,N-WASP是通过VCA结构域激活下游信号通路来调控神经元迁移,并且主要通过polyPro结构域接收上游信号调控神经元迁移,而Cdc42激活N-WASP进而打开VCA的这条信号通路只是部分参与对神经元迁移的调控。此外,WH1结构域可能不参与N-WASP对神经元迁移的调控。
3 讨 论
大脑皮层的正常发育对于其执行记忆、学习和感觉的功能是非常重要的,其中神经元迁移是大脑皮层发育中的一个重要过程。神经元迁移是一个高度动态化的协同调控过程,通过细胞骨架中肌动蛋白的动态组装影响神经元前体的形态进而调控神经元迁移[28]。WASP家族中的WAVE蛋白和其他4个蛋白可形成一个五聚体复合物(pentameric WAVEregulatory complex, WRC),然后被大约120种膜蛋白招募到细胞膜附近,进而引起肌动蛋白的组装[21]。这些膜蛋白包括很多原钙粘蛋白(protocadherin, PCDH)、Celsr3 (cadherin EGF LAG seven-pass G-type receptor 3)、Fat3 (FAT atypical cadherin 3)等[18,21]。原钙粘蛋白通过抑制Pyk2 (proline-rich tyrosine kinase 2)的活性而激活Rac1 (Ras-related C3 botulinum toxin substrate 1)来调控神经元的树突与树突棘的形态生成[16]。此外,N-WASP能够调节神经元突起(neurites)以及突起的生长锥导向[49,50],然而其在神经元迁移中的功能和机制依然是不清楚的。本研究使用在体子宫内胚胎电转技术研究N-WASP蛋白在小鼠大脑皮层神经元迁移中所起的作用,发现N-WASP调控神经元迁移主要依赖于polyPro和VCA结构域。通过提取不同时期的胚胎大脑皮层组织(E12.5、E14.5、E16.5、E18.5和 P1)进行蛋白免疫印迹实验以及分析胚胎发育不同时期前脑(E12.5、E14.5、E16.5和P0)的RNA-seq数据,发现在胚胎时期的大脑皮层发育过程中,N-WASP在mRNA和蛋白质水平上均有表达,并且其表达随着大脑发育逐渐下降。然后通过在体子宫内胚胎电转技术过表达和敲低N-WASP,发现过表达和敲低N-WASP均造成神经元迁移障碍,说明其在调控神经元迁移中可能起到了重要作用。本研究观察到敲低N-WASP的表型没有过表达明显,这可能是因为N-WASP没有被完全敲除。
先前研究表明,Lis1 (lissencephaly-1)过表达对神经元迁移没有明显影响,而Lis1敲低会导致神经元迁移障碍[8,51];双皮质素(doublecortin, DCX)过表达会促进神经元迁移,而双皮质素敲低会阻止神经元迁移[8,52]。有趣的是,本研究发现过表达和敲低N-WASP均会造成神经元迁移障碍。神经元迁移是一个高度动态的过程,需要肌动蛋白骨架动态调控,所以N-WASP的表达量的平衡对这一过程显得非常重要。此外,有报道称在患有癫痫的人脑组织中N-WASP的表达量高于正常水平[53],预示N-WASP的表达量过多产生的病理学效应可能参与了疾病的进程。
N-WASP在未激活时通过分子内的GBD与VCA相互作用保持自抑制状态。在激活时,其通过接收上游信号刺激释放出VCA结构域,暴露出来的VCA结构域通过激活Arp2/3复合体来引起肌动蛋白的动态化组装。本研究表明在正常的大脑皮层发育状态下,N-WASP蛋白可能参与调控神经元迁移,而且其表达量的平衡对神经元的迁移也是至关重要的。进一步的研究结果表明分别过表达VCA结构域和ΔpolyPro (含有VCA结构域)突变体均会造成神经元迁移障碍,说明VCA结构域可能竞争性抑制内源的N-WASP蛋白发挥作用。同理,分别过表达polyPro结构域和ΔVCA (含有polyPro结构域)突变体均会造成神经元迁移障碍,说明polyPro结构域也可能竞争性抑制内源的N-WASP蛋白发挥作用。推测这些突变体可能竞争性地结合了一些与内源N-WASP相互作用的蛋白(如含有SH3功能域的接头蛋白),从而抑制内源的N-WASP发挥作用。与此同时,过表达WH1-GBD结构域(不含有polyPro和VCA结构域)却对神经元迁移没有明显影响。总之,这些结果说明N-WASP的polyPro和VCA结构域参与调控神经元迁移,可能是通过polyPro结构域接收上游信号释放出VCA结构域,再激活Arp2/3复合体影响肌动蛋白的组装进而调控神经元迁移。
N-WASP的WH1结构域主要与WIP家族蛋白相互作用,其GBD结构域主要通过与Cdc42 GTPase结合接收上游信号。本研究发现过表达WH1结构域对神经元迁移没有明显影响,而过表达ΔWH1则会造成严重的神经元迁移障碍,说明过表达WH1结构域不能竞争性抑制内源的N-WASP蛋白发挥作用,而ΔWH1(含有polyPro和VCA结构域)依然能够竞争性抑制内源的N-WASP蛋白发挥作用,可能是因为N-WASP调控神经元迁移不需要WH1结构域的参与。之前有报道称N-WASP通过WH1结构域参与调控上皮细胞之间连接的肌动蛋白骨架,而经典的VCA结构域并不参与其中,这种调控作用主要是通过WH1结构域维持肌动蛋白组装后的稳定性[54]。此结论与本文的研究结果相一致,因为神经元迁移是一个时刻在变化的过程,其需要肌动蛋白组装的动态平衡来形成合适的细胞骨架网络,而细胞骨架网络对于神经细胞的形态至关重要。N-WASP的WH1结构域可能更侧重于影响肌动蛋白组装后的静态稳定性,而不是调控肌动蛋白的动态组装[54]。此外,本研究还发现过表达H208D突变的N-WASP对神经元迁移有影响,但并非像其他突变体产生明显的影响。由于H208D突变的N-WASP不能结合Cdc42,因此推测Cdc42激活N-WASP引起肌动蛋白的动态调控可能参与了神经元迁移,但并不是主要的信号通路。
N-WASP在参与信号通路发挥作用时,需要从未激活状态转变成激活状态。N-WASP的高度激活需要两个步骤,首先通过接收上游信号分子(如Cdc42或含有SH3结构域的接头蛋白)的激活进行变构调节,释放出VCA结构域结合Arp2/3复合体,然后通过连接蛋白使得N-WASP进行二聚化或者寡聚化增加与Arp2/3复合体的亲和性来调控肌动蛋白的组装[39]。结合最近的研究发现一个VCA结构域结合Arp2/3复合体的背面而另一个VCA结构域结合该复合体的底面[55],本文提出一个可能的N-WASP调控神经元迁移的模型:Cdc42和含有SH3结构域的蛋白可能协同结合N-WASP蛋白使其进行变构释放出VCA结构域,再通过多价的含有SH3结构域的蛋白结合polyPro结构域使得N-WASP进行二聚化或者寡聚化,最终激活肌动蛋白的组装调控神经元的迁移(图4)。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4N-WASP蛋白调控大脑皮层神经元迁移机制的模式图
N-WASP由上游信号分子(含有SH3结构域的接头蛋白和Cdc42)激活,释放出VCA结构域,结合Arp2/3复合体,再通过含有SH3结构域的接头蛋白进行二聚化或者寡聚化[39],释放出来的两个VCA结构域分别结合Arp2/3复合体的背面和底面[55],从而高度激活Arp2/3复合体引起肌动蛋白的组装来调控神经元的迁移。 MZ:marginal zone,边缘区;CP:cortical plate,皮质区;IZ:intermediate zone,中间区;SVZ:subventricular zone,室下区;VZ:ventricular zone,脑室区;n:neuron,神经元;R:radial glial cell,放射状胶质细胞。
Fig. 4A model for N-WASP regulating cortical neuron migration
综上所述,本研究发现N-WASP在大脑皮层神经元前体放射状迁移中起到重要作用,并且其主要是通过polyPro和VCA结构域动态调控细胞骨架中肌动蛋白丝状分支的构建影响神经元迁移。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 2]
URL [本文引用: 1]
URLPMID:19523518 [本文引用: 1]

Neocortex, a distinct six-layered neural structure, is one of the most exquisite nerve tissues in the human body. Proper assembly of neocortex requires precise regulation of neuronal migration and abnormalities can result in severe neurological diseases. Three major types of neuronal migration have been implicated in corticogenesis: radial migration of excitatory neuron precursors and tangential migration of interneurons as well as Cajal etzius cells. In the past several years, significant progress has been made in understanding how these parallel events are regulated and coordinated during corticogenesis. New insights have been gained into regulation of radial neuron migration by the well-known Reelin signal. New pathways have also been identified that regulate radial as well as tangential migration. Equally important, better understandings have been obtained on the cellular and molecular mechanics of cell migration by both projection neurons and interneurons. These findings have not only enhanced our understanding of normal neuron migration but also revealed insights into the etiologies of several neurological diseases where these processes go awry.
URLPMID:23839596 [本文引用: 1]

Abstract Planar cell polarity (PCP) is complementary to the intrinsic polarization of single cells and refers to the global coordination of cell behaviour in the plane of a tissue and, by extension, to the signalling pathways that control it. PCP is most evident in cell sheets, and research into PCP was for years confined to studies in Drosophila melanogaster. However, PCP has more recently emerged as an important phenomenon in vertebrates, in which it regulates various developmental processes and is associated with multiple disorders. In particular, core PCP genes are crucial for the development and function of the nervous system. They are involved in neural tube closure, ependymal polarity, neuronal migration, dendritic growth and axon guidance.
URLPMID:16713637 [本文引用: 3]

Abstract The genetic basis is now known for several disorders of neuronal migration in the developing cerebral cortex. Identification of the cellular processes mediated by the implicated genes is revealing crucial stages of neuronal migration and has the potential to reveal common cellular causes of neuronal migration disorders. We hypothesize that a newly recognized morphological stage of neuronal migration, the multipolar stage, is vulnerable and is disrupted in several disorders of neocortical development. The multipolar stage occurs as bipolar progenitor cells become radially migrating neurons. Several studies using in utero electroporation and RNAi have revealed that transition out of the multipolar stage depends on the function of filamin A, LIS1 and DCX. Mutations in the genes encoding these proteins in humans cause distinct neuronal migration disorders, including periventricular nodular heterotopia, subcortical band heterotopia and lissencephaly. The multipolar stage therefore seems to be a critical point of migration control and a vulnerable target for disruption of neocortical development. This review is part of the INMED/TINS special issue "Nature and nurture in brain development and neurological disorders", based on presentations at the annual INMED/TINS symposium (http://inmednet.com/).
URLPMID:3610574 [本文引用: 1]

The size and surface area of the mammalian brain are thought to be critical determinants of intellectual ability. Recent studies show that development of the gyrated human neocortex involves a lineage of neural stem and transit-amplifying cells that forms the outer subventricular zone (OSVZ), a proliferative region outside the ventricular epithelium. We discuss how proliferation of cells within the OSVZ expands the neocortex by increasing neuron number and modifying the trajectory of migrating neurons. Relating these features to other mammalian species and known molecular regulators of the mouse neocortex suggests how this developmental process could have emerged in evolution.
URLPMID:14602813 [本文引用: 1]

Two distinct modes of radial neuronal migration, locomotion and somal translocation, have been reported in the developing cerebral cortex. Although these two modes of migration have been well documented, the cortical intermediate zone contains abundant multipolar cells, and they do not resemble the cells migrating by locomotion or somal translocation. Here, we report that these multipolar cells express neuronal markers and extend multiple thin processes in various directions independently of the radial glial fibers. Time-lapse analysis of living slices revealed that the multipolar cells do not have any fixed cell polarity, and that they very dynamically extend and retract multiple processes as their cell bodies slowly move. They do not usually move straight toward the pial surface during their radial migration, but instead frequently change migration direction and rate; sometimes they even remain in almost the same position, especially when they are in the subventricular zone. Occasionally, the multipolar cells jump tangentially during their radial migration. Because the migration modality of these cells clearly differs from locomotion or somal translocation, we refer to their novel type of migration as "multipolar migration." In view of the high proportion of cells exhibiting multipolar migration, this third mode of radial migration must be an important type of migration in the developing cortex.
URL [本文引用: 1]

大脑是人体内结构和功能最为复杂的器官,它实现人类的感知、情感、思维、记忆并控制其他器官的活动。神经元是大脑结构的基本单元,大约1000亿个神经元通过神经突起相互连接组成复杂而有序的神经网络。神经元是多种多样的,神经元的多样性决定了大脑处理储存信息的容量。科学家们发现神经元的多样性与一种膜蛋白分子一原钙粘蛋白的分子多样性有关,不同原钙粘蛋白分子在神经元内随机组合表达赋予每个神经元自己的个性。
URL [本文引用: 1]

大脑是人体内结构和功能最为复杂的器官,它实现人类的感知、情感、思维、记忆并控制其他器官的活动。神经元是大脑结构的基本单元,大约1000亿个神经元通过神经突起相互连接组成复杂而有序的神经网络。神经元是多种多样的,神经元的多样性决定了大脑处理储存信息的容量。科学家们发现神经元的多样性与一种膜蛋白分子一原钙粘蛋白的分子多样性有关,不同原钙粘蛋白分子在神经元内随机组合表达赋予每个神经元自己的个性。
URLPMID:1436919 [本文引用: 1]

Interneurons are extremely diverse in the mammalian brain and provide an essential balance for functional neural circuitry. The vast majority of murine cortical interneurons are generated in the subpallium and migrate tangentially over a long distance to acquire their final positions. By using a mouse line with a deletion of the Celsr3 (Flamingo, or Fmi1) gene and a knock-in of the green fluorescent protein reporter, we find that Celsr3, a member of the nonclustered protocadherin (Pcdh) family, is predominantly expressed in the cortical interneurons in adults and in the interneuron germinal zones in embryos. We show that Celsr3 is crucial for interneuron migration in the developing mouse forebrain. Specifically, in Celsr3 knockout mice, calretinin-positive interneurons are reduced in the developing neocortex, accumulated in the corticostriatal boundary, and increased in the striatum. Moreover, the laminar distribution of cortical calbindin-positive cells is altered. Finally, we found that expression patterns of NRG1 (neuregulin-1) and its receptor ErbB4, which are essential for interneuron migration, are changed in Celsr3 mutants. These results demonstrate that the protocadherin Celsr3 gene is essential for both tangential and radial interneuron migrations in a class-specific manner.
URLPMID:28631008 [本文引用: 2]

The protocadherins (Pcdhs), which make up the most diverse group within the cadherin superfamily, were first discovered in the early 1990s. Data implicating the Pcdhs, including ~60 proteins encoded b
In: Qiao ZD, He L, ed.
URL [本文引用: 1]

react-text: 140 A rare class of introns in higher eukaryotes is processed by the recently discovered AT-AC spliceosome. AT-AC introns are processed inefficiently in vitro, but the reaction is stimulated by exon-definition interactions involving binding of U1 snRNP to the 5' splice site of the downstream conventional intron. We report that purine-rich exonic splicing enhancers also strongly stimulate sodium... /react-text react-text: 141 /react-text [Show full abstract]
见: 乔中东, 贺林.
URL [本文引用: 1]

react-text: 140 A rare class of introns in higher eukaryotes is processed by the recently discovered AT-AC spliceosome. AT-AC introns are processed inefficiently in vitro, but the reaction is stimulated by exon-definition interactions involving binding of U1 snRNP to the 5' splice site of the downstream conventional intron. We report that purine-rich exonic splicing enhancers also strongly stimulate sodium... /react-text react-text: 141 /react-text [Show full abstract]
URLPMID:22542181 [本文引用: 1]

Abstract The 22 0206-protocadherins (0206-Pcdhs) potentially specify thousands of distinct homophilic adhesive interactions in the brain. Neonatal lethality of mice lacking the Pcdh-0206 gene cluster has, however, precluded analysis of many brain regions. Here, we use a conditional Pcdh-0206 allele to restrict mutation to the cerebral cortex and find that, in contrast to other central nervous system phenotypes, loss of 0206-Pcdhs in cortical neurons does not affect their survival or result in reduced synaptic density. Instead, mutant cortical neurons exhibit severely reduced dendritic arborization. Mutant cortices have aberrantly high levels of protein kinase C (PKC) activity and of phosphorylated (inactive) myristoylated alanine-rich C-kinase substrate, a PKC target that promotes arborization. Dendrite complexity can be rescued in Pcdh-0206 mutant neurons by inhibiting PKC, its upstream activator phospholipase C, or the 0206-Pcdh binding partner focal adhesion kinase. Our results reveal a distinct role for the 0206-Pcdhs in cortical development and identify0002a signaling pathway through which they play this role. Copyright 0008 2012 Elsevier Inc. All rights reserved.
URLPMID:22730554 [本文引用: 2]

Abstract Dendritic patterning and spine morphogenesis are crucial for the assembly of neuronal circuitry to ensure normal brain development and synaptic connectivity as well as for understanding underlying mechanisms of neuropsychiatric diseases and cognitive impairments. The Rho GTPase family is essential for neuronal morphogenesis and synaptic plasticity by modulating and reorganizing the cytoskeleton. Here, we report that protocadherin (Pcdh) clusters and cell adhesion kinases (CAKs) play important roles in dendritic development and spine elaboration. The knockout of the entire Pcdh02± cluster results in the dendritic simplification and spine loss in CA1 pyramidal neurons in vivo and in cultured primary hippocampal neurons in vitro. The knockdown of the whole Pcdh0206 cluster or in combination with the Pcdh02± knockout results in similar dendritic and spine defects in vitro. The overexpression of proline-rich tyrosine kinase 2 (Pyk2, also known as CAK0205, RAFTK, FAK2, and CADTK) recapitulates these defects and its knockdown rescues the phenotype. Moreover, the genetic deletion of the Pcdh02± cluster results in phosphorylation and activation of Pyk2 and focal adhesion kinase (Fak) and the inhibition of Rho GTPases in vivo. Finally, the overexpression of Pyk2 leads to inactivation of Rac1 and, conversely, the constitutive active Rac1 rescues the dendritic and spine morphogenesis defects caused by the knockout of the Pcdh02± cluster and the knockdown of the Pcdh0206 cluster. Thus, the involvement of the Pcdh-CAK-Rho GTPase pathway in the dendritic development and spine morphogenesis has interesting implications for proper assembly of neuronal connections in the brain.
URL [本文引用: 1]
URLPMID:25113559 [本文引用: 2]

Abstract The globus pallidus (GP) is a central component of basal ganglia whose malfunctions cause a variety of neuropsychiatric disorders as well as cognitive impairments in neurodegenerative diseases such as Parkinson's disease. Here we report that the protocadherin gene Celsr3 is regulated by the insulator CCCTC-binding factor (CTCF) and the repressor neuron-restrictive silencer factor (NRSF, also known as REST) and is required for the development and connectivity of GP. Specifically, CTCF/cohesin and NRSF inhibit the expression of Celsr3 through specific binding to its promoter. In addition, we found that the Celsr3 promoter interacts with CTCF/cohesin-occupied neighboring promoters. In Celsr3 knockout mice, we found that the ventral GP is occupied by aberrant calbindin-positive cholinergic neurons ectopic from the nucleus basalis of Meynert. Furthermore, the guidepost cells for thalamocortical axonal development are missing in the caudal GP. Finally, axonal connections of GP with striatum, subthalamic nucleus, substantia nigra, and raphe are compromised. These data reveal the essential role of Celsr3 in GP development in the basal forebrain and shed light on the mechanisms of the axonal defects caused by the Celsr3 deletion. Copyright 2014, American Society for Microbiology. All Rights Reserved.
URLMagsci [本文引用: 1]

<p><span >该文通过免疫组化及蛋白免疫印迹的方法分别对</span><em><span >Pcdh</span><span >a</span></em><span >基因敲除和对照组小鼠的中枢神经系统内的髓鞘碱性蛋白表达以及少突胶质细胞的发育进行了测定。结果表明:</span><span >1) <em>Pcdh</em></span><em><span >a</span></em><span >基因缺失<a name="OLE_LINK1">小鼠</a>中枢神经系统中的髓鞘碱性蛋白较对照组小鼠明显减少;</span><span >2) <em>Pcdh</em></span><em><span >a</span></em><span >基因敲除可导致少突胶质细胞发育异常:在小脑中</span><span >, </span><span >处于成熟期的少突胶质细胞减少</span><span >, </span><span >而处于前体细胞阶段的少突胶质细胞增多。上述结果提示</span><span >Pcdh</span><span >a</span><span >可以通过调控少突胶质细胞的成熟过程进而影响髓鞘的形成。</span></p>
URLMagsci [本文引用: 1]

<p><span >该文通过免疫组化及蛋白免疫印迹的方法分别对</span><em><span >Pcdh</span><span >a</span></em><span >基因敲除和对照组小鼠的中枢神经系统内的髓鞘碱性蛋白表达以及少突胶质细胞的发育进行了测定。结果表明:</span><span >1) <em>Pcdh</em></span><em><span >a</span></em><span >基因缺失<a name="OLE_LINK1">小鼠</a>中枢神经系统中的髓鞘碱性蛋白较对照组小鼠明显减少;</span><span >2) <em>Pcdh</em></span><em><span >a</span></em><span >基因敲除可导致少突胶质细胞发育异常:在小脑中</span><span >, </span><span >处于成熟期的少突胶质细胞减少</span><span >, </span><span >而处于前体细胞阶段的少突胶质细胞增多。上述结果提示</span><span >Pcdh</span><span >a</span><span >可以通过调控少突胶质细胞的成熟过程进而影响髓鞘的形成。</span></p>
URLPMID:28450636 [本文引用: 1]

Serotonergic neurons project their axons pervasively throughout the brain and innervate various target fields in a space-filling manner, leading to tiled arrangements of their axon terminals to allow optimal allocation of serotonin among target neurons. Here we show that conditional deletion of the mouse protocadherin α (Pcdhα) gene cluster in serotonergic neurons disrupts local axonal tiling and global assembly of serotonergic circuitries and results in depression-like behaviors. Genetic dissection and expression profiling revealed that this role is specifically mediated by Pcdhαc2, which is the only Pcdhα isoform expressed in serotonergic neurons. We conclude that, in contrast to neurite self-avoidance, which requires single-cell identity mediated by Pcdh diversity, a single cell-type identity mediated by the common C-type Pcdh isoform is required for axonal tiling and assembly of serotonergic circuitries.
URLPMID:24439376 [本文引用: 3]

The WAVE regulatory complex (WRC) controls actin cytoskeletal dynamics throughout the cell by stimulating the actin-nucleating activity of the Arp2/3 complex at distinct membrane sites. However, the factors that recruit the WRC to specific locations remain poorly understood. Here, we have identified a large family of potential WRC ligands, consisting of 120 diverse membrane proteins, including protocadherins, ROBOs, netrin receptors, neuroligins, GPCRs, and channels. Structural, biochemical, and cellular studies reveal that a sequence motif that defines these ligands binds to a highly conserved interaction surface of the WRC formed by the Sra and Abi subunits. Mutating this binding surface in flies resulted in defects in actin cytoskeletal organization and egg morphology during oogenesis, leading to female sterility. Our findings directly link diverse membrane proteins to the WRC and actin cytoskeleton and have broad physiological and pathological ramifications in metazoans.
URLPMID:8001129 [本文引用: 1]

Cell. 1994 Aug 26;78(4):635-44. Research Support, Non-U.S. Gov't
URLPMID:12209132 [本文引用: 1]

Abstract The regulation of many immunological events depends on systems that mediate dynamic actin reorganization in response to signals from the cell membrane. The Wiskott-Aldrich syndrome protein (WASp) is the founding member of a family of proteins that have emerged as crucial effectors of Rho GTPases and activators of the cytoskeletal-organizing complex Arp2/3. Now, WASp has been shown to be intimately involved in many pathways that influence the function of the immune system. Disturbances in these systems result in the complex immunodysregulation of Wiskott-Aldrich syndrome.
URLPMID:13133561 [本文引用: 1]

ALDRICH RA, STEINBERG AG, CAMPBELL DC.
URLPMID:17183359 [本文引用: 5]

Wiskott-Aldrich syndrome protein (WASP) and WASP-family verprolin-homologous protein (WAVE) family proteins are scaffolds that link upstream signals to the activation of the ARP2/3 complex, leading to a burst of actin polymerization. ARP2/3-complex-mediated actin polymerization is crucial for the reorganization of the actin cytoskeleton at the cell cortex for processes such as cell movement, vesicular trafficking and pathogen infection. Large families of membrane-binding proteins were recently found to interact with WASP and WAVE family proteins, therefore providing a new layer of membrane-dependent regulation of actin polymerization.
URLPMID:28646090 [本文引用: 1]

Abstract Proteins of the Wiskott-Aldrich syndrome protein (WASP) family function as nucleation-promoting factors for the ubiquitously expressed Arp2/3 complex, which drives the generation of branched actin filaments. Arp2/3-generated actin regulates diverse cellular processes, including the formation of lamellipodia and filopodia, endocytosis and/or phagocytosis at the plasma membrane, and the generation of cargo-laden vesicles from organelles including the Golgi, endoplasmic reticulum (ER) and the endo-lysosomal network. Recent studies have also identified roles for WASP family members in promoting actin dynamics at the centrosome, influencing nuclear shape and membrane remodeling events leading to the generation of autophagosomes. Interestingly, several WASP family members have also been observed in the nucleus where they directly influence gene expression by serving as molecular platforms for the assembly of epigenetic and transcriptional machinery. In this Cell Science at a Glance article and accompanying poster, we provide an update on the subcellular roles of WHAMM, JMY and WASH (also known as WASHC1), as well as their mechanisms of regulation and emerging functions within the cell. 2017. Published by The Company of Biologists Ltd.
URLPMID:20965607 [本文引用: 1]

Coordinated functions of the actin cytoskeleton and microtubules, which need to be carefully controlled in time and space, are required for the drastic alterations of neuronal morphology during neuromorphogenesis and neuronal network formation. A key process in neuronal actin dynamics is filament formation by actin nucleators, such as the Arp2/3 complex, formins and the brain-enriched, novel WH2 domain-based nucleators Spire and cordon-bleu (Cobl). We here discuss in detail the currently available data on the roles of these actin nucleators during neuromorphogenesis and highlight how their required control at the plasma membrane may be brought about. The Arp2/3 complex was found to be especially important for proper growth cone translocation and axon development. The underlying molecular mechanisms for Arp2/3 complex activation at the neuronal plasma membrane include a recruitment and an activation of N-WASP by lipid- and F-actin-binding adaptor proteins, Cdc42 and phosphatidyl-inositol-(4,5)-bisphosphate (PIP 2 ). Together, these components upstream of N-WASP and the Arp2/3 complex ensure fine-control of N-WASP-mediated Arp2/3 complex activation and control distinct functions during axon development. They are counteracted by Arp2/3 complex inhibitors, such as PICK, which likewise play an important role in neuromorphogenesis. In contrast to the crucial role of the Arp2/3 complex in proper axon development, dendrite formation and dendritic arborization was revealed to critically involve the newly identified actin nucleator Cobl. Cobl is a brain-enriched protein and uses three Wiskott鈥揂ldrich syndrome protein homology 2 (WH2) domains for actin binding and for promoting the formation of non-bundled, unbranched filaments. Thus, cells use different actin nucleators to steer the complex remodeling processes underlying cell morphogenesis, the formation of cellular networks and the development of complex body plans.
URLPMID:18075253 [本文引用: 2]

Neuronal migration is a pivotal step for architectural and functional brain development. Migrating neurons exhibit various morphological changes, based on cytoskeletal organization. In addition to many genetic studies revealing the involvement of several cytoskeletal and signaling molecules in cortical neuronal migration (e.g. doublecortin, Lis1, Filamin A, cyclin-dependent kinase 5, Reelin and Dab1), cell biological studies and recently developed techniques, including in utero electroporation, have uncovered detailed functions of these molecules as well as novel molecules, such as Rho family GTPases, focal adhesion kinase, c-jun N-terminal kinase and p27kip1. In this review, we introduce the molecular pathways underlying cortical neuronal migration and morphological changes, with particular focus on recent findings for the regulatory mechanisms of actin cytoskeleton and microtubules.
URL [本文引用: 2]

Here we identify a 65 kDa protein (N-WASP) from brain that binds the SH3 domains of Ash/Grb2. The sequence is homologous to Wiskott-Aldrich syndrome protein (WASP). N-WASP has several functional motifs, such as a pleckstrin homology (PH) domain and cofilin-homologous region, through which N-WASP depolymerizes actin filaments. When overexpressed in COS 7 cells, the wild-type N-WASP causes several surface protrusions where N-WASP co-localizes with actin filaments. Epidermal growth factor (EGF) treatment induces the complex formation of EGF receptors and N-WASP, and produces microspikes. On the other hand, two mutants, C38W (a point mutation in the PH domain) and deltaVCA (deletion of the actin binding domain), localize predominantly in the nucleus and do not cause a change in the cytoskeleton, irrespective of EGF treatment. Interestingly, the C38W PH domain binds less effectively to phosphatidylinositol 4,5-bisphosphate (PIP2) than the wild-type PH domain. These results suggest the importance of the PIP2 binding ability of the PH domain and the actin binding for retention in membranes. Collectively, we conclude that N-WASP transmits signals from tyrosine kinases to cause a polarized rearrangement of cortical actin filaments dependent on PIP2.
URLPMID:20533885 [本文引用: 4]

The proteins of the Wiskott-Aldrich syndrome protein (WASP) family are activators of the ubiquitous actin nucleation factor, the Arp2/3 complex. WASP family proteins contain a C-terminal VCA domain that binds and activates the Arp2/3 complex in response to numerous inputs, including Rho family GTPases, phosphoinositide lipids, SH3 domain-containing proteins, kinases, and phosphatases. In the archetypal members of the family, WASP and N-WASP, these signals are integrated through two levels of regulation, an allosteric autoinhibitory interaction, in which the VCA is sequestered from the Arp2/3 complex, and dimerization/oligomerization, in which multi-VCA complexes are better activators of the Arp2/3 complex than monomers. Here, we review the structural, biochemical, and biophysical details of these mechanisms and illustrate how they work together to control WASP activity in response to multiple inputs. These regulatory principles, derived from studies of WASP and N-WASP, are likely to apply broadly across the family. [References: 135]
URLPMID:10878810 [本文引用: 2]

Wiskott-Aldrich syndrome protein (WASP) and N-WASP have emerged as key proteins connecting signalling cascades to actin polymerization. Here we show that the amino-terminal WH1 domain, and not the polyproline-rich region, of N-WASP is responsible for its recruitment to sites of actin polymerization during Cdc42-independent, actin-based motility of vaccinia virus. Recruitment of N-WASP to vaccinia is mediated by WASP-interacting protein (WIP), whereas in Shigella WIP is recruited by N-WASP. Our observations show that vaccinia and Shigella activate the Arp2/3 complex to achieve actin-based motility, by mimicking either the SH2/SH3-containing adaptor or Cdc42 signalling pathways to recruit the N-WASP-WIP complex. We propose that the N-WASP-WIP complex has a pivotal function in integrating signalling cascades that lead to actin polymerization.
URLPMID:10467124 [本文引用: 2]

Abstract The molecular link between the signalling pathway regulating the formation of filopodia and the initiation of local actin polymerization has been elucidated: N-WASP, a close homologue of WASP, which is the product of the gene responsible for the Wiskott-Aldrich syndrome, mediates a direct connection between the small G-protein Cdc42 and the Arp2/3 complex.
URLPMID:10724160 [本文引用: 2]

The Rho-family GTPase, Cdc42, can regulate the actin cytoskeleton through activation of Wiskott-Aldrich syndrome protein (WASP) family members. Activation relieves an autoinhibitory contact between the GTPase-binding domain and the carboxy-terminal region of WASP proteins. Here we report the autoinhibited structure of the GTPase-binding domain of WASP, which can be induced by the C-terminal region or by organic co-solvents. In the autoinhibited complex, intramolecular interactions with the GTPase-binding domain occlude residues of the C terminus that regulate the Arp2/3 actin-nucleating complex. Binding of Cdc42 to the GTPase-binding domain causes a dramatic conformational change, resulting in disruption of the hydrophobic core and release of the C terminus, enabling its interaction with the actin regulatory machinery. These data show that 'intrinsically unstructured' peptides such as the GTPase-binding domain of WASP can be induced into distinct structural and functional states depending on context.
URLPMID:9422512 [本文引用: 2]

Abstract Cdc42 is a small GTPase of the Rho family which regulates the formation of actin filaments to generate filopodia. Although there are several proteins such as PAK, ACK and WASP (Wiskott-Aldrich syndrome protein) that bind Cdc42 directly, none of these can account for the filopodium formation induced by Cdc42. Here we demonstrate that before it can induce filopodium formation, Cdc42 must bind a WASP-related protein, N-WASP, that is richest in neural tissues but is expressed ubiquitously. N-WASP induces extremely long actin microspikes only when co-expressed with active Cdc42, whereas WASP, which is expressed in haematopoietic cells, does not, despite the structural similarities between WASP and N-WASP. In a cell-free system, addition of active Cdc42 significantly stimulates the actin-depolymerizing activity of N-WASP, creating free barbed ends from which actin polymerization can then take place. This activation seems to be caused by exposure of N-WASP's actin-depolymerizing region induced by Cdc42 binding.
URLPMID:10219243 [本文引用: 1]

Although small GTP-binding proteins of the Rho family have been implicated in signaling to the actin cytoskeleton, the exact nature of the linkage has remained obscure. We describe a novel mechanism that links one Rho family member, Cdc42, to actin polymerization. N-WASP, a ubiquitously expressed Cdc42-interacting protein, is required for Cdc42-stimulated actin polymerization in Xenopus egg extracts. The C terminus of N-WASP binds to the Arp2/3 complex and dramatically stimulates its ability to nucleate actin polymerization. Although full-length N-WASP is less effective, its activity can be greatly enhanced by Cdc42 and phosphatidylinositol (4,5) bisphosphate. Therefore, N-WASP and the Arp2/3 complex comprise a core mechanism that directly connects signal transduction pathways to the stimulation of actin polymerization.
URL [本文引用: 1]
URLPMID:2150699 [本文引用: 1]

Neuronal Wiskott-Aldrich Syndrome protein (N-WASP) transmits signals from Cdc42 to the nucleation of actin filaments by Arp2/3 complex. Although full-length N-WASP is a weak activator of Arp2/3 complex, its activity can be enhanced by upstream regulators such as Cdc42 and PI(4,5)P2. We dissected this activation reaction and found that the previously described physical interaction between the NH2-terminal domain and the COOH-terminal effector domain of N-WASP is a regulatory interaction because it can inhibit the actin nucleation activity of the effector domain by occluding the Arp2/3 binding site. This interaction between the NH2- and COOH termini must be intramolecular because in solution N-WASP is a monomer. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) influences the activity of N-WASP through a conserved basic sequence element located near the Cdc42 binding site rather than through the WASp homology domain 1. Like Cdc42, PI(4,5)P2reduces the affinity between the NH2- and COOH termini of the molecule. The use of a mutant N-WASP molecule lacking this basic stretch allowed us to delineate a signaling pathway inXenopusextracts leading from PI(4,5)P2to actin nucleation through Cdc42, N-WASP, and Arp2/3 complex. In this pathway, PI(4,5)P2serves two functions: first, as an activator of N-WASP; and second, as an indirect activator of Cdc42.
URLPMID:26554011 [本文引用: 1]

Actin filament networks assemble on cellular membranes in response to signals that locally activate neural Wiskott-Aldrich-syndrome protein (N-WASP) and the Arp2/3 complex. An inactive conformation of N-WASP is stabilized by intramolecular contacts between the GTPase binding domain (GBD) and the C helix of the verprolin-homology, connector-helix, acidic motif (VCA) segment. Multiple SH3 domain-containing adapter proteins can bind and possibly activate N-WASP, but it remains unclear how such binding events relieve autoinhibition to unmask the VCA segment and activate the Arp2/3 complex. Here, we have used purified components to reconstitute a signaling cascade driven by membrane-localized Src homology 3 (SH3) adapters and N-WASP, resulting in the assembly of dynamic actin networks. Among six SH3 adapters tested, Nck was the most potent activator of N-WASP-driven actin assembly. We identify within Nck a previously unrecognized activation motif in a linker between the first two SH3 domains. This linker sequence, reminiscent of bacterial virulence factors, directly engages the N-WASP GBD and competes with VCA binding. Our results suggest that animals, like pathogenic bacteria, have evolved peptide motifs that allosterically activate N-WASP, leading to localized actin nucleation on cellular membranes.
URLPMID:18995840 [本文引用: 3]

Members of the Wiskott-Aldrich syndrome protein (WASP) family control actin dynamics in eukaryotic cells by stimulating the actin nucleating activity of the Arp2/3 complex. The prevailing paradigm for WASP regulation invokes allosteric relief of autoinhibition by diverse upstream activators. Here we demonstrate an additional level of regulation that is superimposed upon allostery: dimerization increases the affinity of active WASP species for Arp2/3 complex by up to 180-fold, greatly enhancing actin assembly by this system. This finding explains a large and apparently disparate set of observations under a common mechanistic framework. These include WASP activation by the bacterial effector EspFu and a large number of SH3 domain proteins, the effects on WASP of membrane localization/clustering and assembly into large complexes, and cooperativity between different family members. Allostery and dimerization act in hierarchical fashion, enabling WASP/WAVE proteins to integrate different classes of inputs to produce a wide range of cellular actin responses.
[本文引用: 1]
URLPMID:24462670 [本文引用: 1]

Cerebrospinal fluid (CSF) is produced by the choroid plexus and moved by multi-ciliated ependymal cells through the ventricular system of the vertebrate brain. Defects in the ependymal layer functionality are a common cause of hydrocephalus. N-WASP (Neural-Wiskott Aldrich Syndrome Protein) is a brain-enriched regulator of actin cytoskeleton and N-WASP knockout caused embryonic lethality in mice with neural tube and cardiac abnormalities. To shed light on the role of N-WASP in mouse brain development, we generated N-WASP conditional knockout mouse model N-WASPfl/fl; Nestin-Cre (NKO-Nes). NKO-Nes mice were born with Mendelian ratios but exhibited reduced growth characteristics compared to their littermates containing functional N-WASP alleles. Importantly, all NKO-Nes mice developed cranial deformities due to excessive CSF accumulation and did not survive past weaning. Coronal brain sections of these animals revealed dilated lateral ventricles, defects in ciliogenesis, loss of ependymal layer integrity, reduced thickness of cerebral cortex and aqueductal stenosis. Immunostaining for N-cadherin suggests that ependymal integrity in NKO-Nes mice is lost as compared to normal morphology in the wild-type controls. Moreover, scanning electron microscopy and immunofluorescence analyses of coronal brain sections with anti-acetylated tubulin antibodies revealed the absence of cilia in ventricular walls of NKO-Nes mice indicative of ciliogenesis defects. N-WASP deficiency does not lead to altered expression of N-WASP regulatory proteins, Fyn and Cdc42, which have been previously implicated in hydrocephalus pathology. Taken together, our results suggest that N-WASP plays a critical role in normal brain development and implicate actin cytoskeleton regulation as a vulnerable axis frequently deregulated in hydrocephalus. 2014 Elsevier Inc.
[本文引用: 1]
URLPMID:15361067 [本文引用: 1]

Abstract Migration of cells is critical to development of the central nervous system. Reelin, which was identified from the reeler mutant mice having a defect in the multilamellar structure of the brain, is thought to be a key signalling molecule that functions as a cue for determination of cell position. mDab1 (mouse Disabled homologue 1) functions downstream of Reelin. However, the mechanism by which mDab1 regulates cell migration during brain development is unknown. In the present paper, we show that mDab1 associates with N-WASP (neuronal Wiskott-Aldrich syndrome protein) in vitro and in brains of embryonic mice. mDab1 activates N-WASP directly, and induces actin polymerization through the Arp2/3 (actin-related protein 2/3) complex. mDab1 induces formation of filopodia when it is overexpressed in COS-7 cells. This filopodium formation is dependent on N-WASP, because expression of an N-WASP mutant that cannot induce Arp2/3-complex-mediated actin polymerization suppressed filopodium formation. The PTB (phosphotyrosine-binding) domain of mDab1 binds to N-WASP via the NRFY (Asn-Arg-Phe-Tyr) sequence close to the CRIB (Cdc42/Rac-interactive binding) motif of N-WASP and activates N-WASP in vitro. When mDab1 is phosphorylated by Fyn kinase in COS-7 cells, mDab1 is ubiquitinated in a Cbl-dependent manner, and mDab1 does not induce filopodium in the presence of activated Fyn. These findings suggest that mDab1 regulates the actin cytoskeleton through N-WASP, which is negatively regulated by phosphorylation-mediated ubiquitination of mDab1.
URLPMID:22955616 [本文引用: 2]

The human genome encodes the blueprint of life, but the function of the vast majority of its nearly three billion bases is unknown. The Encyclopedia of DNA Elements (ENCODE) project has systematically mapped regions of transcription, transcription factor association, chromatin structure and histone modification. These data enabled us to assign biochemical functions for 80% of the genome, in particular outside of the well-studied protein-coding regions. Many discovered candidate regulatory elements are physically associated with one another and with expressed genes, providing new insights into the mechanisms of gene regulation. The newly identified elements also show a statistical correspondence to sequence variants linked to human disease, and can thereby guide interpretation of this variation. Overall, the project provides new insights into the organization and regulation of our genes and genome, and is an expansive resource of functional annotations for biomedical research.
URL [本文引用: 1]
URLPMID:12456725 [本文引用: 1]

Rho family small GTPases regulate multiple cellular functions through reorganization of the actin cytoskeleton. Among them, Cdc42 and Tc10 induce filopodia or peripheral processes in cultured cells. We have identified a member of the family, designated as RhoT, which is closely related to Tc10. Tc10 was highly expressed in muscular tissues and brain and remarkably induced during differentiation of C2 skeletal muscle cells and neuronal differentiation of PC12 and N1E-115 cells. On the other hand, RhoT was predominantly expressed in heart and uterus and induced during neuronal differentiation of N1E-115 cells. Tc10 exogenously expressed in fibroblasts generated actin-filament-containing peripheral processes longer than the Cdc42-formed filopodia, whereas RhoT produced much longer and thicker processes containing actin filaments. Furthermore, both Tc10 and RhoT induced neurite outgrowth in PC12 and N1E-115 cells, but Cdc42 did not do this by itself. Tc10 and RhoT as well as Cdc42 bound to the N-terminal CRIB-motif-containing portion of N-WASP and activated N-WASP to induce Arp2/3-complex-mediated actin polymerization. The formation of peripheral processes and neurites by Tc10 and RhoT was prevented by the coexpression of dominant-negative mutants of N-WASP. Thus, N-WASP is essential for the process formation and neurite outgrowth induced by Tc10 and RhoT. Neuronal differentiation of PC12 and N1E-115 cells induced by dibutyryl cyclic AMP and by serum starvation, respectively, was prevented by dominant-negative Cdc42, Tc10 and RhoT. Taken together, all these Rho family proteins are required for neuronal differentiation, but they exert their functions differentially in process formation and neurite extension. Consequently, N-WASP activated by these small GTPases mediates neuronal differentiation in addition to its recently identified role in glucose uptake.
URLPMID:17635999 [本文引用: 1]

Abstract NGF-induced differentiation of PC12 cells is mediated by actin-polymerisation-driven membrane protrusion, involving GTPase signalling pathways that activate actin nucleation promoting factors such as the neural Wiskott-Aldrich syndrome protein (N-WASP). Expression of the exocyst subunit Exo70 in PC12 cells and neurons leads to the generation of numerous membrane protrusions, an effect that is strongly potentiated upon NGF-induced differentiation. F枚rster resonance energy transfer (FRET) imaging by fluorescence lifetime microscopy (FLIM) reveals an NGF-induced interaction of activated TC10 with Exo70. Expression of dominant-negative mutants and siRNA-mediated knockdown implicates N-WASP in NGF-induced Exo70-TC10-mediated membrane protrusion. However, FRET imaging of N-WASP activation levels of cells expressing Exo70 and/or constitutively active TC10 reveals that this complex locally antagonises the NGF-induced activation of N-WASP in membrane protrusions. Experiments involving siRNA-mediated knockdown of Cdc42 and overexpression of constitutively active Cdc42 confirm that the Exo70-TC10 complex mainly targets the NGF-induced Cdc42-dependent activation of N-WASP. Our results show that Exo70 is responsible for the correct targeting of the Exo70-TC10 complex to sites of membrane protrusion. The functional uncoupling between both pathways represents a novel regulatory mechanism that enables switching between morphologically distinct - Cdc42- or TC10-dominated - forms of cellular membrane outgrowth.
URLPMID:2414292 [本文引用: 1]

Changes in the number, size, and shape of dendritic spines are associated with synaptic plasticity, which underlies cognitive functions such as learning and memory. This plasticity is attributed to reorganization of actin, but the molecular signals that regulate this process are poorly understood. In this study, we show neural Wiskott-Aldrich syndrome protein (N-WASP) regulates the formation of dendritic spines and synapses in hippocampal neurons. N-WASP localized to spines and active, functional synapses as shown by loading with FM4-64 dye. Knock down of endogenous N-WASP expression by RNA interference or inhibition of its activity by treatment with a specific inhibitor, wiskostatin, caused a significant decrease in the number of spines and excitatory synapses. Deletion of the C-terminal VCA region of N-WASP, which binds and activates the actin-related protein 2/3 (Arp2/3) complex, dramatically decreased the number of spines and synapses, suggesting activation of the Arp2/3 complex is critical for spine and synapse formation. Consistent with this, Arp3, like N-WASP, was enriched in spines and excitatory synapses and knock down of Arp3 expression impaired spine and synapse formation. A similar defect in spine and synapse formation was observed when expression of an N-WASP activator, Cdc42, was knocked down. Thus, activation of N-WASP and, subsequently, the Arp2/3 complex appears to be an important molecular signal for regulating spines and synapses. Arp2/3-mediated branching of actin could be a mechanism by which dendritic spine heads enlarge and subsequently mature. Collectively, our results point to a critical role for N-WASP and the Arp2/3 complex in spine and synapse formation.
URLPMID:3283256533400535085521348360 [本文引用: 1]

Neuritogenesis, or neurite outgrowth, is a critical process for neuronal differentiation and maturation in which growth cones are formed from highly dynamic actin structures. Gas7 (growth arrest-specific gene 7), a new member of the PCH (Pombe Cdc15 homology) protein family, is predominantly expressed in neurons and is required for the maturation of primary cultured Purkinje neurons as well as the neuron-like differentiation of PC12 cells upon nerve growth factor stimulation. We report that Gas7 co-localizes and physically interacts with N-WASP, a key regulator of Arp2/3 complex-mediated actin polymerization, in the cortical region of Gas7-transfected Neuro-2a cells and growth cones of hippocampal neurons. The interaction between Gas7 and N-WASP is mediated by WW-Pro domains, which is unique in the PCH protein family, where most interactions are of the SH3-Pro kind. The interaction contributes to the formation of membrane protrusions and processes by recruiting the Arp2/3 complex in a Cdc42-independent manner. Importantly, specific interaction between Gas7 and N-WASP is required for regular neurite outgrowth of hippocampal neurons. The data demonstrate an essential role of Gas7 through its interaction with N-WASP during neuronal maturation/differentiation.
URLPMID:22383893 [本文引用: 1]

Abstract The Eph receptor tyrosine kinases (RTKs) are regulators of cell migration and axon guidance. However, our understanding of the molecular mechanisms by which Eph RTKs regulate these processes is still incomplete. To understand how Eph receptors regulate axon guidance in Caenorhabditis elegans, we screened for suppressors of axon guidance defects caused by a hyperactive VAB-1/Eph RTK. We identified NCK-1 and WSP-1/N-WASP as downstream effectors of VAB-1. Furthermore, VAB-1, NCK-1, and WSP-1 can form a complex in vitro. We also report that NCK-1 can physically bind UNC-34/Enabled (Ena), and suggest that VAB-1 inhibits the NCK-1/UNC-34 complex and negatively regulates UNC-34. Our results provide a model of the molecular events that allow the VAB-1 RTK to regulate actin dynamics for axon guidance. We suggest that VAB-1/Eph RTK can stop axonal outgrowth by inhibiting filopodia formation at the growth cone by activating Arp2/3 through a VAB-1/NCK-1/WSP-1 complex and by inhibiting UNC-34/Ena activity.
URLPMID:1350777 [本文引用: 1]

Abstract Mutations in the human LIS1 gene cause the smooth brain disease classical lissencephaly. To understand the underlying mechanisms, we conducted in situ live cell imaging analysis of LIS1 function throughout the entire radial migration pathway. In utero electroporation of LIS1 small interference RNA and short hairpin dominant negative LIS1 and dynactin cDNAs caused a dramatic accumulation of multipolar progenitor cells within the subventricular zone of embryonic rat brains. This effect resulted from a complete failure in progression from the multipolar to the migratory bipolar state, as revealed by time-lapse analysis of brain slices. Surprisingly, interkinetic nuclear oscillations in the radial glial progenitors were also abolished, as were cell divisions at the ventricular surface. Those few bipolar cells that reached the intermediate zone also exhibited a complete block in somal translocation, although, remarkably, process extension persisted. Finally, axonal growth also ceased. These results identify multiple distinct and novel roles for LIS1 in nucleokinesis and process dynamics and suggest that nuclear position controls neural progenitor cell division.
URLPMID:14625554 [本文引用: 1]

Mutations in the doublecortin gene (DCX) in humans cause malformation of the cerebral neocortex. Paradoxically, genetic deletion of Dcx in mice does not cause neocortical malformation. We used electroporation of plasmids encoding short hairpin RNA to create interference (RNAi) of DCX protein in utero, and we show that DCX is required for radial migration in developing rat neocortex. RNAi of DCX causes both cell-autonomous and non-cell autonomous disruptions in radial migration, and creates two disruptions in neocortical development. First, many neurons prematurely stop migrating to form subcortical band heterotopias within the intermediate zone and then white matter. Second, many neurons migrate into inappropriate neocortical lamina within normotopic cortex. In utero RNAi can therefore be effectively used to study the specific cellular roles of DCX in neocortical development and to produce an animal model of double cortex syndrome.
URLPMID:18708039 [本文引用: 1]

Neuronal circuit remodeling is the most critical pathological characteristic closely associated with the initiation and maintenance of epilepsy; however, the exact mechanisms of neuronal remodeling need further elucidation. Neuronal Wiskott鈥揂ldrich syndrome protein (N-WASP) is a key regulator of the actin cytoskeleton that causes actin polymerization and thus neurite extension. Our previous research demonstrated that the upstream regulator of N-WASP, cell division cycle 42 GTP-binding protein (Cdc42), is significantly upregulated in the brains of patients with intractable epilepsy (IE). In addition, cDNA microarray analysis has shown that gene expression of N-WASP is notably enhanced in the epileptic brain, suggesting a possible role for N-WASP in epileptogenesis. Here, we investigated the expression of N-WASP and its downstream effector, actin-related protein 2/3 (Arp2/3), at the protein level in the temporal lobe of IE patient brains to explore its possible role in the genesis of IE. Forty surgical samples from brains of patients with IE and 20 control brain tissues were obtained for this study. The expression of N-WASP in the anterior temporal neocortex was detected using immunohistochemistry, immunofluorescence and western blotting; Arp2/3 expression was detected by western blotting. Compared with controls, N-WASP expression in brains of IE patients was significantly higher; similarly, Arp2/3 level was markedly increased in the IE patient group. These results suggest that increased expression of N-WASP in the human brain may be associated with human IE.
URLPMID:21785420 [本文引用: 2]

Abstract N-WASP is a major cytoskeletal regulator that stimulates Arp2/3-mediated actin nucleation. Here, we identify a nucleation-independent pathway by which N-WASP regulates the cytoskeleton and junctional integrity at the epithelial zonula adherens. N-WASP is a junctional protein whose depletion decreased junctional F-actin content and organization. However, N-WASP (also known as WASL) RNAi did not affect junctional actin nucleation, dominantly mediated by Arp2/3. Furthermore, the junctional effect of N-WASP RNAi was rescued by an N-WASP mutant that cannot directly activate Arp2/3. Instead, N-WASP stabilized newly formed actin filaments and facilitated their incorporation into apical rings at the zonula adherens. A major physiological effect of N-WASP at the zonula adherens thus occurs through a non-canonical pathway that is distinct from its capacity to activate Arp2/3. Indeed, the junctional impact of N-WASP was mediated by the WIP-family protein, WIRE, which binds to the N-WASP WH1 domain. We conclude that N-WASP-WIRE serves as an integrator that couples actin nucleation with the subsequent steps of filament stabilization and organization necessary for zonula adherens integrity.
URLPMID:29386393 [本文引用: 2]

Arp2/3 complex is a key actin cytoskeletal regulator that creates branched actin filament networks in response to cellular signals. WASP-activated Arp2/3 complex assembles branched actin networks by nucleating new filaments from the sides of pre-existing ones. WASP-mediated activation requires seed filaments, to which the WASP-bound Arp2/3 complex can bind to form branches, but the source of... [Show full abstract]
