 ,*首都师范大学生命科学学院, 北京 100048
,*首都师范大学生命科学学院, 北京 100048Morphology and Genetic Diversity of Phragmites australis in Beijing
Xi Zhang, Tianhang Qiu, Anan Wang, Huajian Zhou, Min Yuan, Li Li, Sulan Bai, Suxia Cui ,*College of Life Sciences, Capital Normal University, Beijing 100048, China
,*College of Life Sciences, Capital Normal University, Beijing 100048, China通讯作者: *E-mail:sxcui@cnu.edu.cn
责任编辑: 白羽红
收稿日期:2020-01-14接受日期:2020-08-26网络出版日期:2020-11-01
| 基金资助: |
Corresponding authors: *E-mail:sxcui@cnu.edu.cn
Received:2020-01-14Accepted:2020-08-26Online:2020-11-01

摘要
为探明北京地区芦苇(Phragmites australis)的资源状态和多样性, 实地考察北京主要河流、湿地和水库, 发现北京地区芦苇总生长面积已超过600 hm2。芦苇染色体倍性以八倍体为主, 四倍体次之。在面积较大的湿地内, 八倍体单一芦苇群落占据优势地位; 而在城市的浅河内有形态和遗传性多样的混合种群。研究表明, 植物性状和倍性水平之间无显著相关性。在小清河发现了6种形态各异的芦苇克隆, 均属于叶绿体DNA片段的P单倍型; 其单倍体基因组大小为(0.499±0.019) pg, 变异系数为3.8%。这表明表型与单倍型之间也不具相关性。此外, 发现1个具有变叶特性的芦苇, 将其命名为金条芦苇。北京地区芦苇形态和遗传多样性为研究芦苇基因型与环境适应性之间的关系提供了珍贵的资源。
关键词:
Abstract
To understand the distribution and diversity of Phragmites australis in Beijing, we carried out field investigation to Beijing’s major rivers, wetlands and reservoirs, which reveals that the total area of reed populations has reached more than 600 hm 2 in Beijing. The ploidy level is dominated by octaploid, followed by tetraploid. In larger wetlands, the single community of octoploid occupies a dominant position; while in shallow urban rivers, the mixed populations with different morphological, ploidy and genetic diversity are common. There is no significant correlation between the plant traits and ploidy level variation. Six different reed clones were found in Xiaoqing River, all belonging to P haplotypes. The haploid genome size of all clones ranged from (0.499±0.019) pg, with a coefficient of variation of 3.8%. These results show that there is no correlation between phenotype and haplotype. In addition, a reed variant with versicolor leaf characteristics was discovered and named as Phragmites australis var. australis f. Goldstripe. The morphology and genetic diversity of Phragmites in Beijing provide valuable resources for future study of the relationship between reed genotypes and environmental adaptability.
Keywords:
PDF (15803KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
张茜, 裘天航, 王安安, 周华健, 袁敏, 李利, 白素兰, 崔素霞. 北京地区芦苇资源状态及其多样性. 植物学报, 2020, 55(6): 693-704 doi:10.11983/CBB20006
Zhang Xi, Qiu Tianhang, Wang Anan, Zhou Huajian, Yuan Min, Li Li, Bai Sulan, Cui Suxia.

芦苇(Phragmites australis)是一种典型的湿地水生植物, 但能生长在盐渍干旱的恶劣环境以及富营养化的污染水域, 并成为其生境内的优势物种(Clevering and Lissner, 1999; Saltonstall, 2002; Cui et al., 2009)。由于强大的环境适应能力, 芦苇成功地分布于全世界。鉴于芦苇生境的广布性、强大的环境适应性以及对环境的高度敏感性, 使其成为一个可选的环境监测物种。一项大规模的综合研究证明, 芦苇的一些生态种群能对全球生态变化, 如温度、大气中CO2浓度、干旱、洪涝、盐渍以及富营养化做出响应(Eller et al., 2017)。北京地处海河流域, 境内有永定河、潮白河、北运河、大清河和蓟运河五大水系, 自古以来就有大面积的芦苇分布。近年来, 随着湿地恢复、河流整治、污染治理的需求增大, 芦苇的生长恢复面积越来越大。但对北京地区芦苇的研究尚属空白, 其分布面积、生境、染色体倍性、遗传多样性和环境可塑性等生物学问题基本无资料记述, 成为环境监测和开发利用的首要障碍。
芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(Dykyjová and Pazourková, 1979)到三倍体、四倍体、六倍体、八倍体、十倍体, 甚至十二倍体的芦苇均有报道(Gorenflflot, 1986; Clevering and Lissner, 1999)。四倍体和八倍体芦苇占据主要地位。在美洲, 芦苇常被列为入侵物种, 这与其染色体组的高倍性以及强大的环境适应性密切相关(Meyerson et al., 2009)。在中国, 芦苇的生长和分布面积极广, 有14个主要分布区, 总面积约1.3×106 hm2 (郭春秀等, 2012), 其中八倍体芦苇占绝对优势(Clevering and Lissner, 1999; Cui et al., 2002)。然而, 这种全球分布广泛、自然生长面积巨大的物种在种内的分类依然不清楚。有的以生境特点命名为各类生态型, 如沼泽芦苇和沙丘芦苇(张承烈和陈国仓, 1991); 有些则直接以栖息地为名, 如白洋淀苇、博斯腾苇、黑龙江苇和盘锦苇(李建国等, 2004)。其主要原因是芦苇在株高、叶片形态、花序及群落等方面都有极高的环境可塑性(Haslam, 1970), 在种内多呈现形态上的重叠, 因此很难依据形态特征加以区分(Clayton, 1967)。近年来, 以分子标记为分类依据的研究已逐渐展开。其中, 以叶绿体基因组非编码区片段变异的单倍型分类方法(Saltonstall, 2002)受到普遍认可。在此基础上, 研究者完成了中国北方芦苇和西南芦苇的叶绿体DNA片段单倍型分类, 发现西北地区的芦苇以单倍型O和M型为主; 东北和华北地区则主要以M和P型为主(An et al., 2012); 但西南芦苇以I、P、Q和U型为主(Tanaka et al., 2017)。遗憾的是, 这种分类方式缺乏芦苇单倍型与表型之间的对应关系描述, 在研究和实践中都难以应用。
芦苇的生态和经济价值很高。在经济领域, 芦苇是传统的造纸原料, 亦可用于建筑材料、饲料和燃料, 此外还是不可缺少的景观植物。然而, 芦苇更重要的作用是在生态领域, 它是湿地生态系统中的优势种群, 可以涵养水源、维护生态系统平衡(张兵, 2008; 程杰, 2013)。近年来, 由于人类活动的侵扰, 工业、农业和生活污水排放加剧了环境水体的污染, 芦苇又成为最常用的湿地和水体修复植物(Ahmed, 2017)。综上, 芦苇因其生境的广布性、巨大的生物量以及对环境的高度敏感性和适应性, 加之其复杂的遗传多样性和庞大的基因资源, 成为当前研究的热点。本文详细阐述了北京地区芦苇的资源状态。通过对五大水系的主要河流、湿地、水库和大型公园进行实地考察, 发现芦苇是北京地区占地面积非常大的植物种类, 总生长面积已超过600 hm2, 染色体组以八倍体为主, 四倍体次之, 并且具有形态和遗传多样性。
1 材料与方法
1.1 芦苇的生长面积和形态指标检测
测算芦苇(Phragmites australis (Cav.) Trin. ex Steud var. australis)的生长面积以公顷为单位。在植物生长季节(5-10月)实地考察北京地区五大水系(永定河水系、大清河水系、潮白河水系、北运河水系和蓟运河水系)中的湿地、河流和水库中的芦苇生长范围, 利用卫星地图和地图尺App计算其生长面积, 同时记录地理位置坐标。在芦苇开花后期, 选择成熟植株(每采样点n=10)测量株高、基径和旗叶等各类形态指标, 并采集花序于体视显微镜下观察小穗和小花形态。1.2 用流式细胞仪分析基因组大小和染色体倍性
采用流式细胞仪分析芦苇基因组大小及染色体倍性 (Dole?el, 2007; Meyerson et al., 2016)。在野外采集幼嫩的芦苇叶片, 用湿纱布包裹后置于4°C冰箱。分析时用刀片切碎叶片, 加入特制的缓冲液(北京蔬菜研究所提供)混匀并过滤以获得细胞核, 进行DAPI染色。测试仪器为FACSCalibur流式细胞仪(BD公司, 美国), 用CellQuest软件获取数据, 并用ModFit软件(Verity Software House公司)进行结果分析。因芦苇群体之间基因组差异较大, 本研究采用二次内参法计算基因组大小并推算染色体倍性。首先用基因组大小已知的水稻日本晴(Oryza sativa L. cv. ‘Nipponbare’)为内参(Wang et al., 2018, 2C=0.795 pg), 检测并计算出1个四倍体芦苇的基因组大小(4x=2.253 pg); 再以此为内参, 检测八倍体芦苇。以染色体压片确定的四倍体芦苇(4x=48)和八倍体芦苇(8x=96)为参考(Cui et al., 2002), 推测所有分析样品的倍性水平。基因组大小(pg DNA)=(样品G1峰平均值/内参G1峰平均值)×内参基因组大小; 单倍体基因组大小(即单倍体染色体组的DNA含量Cx值)为基因组大小/倍性水平。1.3 小清河样地芦苇表型特征和单倍体分型
小清河样地位于北京西南的小清河中段(116.21°E, 39.70°N)。取样时检测水深、水温、pH值以及溶解氧水平(HI9142溶解氧测量仪, HANNA, 意大利)。在芦苇开花后期, 选择6个不同形态的芦苇克隆群体(C1-C6)进行形态指标的检测, 并进行主成分分析(PCA)。同时采集新鲜叶片于-80°C冻存, 采用CTAB法(Porebski et al., 1997)提取基因组DNA。叶绿体DNA片段单倍型的鉴别按照Taberlet等(1991)和Saltonstall (2001)的方法, 针对叶绿体基因组中的2段非编码区trnT-trnL及rbcL-psaI设计2对引物。即TF (5'-CATTACAAATGCGATGCTCT-3')和TR (5'- TCTACCGATTTCGCCATATC-3')以及RF (5'-TG-TACAAGCTCGTAACGAAGG-3')和RR (5'-CTAAG-CCTACTAAAGGCACG-3')。获得长度分别为906和1 115 bp的片段后进行测序(金唯智公司)。用CLUSTAL X软件完成序列对比, 单倍型的命名按Saltonstall (2016)的方式完成。1.4 金条芦苇的鉴定及标本制作
金条芦苇的鉴定根据表型、基因组大小和叶绿体基因组部分区段的大小来完成。基因组大小用流式细胞仪进行检测, 并推测染色体倍性。金条芦苇(Phragmites australis f. Goldstripes)和园艺植物变叶芦竹(Arundo donax L. var. versicolor)在叶片形态上有些相似, 但在其叶绿体基因组中有一段长度差异非常显著的序列。通过设计特异性引物, 即F-trnG-UCC (5'-CATAAATCCCTTTCTTCC-3')和R-trnT-GGU (5'-GATCTTTAGGCTATTCACTT-3'), 可以在分子水平上区分这2种植物。采用CTAB法提取基因组DNA, 然后进行PCR扩增。金条芦苇和变叶芦竹PCR产物的片段大小分别为1 069和607 bp。金条芦苇标本由首都师范大学植物标本馆(BGTC004950)进行压片制作并保存。2 结果与讨论
2.1 芦苇的分布、面积及染色体倍性
经实地勘测, 结合卫星地图和地图尺App的测算, 结果显示, 北京地区芦苇分布广泛, 总面积达645 hm2。在北京市5个主要水系中, 永定河水系和潮白河水系芦苇分布最多, 占总面积的78%, 是芦苇主要分布区; 其它3个水系(即大清河、北运河和蓟运河水系)中芦苇分布相对较少, 是次要分布区。从具体地点来看, 分布面积最大的区域是野鸭湖国家湿地公园, 其芦苇面积占北京市芦苇总面积的39.9%; 其次是汉石桥湿地公园(占13.0%)和翠湖湿地公园(占7.0%)。按河流计算, 芦苇分布较多的有潮河、潮白河、永定河和小清河(图1A; 表1)。图1
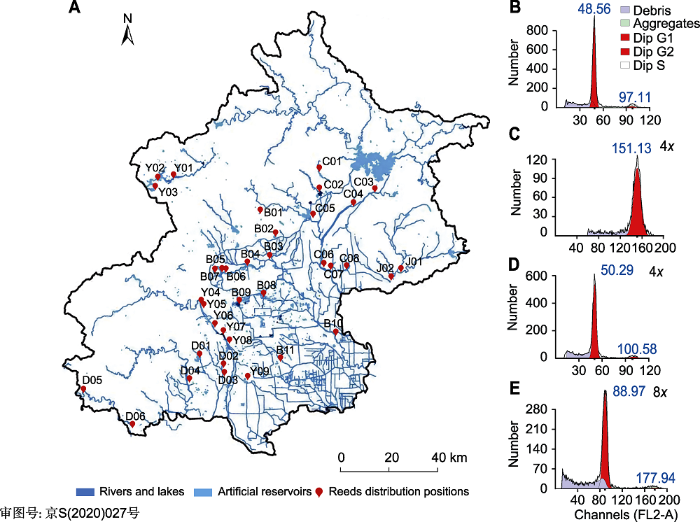 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1北京地区的芦苇分布和染色体倍性分析
(A) 北京地区芦苇分布(芦苇的主要分布区域以红点标注, 并依照水系进行编号: 永定河水系(Y)、潮白河水系(C)、北运河水系(B)、大运河水系(D)和蓟运河水系(J); 蓝色表示河流、湖泊或人造水库); (B)-(E) 用流式细胞术检测芦苇染色体倍性(横坐标为通道的荧光强度, 纵坐标为细胞核数目; G1和G2期分别用深红色和浅红色表示; 碎片和聚集体分别用灰紫色和绿色表示)。图(B)和(C)以水稻为内参, 检测四倍体芦苇; 图(D)和(E)以四倍体芦苇为内参, 检测八倍体芦苇。
Figure 1Distribution and chromosome ploidy of Phragmites australis in Beijing
(A) Distribution of reeds in Beijing (The main distribution areas of reeds are marked by red dots and numbered according to the water system: Yongding River System (Y), Chaobai River System (C), North Canal System (B), Grand Canal System (D), Ji Canal System (J); Blue indicates rivers, lakes or artificial reservoirs); (B)-(E) Detection of reed chromosome ploidy by flow cytometry (The abscissa is the fluorescence intensity of the channel, and the ordinate is the number of nuclei; G1 and G2 phases are indicated by dark red and light red, respectively; fragments and aggregates are indicated by gray-purple and green, respectively). Figures (B) and (C) detected the tetraploid reeds using rice as the internal reference; Figures (D) and (E) detected the octaploid reeds using the tetraploid reeds as the internal reference.
Table 1
表1
表1北京地区芦苇的分布地点、面积和染色体倍性
Table 1
| Water system | Distribution location | Distribution description | Area (hm2) | Proportion (%) | Ploidy level | Origin | |||
|---|---|---|---|---|---|---|---|---|---|
| Yongding River System | 304.11 | 47.16 | |||||||
| Y01 | Weishui River | Distributed at both sides of the west of the Weishui River Tunnel passes th- rough the World Horticultural, exposition to Yankang Road | 5.14 | 0.80 | NA | 115.95°E, 40.45°N | |||
| Y02 | Guanting Reservoir (in Beijing) | Scattered along the bank about 15 km west of the Guanting Bridge | 0.21 | 0.03 | 8x/4x | 115.87°E, 40.44°N | |||
| Y03 | Wild Duck Lake National Wetland Park | Distributed in patches in the core area, buffer zone, and test area | 257.40 | 39.92 | 8x | 115.86°E, 40.41°N | |||
| Water system | Distribution location | Distribution description | Area (hm2) | Proportion (%) | Ploidy level | Origin | |||
| Y04 | Shanxia Section of Yongding River | Distributed along the river bank | 8.31 | 1.29 | NA | 116.09°E, 39.99°N | |||
| Y05 | Sanjiadian Rush Wetland Park | Distributed in patches | 4.60 | 0.71 | 8x | 116.10°E, 39.97°N | |||
| Y06 | Lianshi Lake Park | Distributed along both banks of the river | 2.12 | 0.33 | 8x | 116.15°E, 39.90°N | |||
| Y07 | Yuanbo Lake Wetland | Distributed in patches | 16.30 | 2.53 | NA | 116.21°E, 39.87°N | |||
| Y08 | Ludi Park | Distributed in patches along the coast | 9.71 | 1.51 | NA | 116.22°E, 39.84°N | |||
| Y09 | Niantan Park | Distributed around the shore of a lake in the park | 0.32 | 0.05 | NA | 116.31°E, 39.71°N | |||
| Daqing River System | 58.63 | 9.09 | |||||||
| D01 | Chongqing Reservoir | Distributed in patches in the southwest corner of the reservoir | 13.17 | 2.04 | NA | 116.08°E, 39.79°N | |||
| D02 | Jiuzi River | Distributed in the upper reaches of Xiaoqing River | 5.42 | 0.84 | 8x | 116.19°E, 39.75°N | |||
| D03 | Xiaoqing River | Distributed in patches along the river from the south of Shuisi Road to the junction of Hedgehog River | 20.02 | 3.10 | 8x/4x | 116.20°E, 39.72°N | |||
| D04 | Dashi River | Distributed along the north and south rivers of Jingzhou Road | 16.41 | 2.54 | NA | 116.03°E, 39.70°N | |||
| D05 | Juma River | Sporadic distribution from Shidu to Yesanpo | 0.31 | 0.05 | 8x | 115.51°E, 39.66°N | |||
| D06 | Beijuma River | Distributed in patches at the Beijing border of the North Juma River Estuary | 3.30 | 0.51 | NA | 115.76°E, 39.53°N | |||
| Chaobai River System | 196.83 | 30.52 | |||||||
| C01 | Qinglong Gorge | Sporadically distributed from the upper reaches of Qinglong Gorge to Baiquan Mountain | 0.06 | 0.01 | 4x | 116.66°E, 40.48°N | |||
| C02 | Yanqi Lake | Artificial reed landscape at the northwest corner of the lake | 0.83 | 0.13 | 8x | 116.60°E, 40.40°N | |||
| C03 | Chao River | Distributed in patches about 7 km along the river near Miyun Service Area of Dachang Expressway | 38.23 | 5.93 | 8x/4x | 116.94°E, 40.40°N | |||
| C04 | The estuary of Chaobai River | Upper reaches of Chao River and Bai River cross estuary | 7.08 | 1.10 | 8x | 116.83°E, 40.35°N | |||
| C05 | Huai River | About 4.5 km from Huairou Reservoir to Yanqi River junction | 10.83 | 1.68 | 8x | 116.63°E, 40.31°N | |||
| C06 | Chaobai River Bridge | Piled up along the bank of Fuxing Bridge/Fengbo Bridge/Chaobai River Bridge in Shunping Road | 18.94 | 2.94 | NA | 116.69°E, 40.12°N | |||
| C07 | Hanshiqiao Wetland Park | Distributed in pieces of the core area, buffer zone and recovery area | 83.95 | 13.02 | 8x | 116.80°E, 40.12°N | |||
| C08 | Binhe Forest Park | Distributed along the north and south banks of Chaobai River | 36.91 | 5.72 | NA | 116.72°E, 40.11°N | |||
| North Canal Water System | 82.91 | 12.86 | |||||||
| B01 | Lily Reservoir | Distributed in upstream area of the reservoir | 0.05 | 0.01 | 8x | 116.38°E, 40.32°N | |||
| B02 | Taoyukou Reservoir | Distributed in periphery and upstream areas of the east and west sides of the reservoir | 9.46 | 1.47 | 8x | 116.45°E, 40.24°N | |||
| B03 | Wenyu River | Scattered along the middle and upper banks of Wenyu River | 0.21 | 0.03 | NA | 116.42°E, 40.15°N | |||
| B04 | Shahezha Park | Distributed in patches in the west bank of the lake in the park | 0.34 | 0.05 | 8x | 116.31°E, 40.13°N | |||
| Water system | Distribution location | Distribution description | Area (hm2) | Proportion (%) | Ploidy level | Origin | |||
| B05 | Cuihu Wetland Park | Uneven distribution on the wet land in protected areas and open areas | 45.38 | 7.04 | 8x | 116.19°E, 40.11°N | |||
| B06 | Shangzhuang Reservoir | Evenly distributed along the bank of Shangzhuang Reservoir and scattered along the downstream bank | 0.81 | 0.13 | 8x/4x | 116.21°E, 40.11°N | |||
| B07 | Nansha River | Discontinuous distribution in the 10 km section near Daoxiang Lake | 8.29 | 1.29 | 8x | 116.15°E, 40.10°N | |||
| B08 | Olympic Forest Park | Distributed at the west side of Wetland in Southern District and the east side around the lake | 5.04 | 0.78 | 8x/4x | 116.39°E, 40.01°N | |||
| B09 | Summer Palace | Mixed distribution of calamus on the shore near Zaojiantang site | 0.12 | 0.02 | NA | 116.27°E, 39.99°N | |||
| B10 | Grand Canal Forest Park | Distributed on the semi-arid coastal wetlands in popular reeds | 6.51 | 1.01 | NA | 116.75°E, 39.87°N | |||
| B11 | Nanhaizi Park | Distribution of small pieces of lakeshore in the park | 6.70 | 1.04 | 8x/4x | 116.47°E, 39.77°N | |||
| Jiyun River System | 2.37 | 0.37 | |||||||
| J01 | Ju River and Ru River | Sporadically distributed at the confluence of the Ru River and the Ju River | 2.03 | 0.31 | NA | 117.06°E, 40.11°N | |||
| J02 | Jinji River | The junction of Jinji River and Yanghe River to the vicinity of Yingcheng Village | 0.34 | 0.05 | NA | 117.02°E, 40.08°N | |||
新窗口打开|下载CSV
用流式细胞仪检测了21个芦苇生长点中94个样品的基因组大小, 并获得它们的染色体倍性。结果表明, 北京市的芦苇以八倍体为主(占总样品数62.5%), 而四倍体芦苇比较少见(图1; 表1)。在芦苇分布面积最大的3个湿地公园中, 单一的八倍体芦苇群体占绝对优势。反之, 四倍体芦苇多散生于八倍体芦苇群体中, 少有独立的单一种群存在(表1)。这一特点成为研究北京地区芦苇倍性和表型之间关系的一大障碍。
2.2 芦苇的形态和遗传多态性
北京地区芦苇具有多种形态多态性。在分布量较大的湿地公园, 通常都有2-3种形态多态型。例如, 在汉石桥湿地, 有茎秆粗细差异明显但均为八倍体的2种芦苇(粗苇、细苇); 在翠湖湿地公园和奥森公园湿地, 则存在株高差异巨大亦都是八倍体的2种芦苇(高苇、矮苇)。在人工景观公园和湿地, 花序颜色的差异明显可见。例如, 在奥森公园、南海子公园和莲石湖公园都存在黄色花序和紫色花序的芦苇(黄苇、紫苇)。特别值得关注的是, 在城市边缘的一些自然河流之中, 芦苇的形态多态性更加丰富。调查结果显示, 属于大清河流域的小清河部分河段中, 芦苇的形态多态性以及遗传多态性都非常丰富。我们发现在一个面积仅为0.15 hm2的小样地中就分布着表型差异极显著的6个芦苇克隆群体(C1-C6, 图2)。该小样地位于小清河下游, 一年之中水深变化范围为0.5-1 m, 7月中旬平均水温为27.3°C, pH7.8, 溶解氧为5.7 mg·L-1。由于上下游有橡胶坝, 小样地的水流缓慢。鉴于小样地生态因子的一致性, 我们认为其适于进行芦苇群体的形态差异和遗传学差异研究。图2
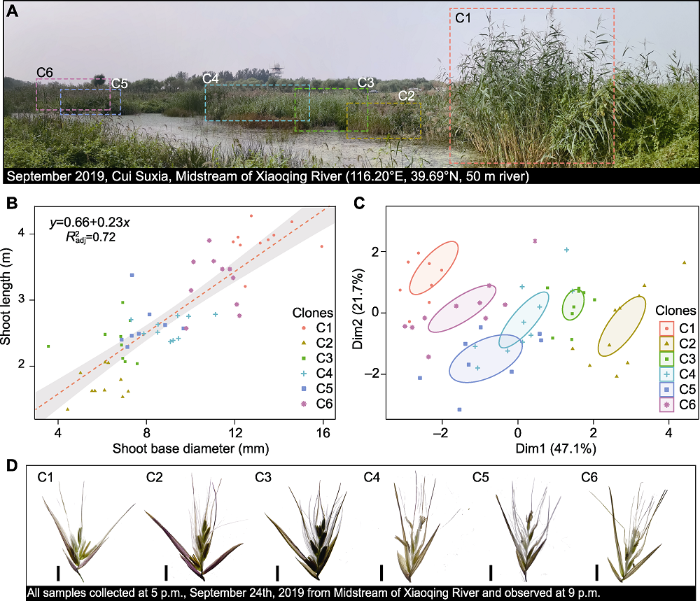 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2北京地区小清河样地芦苇多态性分析
(A) 小清河样地全景图(6个芦苇克隆群体C1-C6分别用不同颜色的矩形虚线框标出); (B) 6个芦苇群体的株高和基径的相关性分析(灰色阴影部分为回归区间, 红色虚线为最佳回归曲线); (C) 芦苇多态性的主成分分析(依据
Figure 2Polymorphism analysis of Phragmites australis in the Xiaoqing River plot
(A) Panoramic view of the Xiaoqing River plot (six different reed clones C1-C6 are marked by rectangular dashed boxes of different colors); (B) Correlation analysis of height and base diameter of six different reed clones (The gray shaded part is the regression interval, and the red dotted line is the best regression curve); (C) Principal component analysis of polymorphism in the reed plot of Xiaoqing River (based on eight morphological indicators of six species of reed clones in
通过流式细胞术分析, 我们从该小样地中鉴定出5个八倍体克隆和1个四倍体克隆(表2)。值得一提的是, 小清河四倍体克隆是我们在北京地区自然生境下找到的唯一四倍体芦苇群体, 极具研究价值。6个芦苇克隆的形态学指标, 包括株高、茎秆基径、第六叶和旗叶的长与宽以及花序大小(表2)。通过对形态学指标进行成对的数据相关性及回归分析, 我们发现芦苇的株高和茎秆基径之间呈线性正相关(图2B), 但四倍体芦苇C1在株高和基径方面都显著高于其它5个八倍体芦苇(C2-C6)。主成分分析显示, 上述6个芦苇克隆可以清晰地分开, 且四倍体C1独立于其它克隆, 与八倍体C6最为相近, 而与同是八倍体的C2却相差最远(图2C)。此外, 这些芦苇克隆在花序大小方面变化很大。成熟花序的长度从大到小依次为C5>C4> C6>C1>C2>C3, 而宽度从大到小依次为C2>C6> C4>C5>C1>C3。其中, 四倍体C1的花序较小(22.3/ 4.3 cm), 而最小的花序是八倍体C3 (20.3/3.3 cm)。总体上, 八倍体C6的花序比较大(26.5/6.9 cm)。表3显示, 小清河芦苇群体在形态上的变异系数均较大, 其中花序宽度变异最高。
Table 3
表3
表3北京小清河样地芦苇克隆群体的形态多态性和变异系数(n=59)
Table 3
| Phenotype | Mean | Maximum | Minimum | Standard deviation | Variation coefficient (%) |
|---|---|---|---|---|---|
| Height (m) | 2.74 | 4.25 | 1.34 | 0.75 | 27.48 |
| Diameter (mm) | 9.02 | 15.93 | 3.56 | 2.77 | 30.76 |
| Inflorescences length (cm) | 24.21 | 36.20 | 10.40 | 5.77 | 23.85 |
| Inflorescences width (cm) | 5.92 | 17.08 | 1.39 | 3.70 | 62.55 |
| Length of flag leaf (cm) | 31.57 | 53.10 | 15.40 | 7.06 | 22.37 |
| Width of flag leaf (cm) | 1.46 | 2.51 | 0.51 | 0.46 | 31.31 |
| Length of the sixth leaf (cm) | 39.41 | 62.30 | 15.30 | 14.19 | 36.02 |
| Width of the sixth leaf (cm) | 2.82 | 4.98 | 1.31 | 0.86 | 30.63 |
新窗口打开|下载CSV
在体视显微镜下, 不同芦苇克隆的小穗有3-6朵不等的小花, 每朵小花都具有3个雄蕊和1个雌蕊。但小穗的颖片、小花的外稃和内稃以及花药的颜色有明显差异, 常见的有黄色、褐色和紫色(图2D)。值得注意的是, 株高最矮的芦苇C2拥有最宽且短的花序, 因其小穗的颖片、小花的外稃和内稃以及花药都呈紫色, 使得整个花序都表现紫色; 而株高最高的四倍体C1花序较小, 颖片、外稃和内稃仅在基部呈现紫色,因而整个花序的紫色要逊于C2。综合上述形态指标、小穗和花的颜色变化可知, 小清河样地芦苇的形态多态性很高。
形态多态性在一定程度上可反映遗传多态性。小清河6个芦苇克隆的单倍体基因组大小(Cx值)为(0.499±0.019) pg, 变异系数为3.8%。表2显示每个克隆的基因组大小, 其中5个八倍体芦苇的平均基因组大小为(4.02±0.15) pg, 变化范围为3.87-4.22 pg。然而, 根据Saltonstall (2016)建立的叶绿体DNA片段单倍型系统, 对这些形态和基因组大小有明显差异的芦苇群体进行溯源, 发现这6个芦苇克隆均为P型。其中, 利用特异性引物所获得的1个叶绿体非编码区域trnT- trnL为906 bp, 与T1 (AY016324)完全匹配, 而另1个叶绿体非编码区域rbcL-psaI为1 115 bp, 与R5 (AF457382)完全匹配。这说明小清河芦苇群体属于东亚/澳洲芦苇系(Eller et al., 2017)。
2.3 芦苇新变型的发现和鉴定
野外考察时我们发现北京地区存在一种变叶芦苇, 叶片上具有明显纵长的浅黄色或白色条纹(图3)。与正常的绿叶芦苇相比, 变叶芦苇的地上部分株高降低约22%, 花期延迟1-2周, 小穗的颖片、外稃以及花药均为紫色, 结实率和萌发率极低(表4; 图3)。但这种变叶芦苇既可通过种子繁殖, 也可通过茎节进行无性繁殖, 所产生的新个体也保持着变叶特性。在《中国植物志》以及其它文献中均未发现这种变叶芦苇的记载。因此, 我们将该变叶芦苇认定为一个新的芦苇变型。根据国际上通用的命名规则, 我们将这个变型命名为金条芦苇(P. australis var. australis f. Gold-stripe)。用流式细胞仪检测发现, 金条芦苇基因组大小为(3.98±0.17) pg, 是八倍体植物(图3C)。目前, 金条芦苇在北京地区还比较少见, 仅零星分布于汉石桥湿地公园、南海子公园和翠湖湿地等。现今, 金条芦苇的模式标本(图3E)保存于首都师范大学植物标本馆(BJTC004950)。具体描述如下:The most significant difference between goldstripe reeds and common reeds is that the leaves have white or light yellow stripes, and a lower growth rate and a late flowering time. Goldstripe reeds are 2-3 meters tall, stem diameter 0.4-1.0 cm, leaves lanceolate linear, 15-28 cm long, 4-10 cm wide, flowering period delayed 1-2 weeks compared to common reeds, panicle purple or brown, and the number of seeds has decreased. Goldstripe reeds have well-developed rhizomes, erect stems, and dense spikelets on panicles. Spikelets are about 8-10 mm long and contain 3-6 florets; glumes on the spikelets, lemma and palea of the floret are purple or brown. The glume and lemma has 3 veins. The apex of lemma is long and acuminate, the base plate is extended, and the dense filamentous pubescence on both sides are slightly longer than the lemma, and it is easy to fall off from the joint when mature; pistil 1, stamen 3, anther 1-1.5 mm, purple or yellow. Mature seeds are brown with filiform pubescence at the base. The presently found goldstripe reeds are all octoploid, with a genome size of (3.98±0.17) pg.
图3
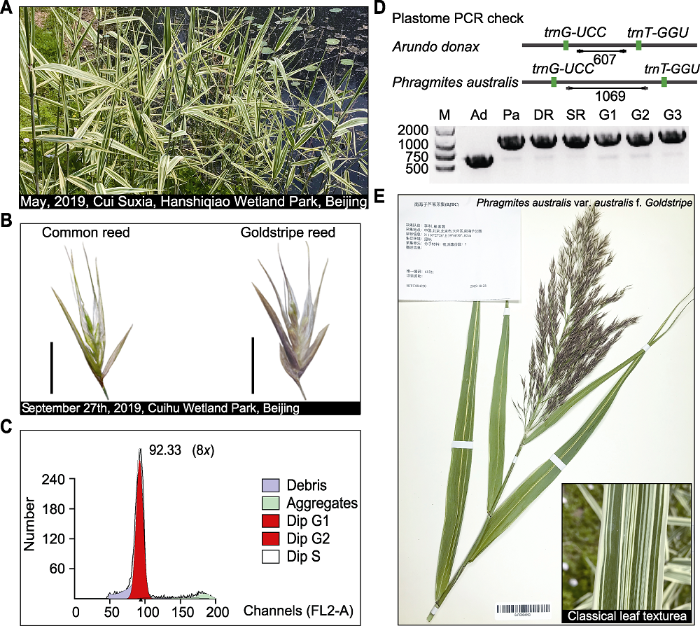 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3芦苇新变型的鉴定
(A) 自然生境中的金条芦苇; (B) 金条芦苇的小穗(bars=5 mm); (C) 金条芦苇细胞核的流式细胞分析结果; (D) 金条芦苇和变叶芦竹叶绿体基因片段(从trnG-UCC到trnT-GGU)大小的差异, 变叶芦竹(Ad)产生607 bp的条带; 芦苇产生1069 bp的条带(Pa为小清河芦苇克隆C6, DR和SR是河西走廊生长的沙丘芦苇和沼泽芦苇, G1-G3均为金条芦苇)(M: 分子标记); (E) 金条芦苇模式标本
Figure 3Identification of a new form of Phragmites australis
(A) Phragmites australis f. Goldstripe in natural habitats; (B) Spikelets of goldstripe reed (bars=5 mm); (C) Identification of ploidy level by the flow cytometric analysis on the goldstripe reed nuclei; (D) The size difference of a DNA fragment (from trnG-UCC to trnT-GGU) as revealed by PCR between goldstripe reed and Arundo donax var. versicolor, A. donax (Ad) produced a 607 bp band; common reeds and goldstripe reed produced 1069 bp bands (Pa indicates Xiaoqinghe reed C6, DR and SR indicate desert dune reeds and swamp reeds growing in the Hexi Corridor, and G1-G3 are all goldstripe reeds)(M: Molecular marker); (E) A type specimen of goldstripe reed
Table 4
表4
表4北京翠湖湿地普通芦苇和金条芦苇表型差异分析(平均值±标准差, n≥16)
Table 4
| Sample | Height (m) | Diameter (mm) | Seed setting rate (%) | Flowering time |
|---|---|---|---|---|
| Common reed | 3.6±0.2 | 8.70±0.80 | 16.8±7.1 | Mid-August |
| Goldstripe reed | 2.8±0.2 | 7.42±0.75 | 0.6±0.2 | Early September |
新窗口打开|下载CSV
2.4 讨论
调查结果显示, 芦苇在北京地区的种群面积非常大, 超过600 hm2, 形态和遗传多态性丰富。北京地区芦苇主要分布于永定河水系和潮白河水系, 约占总面积的78%; 芦苇分布面积最大的地点是野鸭湖湿地(39.9%)和汉石桥湿地(13.0%); 而在潮河、潮白河、永定河和小清河的部分河段中, 芦苇生长繁茂(表1)。通过流式细胞仪检测, 我们发现北京芦苇以八倍体为主, 约占62.5%, 其余为四倍体, 而且2种倍性的芦苇往往混生在一起, 无法简单地从形态上加以区分。但是在大的芦苇分布区, 如野鸭湖湿地和汉石桥湿地, 均为单一倍性芦苇(8x), 形态差异仅限于株高和茎秆粗度, 是北京湿地的优势种群。事实上, 这种单一优势群落称霸的现象正是芦苇在湿地系统中具有强大竞争力的体现(Haslam, 1971; Burdick and Konisky, 2003)。在这种情况下, 其它种类的植物很难与其竞争而逐渐退化。与单一优势种群形成鲜明对比的是芦苇混合种群, 在市内的浅河(潮河和小清河等)河段中生长的芦苇具有极高的形态和遗传多态性(表2, 表3)。利用叶绿体DNA片段进行单倍型溯源时, 发现北京地区的芦苇以P型为主, 属于东亚/澳洲芦苇系(Eller et al., 2017)。至今, 这些形态和遗传多态性很高的芦苇混合种群的形成机制尚不清楚, 推测可能与环境干扰有关。以小清河为例, 我们详细观察并检测芦苇混合种群的情况。在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(图2D)。它们的单倍体基因组大小(Cx值)为(0.499±0.019) pg, 变异系数达3.8%。这与来源于欧洲和北美洲的166个芦苇群体的基因组变异系数相当(Meyerson et al., 2016)。其中, C1克隆是北京地区罕见的四倍体克隆群体, 基因组大小为(2.01±0.09) pg, 是基因组测序的可选材料。据报道, 四倍体芦苇是欧洲和美洲的优势种群, 但在亚洲却以八倍体芦苇为主(Clevering and Lissner, 1999)。由于这6个芦苇群体的生长环境相似, 使得它们在外部形态和内在遗传上都具有可比性。我们发现, 四倍体芦苇C1在株高和基径上显著高于其它5个八倍体芦苇(C2-C6)。上述结果与Meyerson等(2016)对主要源于欧洲和北美洲四倍体芦苇的统计结果一致, 但与Raicu等(1972)报道八倍体芦苇具有较高的株型和粗壮根状茎的结果不符。根据我们的观察结合一些未发表数据, 我们认为芦苇染色体倍性与表型之间不具有较好的对应关系。然而, 值得注意的是, 芦苇花序的多态性非常高。这些花序除了大小和疏密不同之外, 小穗的颖片、小花的外稃和内稃以及花药的颜色都有明显差异, 主要有黄色、褐色或紫色(图2, 图3)。预测这些多态性的花序可以作为芦苇种内分类的基础。遗憾的是, 时至今日芦苇的种内分类依然不清晰。无论是采用叶绿体单倍型分类方法(Saltonstall, 2002)还是微卫星分子标记(Saltonstall, 2003)都很难将表型和遗传信息相统一。我们根据叶绿体DNA片段进行单倍型分类, 结果表明小清河中6个形态差异巨大、染色体倍性不同且基因组大小有变异的芦苇群体都是P型(表2, 表3)。这说明受到普遍认可的单倍型分类法(Saltonstall, 2002; An et al., 2012; Tanaka et al., 2017)只适用于大尺度的种内系统地理学研究。探明芦苇种内多样的表型与遗传特性之间的关系尚需开发新的研究方法。
芦苇是一类具有形态和遗传多态性的禾本科植物, 具有巨大的生物量和全球分布性, 处于生态上的有利位置。由于基因组中丰富的遗传多样性, 其在水生生境、陆地生境、盐碱生境、沙漠边缘和污染水域都可以形成大型单一性种群, 是湿地和城市水域不可替代的景观植物, 更有可能成为监测环境气候变化的有力工具(Eller et al., 2017)。但遗憾的是, 芦苇至今尚无基因组参考序列信息, 这极大地限制了其强大环境适应能力的遗传基础研究(Cui et al., 2009; Li et al., 2017), 也限制了其形态可塑性与遗传信息之间的关联研究。因此, 本研究获得的一些天然四倍体和八倍体材料十分珍贵, 是芦苇基因组测序以及多倍体植物环境适应性研究的好材料。自古以来北京地区芦苇生长繁茂, 随着近些年湿地建设力度加大, 芦苇的恢复和种植面积也越来越大, 但目前尚未见详细的调查报告。随着基因组序列的破解, 各类研究也会逐步展开。这些研究包括芦苇形态多样性和环境可塑性的分子基础、遗传因子与生态因子之间的关系、芦苇对环境修复的能力、芦苇纤维质量的改善和芦苇景观能力的提升等。我们希望北京地区丰富的芦苇资源和多态性研究可以成为解决这一系列问题的开始。
致谢
感谢汉石桥湿地的闫飞先生、野鸭湖湿地的刘均平先生以及翠湖国家湿地公园工作人员在材料采集方面的支持和帮助, 特别感谢吴西堃女士的支持。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
DOI:10.1002/pmic.200800654URLPMID:19701903 [本文引用: 2]

Phragmites communis Trin. (common reed) is a recognized model plant for studying its adaptation to contrasting and harsh environments. To understand the inherent molecular basis for its remarkable resistance to combined stresses, we performed a comprehensive proteomic analysis of the leaf proteins from two ecotypes, i.e. swamp and desert dune, naturally growing in the desert region of northwestern China. First, a proteome reference map of Phragmites was established based on the swamp ecotype. Proteins were resolved by 2-D/SDS-PAGE and identified by MALDI-TOF/TOF MS. In total, 177 spots were identified corresponding to 51 proteins. The major proteins identified are proteins involved in photosynthesis, glutathione and ascorbic acid metabolism as well as protein synthesis and quality control. Second, the 2-DE profiles of the two ecotypes were compared quantitatively via DIGE analysis. Compared with swamp ecotype, 51 proteins spots are higher-expressed and 58 protein spots are lower-expressed by twofold or more in desert dune ecotype. Major differences were found for the proteins involved in light reaction of photosynthesis, protein biosynthesis and quality control and antioxidative reactions. The physiological significance of such differences is discussed in the context of a flow of complex events in relation to plant adaptation to combined environmental stresses.
[本文引用: 2]
URLPMID:17853881 [本文引用: 1]
[本文引用: 1]
URLPMID:29250081 [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3389/fpls.2017.00511URLPMID:28450873 [本文引用: 1]

After a long-term adaptation to desert environment, the perennial aquatic plant Phragmites communis has evolved a desert-dune ecotype. The desert-dune ecotype (DR) of Phragmites communis showed significant differences in water activity and protein distribution compared to its sympatric swamp ecotype (SR). Many proteins that were located in the soluble fraction of SR translocated to the insoluble fraction of DR, suggesting that membrane-associated proteins were greatly reinforced in DR. The unknown phenomenon in plant stress physiology was defined as a proteome translocation response. Quantitative 2D-DIGE technology highlighted these 'bound' proteins in DR. Fifty-eight kinds of proteins were identified as candidates of the translocated proteome in Phragmites. The majority were chloroplast proteins. Unexpectedly, Rubisco was the most abundant protein sequestered by DR. Rubisco activase, various chaperons and 2-cysteine peroxiredoxin were major components in the translocation response. Conformational change was assumed to be the main reason for the Rubisco translocation due to no primary sequence difference between DR and SR. The addition of reductant in extraction process partially reversed the translocation response, implying that intracellular redox status plays a role in the translocation response of the proteome. The finding emphasizes the realistic significance of the membrane-association of biomolecule for plant long-term adaptation to complex stress conditions.
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.032477999URLPMID:11854535 [本文引用: 4]

Cryptic invasions are a largely unrecognized type of biological invasion that lead to underestimation of the total numbers and impacts of invaders because of the difficulty in detecting them. The distribution and abundance of Phragmites australis in North America has increased dramatically over the past 150 years. This research tests the hypothesis that a non-native strain of Phragmites is responsible for the observed spread. Two noncoding chloroplast DNA regions were sequenced for samples collected worldwide, throughout the range of Phragmites. Modern North American populations were compared with historical ones from herbarium collections. Results indicate that an introduction has occurred, and the introduced type has displaced native types as well as expanded to regions previously not known to have Phragmites. Native types apparently have disappeared from New England and, while still present, may be threatened in other parts of North America.
URLPMID:12803624 [本文引用: 1]
[本文引用: 2]
URLPMID:1932684 [本文引用: 1]
[本文引用: 2]
DOI:10.1038/s41586-018-0063-9URLPMID:29695866 [本文引用: 1]

Here we analyse genetic variation, population structure and diversity among 3,010 diverse Asian cultivated rice (Oryza sativa L.) genomes from the 3,000 Rice Genomes Project. Our results are consistent with the five major groups previously recognized, but also suggest several unreported subpopulations that correlate with geographic location. We identified 29 million single nucleotide polymorphisms, 2.4 million small indels and over 90,000 structural variations that contribute to within- and between-population variation. Using pan-genome analyses, we identified more than 10,000 novel full-length protein-coding genes and a high number of presence-absence variations. The complex patterns of introgression observed in domestication genes are consistent with multiple independent rice domestication events. The public availability of data from the 3,000 Rice Genomes Project provides a resource for rice genomics research and breeding.
论中国古代芦苇资源的自然分布、社会利用和文化反映
1
2013
... 芦苇的生态和经济价值很高.在经济领域, 芦苇是传统的造纸原料, 亦可用于建筑材料、饲料和燃料, 此外还是不可缺少的景观植物.然而, 芦苇更重要的作用是在生态领域, 它是湿地生态系统中的优势种群, 可以涵养水源、维护生态系统平衡(
河西走廊芦苇草地资源特征及其保护利用
1
2012
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
白洋淀芦苇资源及其生态功能与利用
1
2004
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
谈芦苇湿地的价值
1
2008
... 芦苇的生态和经济价值很高.在经济领域, 芦苇是传统的造纸原料, 亦可用于建筑材料、饲料和燃料, 此外还是不可缺少的景观植物.然而, 芦苇更重要的作用是在生态领域, 它是湿地生态系统中的优势种群, 可以涵养水源、维护生态系统平衡(
河西走廊不同生态类型芦苇的气体交换特点的研究
1
1991
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Application of raw and activated Phragmites australis as potential adsorbents for wastewater treatments
1
2017
... 芦苇的生态和经济价值很高.在经济领域, 芦苇是传统的造纸原料, 亦可用于建筑材料、饲料和燃料, 此外还是不可缺少的景观植物.然而, 芦苇更重要的作用是在生态领域, 它是湿地生态系统中的优势种群, 可以涵养水源、维护生态系统平衡(
Phylogeographic analyses of Phragmites australis in China: native distribution and habitat preference of the haplotype that invaded North America
2
2012
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
Determinants of expansion for Phragmites australis, common reed, in natural and impacted coastal marshes
1
2003
... 调查结果显示, 芦苇在北京地区的种群面积非常大, 超过600 hm2, 形态和遗传多态性丰富.北京地区芦苇主要分布于永定河水系和潮白河水系, 约占总面积的78%; 芦苇分布面积最大的地点是野鸭湖湿地(39.9%)和汉石桥湿地(13.0%); 而在潮河、潮白河、永定河和小清河的部分河段中, 芦苇生长繁茂(
Studies in the Gramineae: XIV
1
1967
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis
4
1999
... 芦苇(Phragmites australis)是一种典型的湿地水生植物, 但能生长在盐渍干旱的恶劣环境以及富营养化的污染水域, 并成为其生境内的优势物种(
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
... ), 其中八倍体芦苇占绝对优势(
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
Proteomic characterization of Phragmites communis in ecotypes of swamp and desert dune
2
2009
... 芦苇(Phragmites australis)是一种典型的湿地水生植物, 但能生长在盐渍干旱的恶劣环境以及富营养化的污染水域, 并成为其生境内的优势物种(
... 芦苇是一类具有形态和遗传多态性的禾本科植物, 具有巨大的生物量和全球分布性, 处于生态上的有利位置.由于基因组中丰富的遗传多样性, 其在水生生境、陆地生境、盐碱生境、沙漠边缘和污染水域都可以形成大型单一性种群, 是湿地和城市水域不可替代的景观植物, 更有可能成为监测环境气候变化的有力工具(
Plant regeneration from callus cultures in two ecotypes of reed ( Phragmites communis Trinius)
2
2002
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
... 采用流式细胞仪分析芦苇基因组大小及染色体倍性 (
Estimation of nuclear DNA content in plants using flow cytometry
1
2007
... 采用流式细胞仪分析芦苇基因组大小及染色体倍性 (
A diploid form of Phragmites communis, as a possible result of cytogenetical response to ecological stress
1
1979
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Cosmopolitan species as models for ecophysiological responses to global change: the common reed Phragmites australis
4
2017
... 芦苇(Phragmites australis)是一种典型的湿地水生植物, 但能生长在盐渍干旱的恶劣环境以及富营养化的污染水域, 并成为其生境内的优势物种(
... 形态多态性在一定程度上可反映遗传多态性.小清河6个芦苇克隆的单倍体基因组大小(Cx值)为(0.499±0.019) pg, 变异系数为3.8%.
... 调查结果显示, 芦苇在北京地区的种群面积非常大, 超过600 hm2, 形态和遗传多态性丰富.北京地区芦苇主要分布于永定河水系和潮白河水系, 约占总面积的78%; 芦苇分布面积最大的地点是野鸭湖湿地(39.9%)和汉石桥湿地(13.0%); 而在潮河、潮白河、永定河和小清河的部分河段中, 芦苇生长繁茂(
... 芦苇是一类具有形态和遗传多态性的禾本科植物, 具有巨大的生物量和全球分布性, 处于生态上的有利位置.由于基因组中丰富的遗传多样性, 其在水生生境、陆地生境、盐碱生境、沙漠边缘和污染水域都可以形成大型单一性种群, 是湿地和城市水域不可替代的景观植物, 更有可能成为监测环境气候变化的有力工具(
Degrés et niveaux de la variation du nombre chromosomique chez Phragmites australis (Cav.) Trin. ex Steud. Ver?ff. Geobot. Inst. ETH, Stiftung Rübel
1
1986
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Variation of population type in Phragmites communis Trin
1
1970
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Community regulation in Phragmites communis Trin. I. monodominant stands
1
1971
... 调查结果显示, 芦苇在北京地区的种群面积非常大, 超过600 hm2, 形态和遗传多态性丰富.北京地区芦苇主要分布于永定河水系和潮白河水系, 约占总面积的78%; 芦苇分布面积最大的地点是野鸭湖湿地(39.9%)和汉石桥湿地(13.0%); 而在潮河、潮白河、永定河和小清河的部分河段中, 芦苇生长繁茂(
A proteome translocation response to complex desert stress environments in perennial Phragmites sympatric ecotypes with contrasting water availability
1
2017
... 芦苇是一类具有形态和遗传多态性的禾本科植物, 具有巨大的生物量和全球分布性, 处于生态上的有利位置.由于基因组中丰富的遗传多样性, 其在水生生境、陆地生境、盐碱生境、沙漠边缘和污染水域都可以形成大型单一性种群, 是湿地和城市水域不可替代的景观植物, 更有可能成为监测环境气候变化的有力工具(
Do ploidy level and nuclear genome size and latitude of origin modify the expression of Phragmites australis traits and interactions with herbivores?
2
2016
... 采用流式细胞仪分析芦苇基因组大小及染色体倍性 (
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
Phragmites australis in eastern North America: a historical and ecological perspective
1
2009
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components
1
1997
... 小清河样地位于北京西南的小清河中段(116.21°E, 39.70°N).取样时检测水深、水温、pH值以及溶解氧水平(HI9142溶解氧测量仪, HANNA, 意大利).在芦苇开花后期, 选择6个不同形态的芦苇克隆群体(C1-C6)进行形态指标的检测, 并进行主成分分析(PCA).同时采集新鲜叶片于-80°C冻存, 采用CTAB法(
The Phragmites communis Trin. chromosome complement in the Danube delta
1
1972
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
A set of primers for amplification of noncoding regions of chloroplast DNA in the grasses
1
2001
... 小清河样地位于北京西南的小清河中段(116.21°E, 39.70°N).取样时检测水深、水温、pH值以及溶解氧水平(HI9142溶解氧测量仪, HANNA, 意大利).在芦苇开花后期, 选择6个不同形态的芦苇克隆群体(C1-C6)进行形态指标的检测, 并进行主成分分析(PCA).同时采集新鲜叶片于-80°C冻存, 采用CTAB法(
Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America
4
2002
... 芦苇(Phragmites australis)是一种典型的湿地水生植物, 但能生长在盐渍干旱的恶劣环境以及富营养化的污染水域, 并成为其生境内的优势物种(
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
... ).这说明受到普遍认可的单倍型分类法(
Microsatellite variation within and among North American lineages of Phragmites australis
1
2003
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
The naming of Phragmites haplotypes
2
2016
... 小清河样地位于北京西南的小清河中段(116.21°E, 39.70°N).取样时检测水深、水温、pH值以及溶解氧水平(HI9142溶解氧测量仪, HANNA, 意大利).在芦苇开花后期, 选择6个不同形态的芦苇克隆群体(C1-C6)进行形态指标的检测, 并进行主成分分析(PCA).同时采集新鲜叶片于-80°C冻存, 采用CTAB法(
... 形态多态性在一定程度上可反映遗传多态性.小清河6个芦苇克隆的单倍体基因组大小(Cx值)为(0.499±0.019) pg, 变异系数为3.8%.
Universal primers for amplification of three non-coding regions of chloroplast DNA
1
1991
... 小清河样地位于北京西南的小清河中段(116.21°E, 39.70°N).取样时检测水深、水温、pH值以及溶解氧水平(HI9142溶解氧测量仪, HANNA, 意大利).在芦苇开花后期, 选择6个不同形态的芦苇克隆群体(C1-C6)进行形态指标的检测, 并进行主成分分析(PCA).同时采集新鲜叶片于-80°C冻存, 采用CTAB法(
Phylogenetic analyses of Phragmites spp. in southwest China identified two lineages and their hybrids
2
2017
... 芦苇是具有高倍性染色体组的物种, 从已消失的二倍体(
... 以小清河为例, 我们详细观察并检测芦苇混合种群的情况.在面积约0.15 hm2的样地上生长着至少6种形态差异明显的芦苇克隆(
Genomic variation in 3,010 diverse accessions of Asian cultivated rice
1
2018
... 采用流式细胞仪分析芦苇基因组大小及染色体倍性 (
