 ,, 郑林, 杜美霞, 龙俊宏, 何永睿, 陈善春
,, 郑林, 杜美霞, 龙俊宏, 何永睿, 陈善春 ,, 邹修平
,, 邹修平 ,西南大学/中国农业科学院柑桔研究所/国家柑桔工程技术研究中心/国家柑桔品种改良中心,重庆 400712
,西南大学/中国农业科学院柑桔研究所/国家柑桔工程技术研究中心/国家柑桔品种改良中心,重庆 400712Response Characteristics of Plant SAR and Its Signaling Gene CsSABP2 to Huanglongbing Infection in Citrus
ZHAO Ke ,, ZHENG Lin, DU MeiXia, LONG JunHong, HE YongRui, CHEN ShanChun
,, ZHENG Lin, DU MeiXia, LONG JunHong, HE YongRui, CHEN ShanChun ,, ZOU XiuPing
,, ZOU XiuPing ,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences/National Citrus Engineering Research Center/National Center for Citrus Varieties Improvement, Chongqing 400712
,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences/National Citrus Engineering Research Center/National Center for Citrus Varieties Improvement, Chongqing 400712通讯作者:
责任编辑: 岳梅
收稿日期:2020-06-19接受日期:2020-07-24网络出版日期:2021-04-16
| 基金资助: |
Received:2020-06-19Accepted:2020-07-24Online:2021-04-16
作者简介 About authors
赵珂,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2007KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵珂, 郑林, 杜美霞, 龙俊宏, 何永睿, 陈善春, 邹修平. 柑橘SAR及其信号转导基因CsSABP2在黄龙病菌侵染中的响应特征[J]. 中国农业科学, 2021, 54(8): 1638-1652 doi:10.3864/j.issn.0578-1752.2021.08.006
ZHAO Ke, ZHENG Lin, DU MeiXia, LONG JunHong, HE YongRui, CHEN ShanChun, ZOU XiuPing.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】黄龙病(Huanglongbing,HLB)是世界柑橘生产上最具毁灭性的病害[1],目前已危害50多个国家和地区的柑橘产业[2]。HLB在我国大部分柑橘产区发生,严重制约了我国柑橘产业的生存与健康发展[3]。在我国分布的黄龙病菌为韧皮部杆菌属亚洲种(‘Candidatus Liberibacter asiaticus’,CLas)。迄今,柑橘HLB病原尚无法人工分离培养,这严重阻碍了病原菌与寄主互作机制的研究,致使柑橘HLB防控技术和抗病育种研究进展十分缓慢。前期研究表明,植物系统获得性抗性(systemic acquired resistance,SAR)韧皮部长距离传输信号水杨酸甲酯(methyl salicylate,MeSA)在介导柑橘HLB耐性中起重要作用[4],因此,以易感HLB锦橙、耐HLB酸柚、马蜂柑叶片为材料,探究柑橘SAR及其信号转导的关键酶基因CsSABP2在CLas侵染中的响应特征,可为柑橘抗病育种提供新思路和新基因资源。【前人研究进展】SAR是一种植物抵御病原菌侵染的主动防御机制,具有广谱抗病性[4]。植物受到病原菌侵染时,水杨酸(salicylic acid,SA)的合成激活下游防御基因的表达,从而引发局部获得性抗性(local acquired resistance,LAR),再通过长距离信号传导,引发植株的SAR[5]。近年来,从病原和寄主两方面入手广泛研究了柑橘应答黄龙病菌侵染的分子基础[6,7,8,9,10,11,12],发现黄龙病菌通过抑制寄主SAR来促进柑橘感病;SAR介导的基础抗性与柑橘品种HLB耐性紧密相关。在甜橙中超量表达拟南芥SA信号途径关键基因AtNPR1,激活寄主SAR反应,显著增强了转基因植株对HLB的抗性[13]。SA在SAR反应中起着核心作用,当病原菌入侵时,侵染部位合成的SA通过SA信号传导途径激活非侵染部位SAR,其中MeSA是SA信号通过韧皮部长距离传递的关键信使,在激活SAR反应中起至关重要的作用[14]。研究表明,MeSA无任何生物学功能,必须在非侵染部位转换为SA才能有效激活SAR反应[15,16,17]。本实验室前期研究发现,柑橘SA信号途径可能通过MeSA信使正向调控寄主响应黄龙病菌侵染[18],但其关键基因和响应的确切机制有待解析。水杨酸甲酯酶SABP2是MeSA向SA转化的关键酶,是SA激活SAR所必需的[19]。SABP2为水解酶超家族的一员,其活性位点为第81位的丝氨酸(Ser-81)、第238位的组氨酸(His-238)、第210位的天冬氨酸(Asp-210),这3个核心保守氨基酸形成催化三联体,使得SABP2具有MeSA酯酶活性和SA的高亲和性;同时第13位的丙氨酸(Ala-13)若突变,对SABP2的SA高亲和力具有较大影响[20,21]。在杨树中超量表达SABP2能够显著提高植株对溃疡病的抗性[22];沉默SABP2后,烟草即丧失了对烟草花叶病毒(tobacco mosaic virus,TMV)的局部和系统性抗性[23]。SAR的发生伴随着系统性SA含量的增加和病程相关(pathogenesis-related,PR)蛋白基因的表达[24]。PR蛋白能同时在植物受侵染的和未发生侵染部位积累,进而诱导植物产生超敏反应(hypersensitive response,HR)并导致局部细胞死亡,限制病原物扩散,从而使植物产生SAR[25]。同时,研究表明活性氧(reactive oxygen species,ROS)在SAR建成中起着重要作用[26]。SA的积累会抑制过氧化氢酶(catalase,CAT)和抗坏血酸过氧化物酶(ascorbate peroxidase,APX)的活性,从而导致过氧化氢(hydrogen peroxide,H2O2)的积累,诱导防卫反应相关基因的表达。【本研究切入点】目前鲜有MeSA在柑橘与黄龙病菌互作中的功能报道,未见关键基因SABP2在柑橘中调控MeSA应答病原菌侵染的研究。【拟解决的关键问题】解析柑橘不同抗/耐品种SAR应答CLas的特征,在此基础上,筛选和克隆响应CLas侵染的SABP2关键基因,解析其响应的表达特征,为深入研究柑橘MeSA信号途径响应CLas侵染提供候选基因。1 材料与方法
试验于2019年3月至2020年1月在中国农业科学院柑桔研究所国家柑桔品种改良中心完成。1.1 材料
柑橘易感病品种锦橙(Citrus sinensis)及耐病品种酸柚(C. grandis)、马蜂柑(C. hystrix)均取自中国农业科学院柑桔研究所改良中心温室,CLas材料取自广西感病果园。大肠杆菌(Escherichia coli)感受态细胞DH5α、T克隆载体pGEM-T Easy、DNaseI(RNase-free)和反转录试剂盒购自TaKaRa公司;植物总RNA提取试剂盒和植物DNA快速提取试剂盒购自北京艾德莱生物科技有限公司;NovoStart? SYBR qPCR SuperMix Plus试剂盒购自NovoProtein公司;H2O2含量试剂盒购自上海优选生物公司;淀粉含量检测试剂盒购自北京金克隆公司;SA、MeSA均为实验室配制,配制浓度分别为10 μmol·L-1、0.1 mmol·L-1[27]。1.2 CLas侵染
嫁接传毒参照ZOU等[28]的方法进行。对成熟度一致的一年生健康锦橙、酸柚、马蜂柑嫁接病原材料,3个月后提取叶脉DNA。利用引物进行CLas常规PCR检测(表1)。PCR反应条件:95℃预变性5 min;95℃ 30 s,63℃ 30 s,72℃ 2 min,35个循环;72℃延伸10 min;4℃保存。将PCR产物进行琼脂糖凝胶电泳检测,PCR检测为阳性的植株用于下一步研究。Table 1
表1
表1普通PCR引物序列
Table 1
| 引物名称 Primer name | 用途 Amplification | 引物序列 Primer sequence (5′-3′) |
|---|---|---|
| OI1-F | CLas普通PCR检测 CLas common PCR detection | GCGCGTATGCAATACGAGCGGCA |
| OI2C-R | GCCTCGCGACTTCGCAACCCAT | |
| CsSABP2-1-F | 基因克隆 Gene cloning | TCCCCCGGGATGGAAGAAGTAGTAGGCAT |
| CsSABP2-1-R | CCGCTCGAGTTATGCATACTTAAGAGAAA | |
| CsSABP2-2-F | 基因克隆 Gene cloning | TCCCCCGGGATGGCAGAAGCCAAGAAACAGAA |
| CsSABP2-2-R | CCGCTCGAGTTAAGCATACTTATGAGCAA | |
| CsSABP2-3-F | 基因克隆 Gene cloning | TCCCCCGGGATGAAACCAACGGAGAAAAT |
| CsSABP2-3-R | CCGCTCGAGTTAAGCATACTTTTGAGCAA | |
| CsSABP2-4-F | 基因克隆 Gene cloning | TCCCCCGGGATGGGAGAAGAGATTAACAT |
| CsSABP2-4-R | CCGCTCGAGCTAGTTGCAGCCAACAGAAG |
新窗口打开|下载CSV
1.3 柑橘DNA和RNA的提取
用植物DNA快速提取试剂盒提取感病锦橙叶脉DNA,1%琼脂糖凝胶电泳检测,以获得纯度高、完整性好且无RNA污染的DNA,微量分光光度计检测各样品DNA浓度,并稀释至50 ng·μL-1,-20℃保存备用。用植物总RNA快速提取试剂盒提取锦橙叶片RNA,1%琼脂糖凝胶电泳检测,以获得纯度高、完整性好且未降解的RNA,微量分光光度计检测各样品RNA浓度。利用反转录试剂盒将RNA反转录为cDNA。
1.4 柑橘SABP2的筛选与克隆
以本实验前期构建的CLas感染锦橙和酸柚转录组数据为基础,筛选CsSABP2差异表达基因,并从华中农业大学甜橙基因组数据库CAP(http://citrus.hzau. edu.cn/cgi-bin/orange/search)[29]中调取差异基因的编码序列(CDS)和氨基酸序列,然后以烟草、拟南芥、马铃薯等SABP2氨基酸序列为参考,比对筛选CsSABP2。根据CDS序列设计引物(表1),以感染CLas锦橙叶脉cDNA为模板进行PCR扩增目的基因编码序列。反应条件:98℃预变性3 min,94℃ 15 s,58℃ 30 s,72℃ 1.5 min,35个循环,72℃延伸3 min,4℃保存;PCR产物连接pGEM-T easy载体并转化大肠杆菌DH5α,挑取阳性克隆进行测序。1.5 活性氧H2O2含量测定
采成熟度一致的一年生野生型锦橙叶片,经自来水冲洗干净后,用70%的无水乙醇灭菌3—5 s,再用无菌水冲洗,用灭菌滤纸吸干表面水分,将离体叶片浸泡在浓度为10 μmol·L-1的SA溶液、无菌水、0.1 mmol·L-1的MeSA溶液中,处理端与非处理端于0、6、12、24 h取样,每个时间点各取3片叶,分别加入1 mL丙酮,研磨成匀浆后备用。利用H2O2含量测定试剂盒进行测定。1.6 淀粉含量测定
采经检测已带有HLB毒源的锦橙叶片,经自来水冲洗干净后,用70%的无水乙醇灭菌3—5 s,再用无菌水冲洗,用灭菌滤纸吸干表面水分,离体叶片分别喷施无菌水、10 μmol·L-1的SA、0.1 mmol·L-1的MeSA,每周喷施一次。每周无菌水、SA、MeSA处理各取3片叶,利用淀粉含量检测试剂盒进行感病后叶片的淀粉含量测定。1.7 激素处理
采成熟度一致的一年生野生型锦橙叶片经自来水冲洗干净后,用70%的无水乙醇灭菌3—5 s,再用无菌水冲洗,用灭菌滤纸吸干表面水分,离体叶片的叶尖处分别浸泡在浓度为10 μmol·L-1的SA溶液、无菌水、0.1 mmol·L-1的MeSA溶液中,处理端与非处理端于0、6、12、24 h取样,每个时间点各取3片叶,一部分液氮速冻备用,一部分加入1 mL丙酮,研磨成匀浆后备用。采相同成熟度的一年生野生型锦橙叶片,经自来水、无菌水分别清洗后,用灭菌滤纸吸干表面水分。用打孔器将叶片打成直径为7 mm的叶圆片,分别浸泡在无菌水、浓度为10 μmol·L -1的SA、0.1 mmol·L-1的MeSA溶液中,于 0、12、24、36、48 h取样,每个时间点各取15片叶圆片,液氮速冻,提取RNA反转录成cDNA,-20℃冻存备用。
1.8 基因表达的qRT-PCR分析
利用ABI 7500荧光定量PCR仪进行qRT-PCR。利用NCBI中Primer Blast在线设计定量引物(表2)。并以甜橙Actin为内参基因,PCR反应体系12 μL:6 μL 2×SYBRGreen荧光染料、4.4 μL H2O、0.3 μL 10 mmol·L -1引物、1 μL cDNA;反应程序:95℃ 5 min,95℃ 15 s,60℃ 1 min,40个循环。外源MeSA诱导SAR相关基因表达、CLas侵染诱导的基因表达以及激素诱导的基因表达分析均为每个处理进行3次生物学重复和3次平行样重复。相对表达量采用2-ΔΔCt法计算(ΔCt=CtGene-CtActin)。Excel处理数据,SPSS 2.0进行差异显著性分析,P<0.05表示差异显著。Table 2
表2
表2qRT-PCR所用引物序列
Table 2
| 引物名称 Primer name | 用途 Amplification | 引物序列 Primer sequence (5′-3′) |
|---|---|---|
| CsPR1-F | 检测CsPR1的表达量 To detect the expression of CsPR1 | AAATGTGGGTGAATGAGAAAGC |
| CsPR1-R | ATTATTGTTGCACGTCACCTTG | |
| CsPR2-F | 检测CsPR2的表达量 To detect the expression of CsPR2 | TTCCACTGCCATCGAAACTG |
| CsPR2-R | GTAATCTTGTTTAAATGAGCCTCTTG | |
| CsPR5-F | 检测CsPR5的表达量 To detect the expression of CsPR5 | CACCATTGCCAATAACCCTAATG |
| CsPR5-R | GGGACAGTTACCGTTAAGATCAG | |
| CsSABP2-1-F | 检测CsSABP2-1的表达量 To detect the expression of CsSABP2-1 | ATCTCCGTGGCTGTTTTCGT |
| CsSABP2-1-R | CTGCCGTCCTCTTTTCCCAT | |
| CsSABP2-2-F | 检测CsSABP2-2的表达量 To detect the expression of CsSABP2-2 | GCCAGACACCAAACACCAAC |
| CsSABP2-2-R | ACATCTTTCCCAGCTCCACG | |
| CsSABP2-3-F | 检测CsSABP2-3的表达量 To detect the expression of CsSABP2-3 | AGCATTCATGCCAGACACCA |
| CsSABP2-3-R | GTGTCCAACCATTCCCCACT | |
| CsSABP2-4-F | 检测CsSABP2-4的表达量 To detect the expression of CsSABP2-4 | TGACTTATCGGGATTCGGCG |
| CsSABP2-4-R | TTGCGCTGCAATTCTTGCTT | |
| actin-F | 检测actin的表达量 To detect the expression of actin | CATCCCTCAGCACCTTCC |
| actin-R | CCAACCTTAGCACTTCTCC |
新窗口打开|下载CSV
2 结果
2.1 柑橘SAR响应CLas侵染特征分析
为了探讨不同耐病品种SAR响应CLas侵染的反应特征,分析了SAR Marker基因CsPR1、CsPR2和CsPR5在锦橙、酸柚、马蜂柑中受CLas侵染的表达特征。qRT-PCR结果显示,CsPR1在锦橙中受CLas侵染诱导显著上调表达;CsPR2、CsPR5在酸柚和马蜂柑中受CLas侵染诱导显著上调表达,3个基因均明显响应CLas侵染诱导上调表达。CsPR1在易感病锦橙中表达水平显著高于耐病品种酸柚和马蜂柑,且3个品种中CsPR1在叶脉中表达水平显著高于其叶肉。相反,CsPR2在耐病品种酸柚和马蜂柑中表达水平显著高于易感病锦橙,而且其表达水平在3个品种叶肉中均显著高于其叶脉。CsPR5在酸柚和马蜂柑中特别是叶肉组织中表达水平显著高于锦橙(图1)。结果显示耐病酸柚和马蜂柑中SAR响应CLas侵染明显强于易感病锦橙。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1PR基因响应CLas的表达分析
JC:锦橙Jincheng;SP:酸柚Sour pomelo;MFG:马蜂柑Kaffir lime。柱上不同大写字母和小写字母分别表示基因在叶脉和叶肉的表达量在不同品种之间差异显著(P<0.05)Different capital and lowercase letters on the bars indicate that gene expression levels in vein and mesophyll are significantly different among different varieties (P<0.05)。
Fig. 1Expression analysis of PR genes in response to CLas
2.2 外施MeSA对锦橙SAR反应的影响
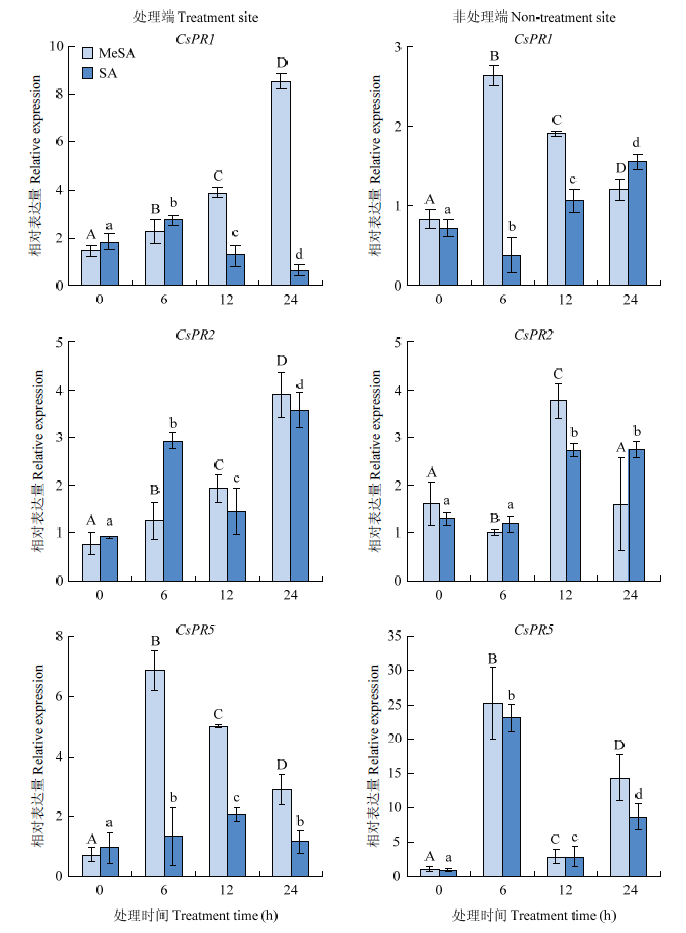
为研究外源MeSA对柑橘SAR的影响,以水和SA为对照,分析了外源MeSA诱导锦橙SAR相关基因表达和活性氧H2O2积累情况。与SA处理相比,在处理端,MeSA处理均上调CsPR1、CsPR2和CsPR5的表达;在非处理端,CsPR2受MeSA处理表达水平变化不明显,但CsPR1上调表达,特别是CsPR5表达水平显著上调。该结果表明外源MeSA能激活系统部位SAR反应(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2外施MeSA和SA诱导SAR相关基因CsPR1、CsPR2和CsPR5表达的qRT-PCR分析
处理端和非处理端分别指同一叶片中激素处理部位和非处理部位,
Fig. 2qRT-PCR analysis of SAR-related genes CsPR1, CsPR2 and CsPR5 induced by MeSA and SA
The treatment site and non-treatment site refer to hormone treated and non-treated sites in the same isolated leaf, the same as
MeSA、SA和水处理均诱导H2O2增加。在处理端,SA处理6 h时H2O2 含量显著低于水对照,但其他时间点MeSA、SA诱导的H2O2积累与水对照无明显差异。在非处理端,MeSA处理6、12、24 h时,H2O2 含量均显著高于水对照,SA仅在6 h时诱导H2O2积累显著高于水对照,其余时间点无明显差异(图3)。结果显示,外源MeSA诱导非处理端H2O2积累强于SA,外施MeSA能显著促进柑橘SAR反应。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3外施MeSA和SA诱导H2O2积累的检测
“*”表示与水对照的差异显著性(P<0.05)
Fig. 3Detection of H2O2 accumulation induced by MeSA and SA
“*” indicates significant difference with water control (P<0.05)
2.3 外施MeSA、SA对感病叶片中淀粉积累的影响
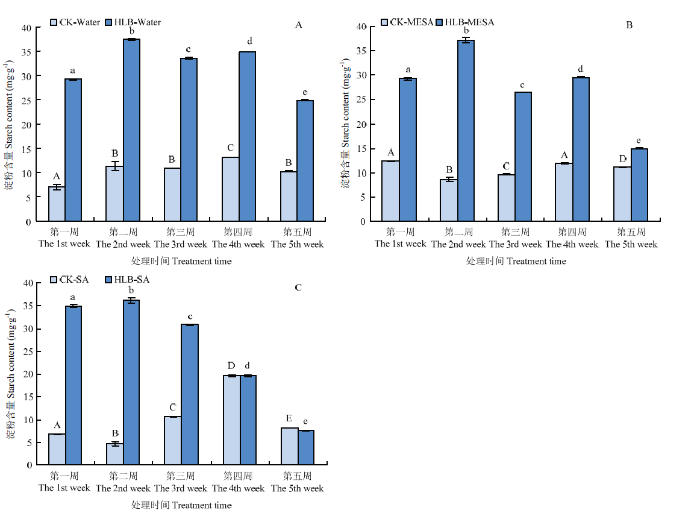
柑橘感染CLas后淀粉含量是其症状程度的一个重要生理指标。因此,进一步分析了外施MeSA对感病锦橙叶片中淀粉含量的影响。如图4所示,5周后,与水处理相比,MeSA、SA处理均使锦橙感病叶片中淀粉含量显著下降,表明外施MeSA抑制了感病叶片中淀粉的积累。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4外施MeSA和SA对感病叶片淀粉积累的影响
A:水处理Water treatment;B:MeSA处理MeSA treatment;C:SA处理SA treatment。CK均表示健康锦橙叶片CK refers to healthy Jincheng leaves。柱上不同大写字母和小写字母分别表示淀粉含量在同一处理不同周之间差异显著(P<0.05)Different capital and lowercase letters on the bars indicate that starch content is significantly different of different weeks in the same treatment (P<0.05)
Fig. 4Effect of MeSA and SA on starch accumulation of infected leaves
2.4 CsSABP2的克隆与生物信息学分析
为了深入了解MeSA应答柑橘HLB侵染机制,比较分析了易感病锦橙和耐病酸柚感染CLas转录组数据(未发表),筛选到6个功能注释为CsSABP2的差异表达基因(表3)。CsSABP2-1和CsSABP2-4在两个品种中均上调表达;CsSABP2-2在酸柚中上调表达,而在锦橙中下调表达;CsSABP2-3在锦橙中上调表达,而在酸柚中下调表达;CsSABP2-5和CsSABP2-6仅在锦橙中差异上调表达,酸柚中无变化。Table 3
表3
表3CsSABP2家族在锦橙和酸柚中应答CLas侵染的转录组比较分析
Table 3
| 基因ID Gene ID | 基因名称 Gene name | SP CLas vs SP mock | JC CLas vs JC mock | 关键氨基酸分析 Analysis of key amino acids | ||
|---|---|---|---|---|---|---|
| log2 fold change | 校正值padj | log2 fold change | 校正值padj | |||
| Cs1g23200 | CsSABP2-1 | 3.44 | 1.88E-14 | 2.67 | 3.87E-16 | 有MeSA脂酶活性 It has MeSA lipase activity |
| Cs7g24820 | CsSABP2-2 | 1.09 | 8.70E-05 | -2.44 | 4.33E-35 | 有MeSA脂酶活性 It has MeSA lipase activity |
| Cs7g24830 | CsSABP2-3 | -2.05 | 3.75E-18 | 3.44 | 1.07E-12 | 有MeSA脂酶活性 It has MeSA lipase activity |
| Cs7g29470 | CsSABP2-4 | 1.13 | 1.47E-03 | 2.17 | 5.33E-32 | 有MeSA脂酶活性 It has MeSA lipase activity |
| Cs2g16450 | CsSABP2-5 | — | — | 1.29 | 5.14E-04 | Ser突变,可能无MeSA脂酶活性 Serine is mutated, may not have MeSA lipase activity |
| Cs7g24810 | CsSABP2-6 | — | — | 3.34 | 8.30E-04 | 缺失突变,可能无MeSA脂酶活性 Deletion mutation, may not have MeSA lipase activity |
新窗口打开|下载CSV
与烟草SABP2(NtSABP2)的氨基酸序列比对发现,CsSABP2-1、CsSABP2-2、CsSABP2-3、CsSABP2-4蛋白均含有保守的酶活性催化三联体核心氨基酸残基Ser-81、His-238和Asp-210,而CsSABP2-6第81位Ser缺失突变。催化三联体的第三个成员Asp-210位于二级结构连接链β5和螺旋αE中[20],CsSABP2-5的二级结构连接链β5中有3个氨基酸缺失突变,预示CsSABP2-1、CsSABP2-2、CsSABP2-3、CsSABP2-4具有SABP2蛋白酶活性的功能(图5)。CsSABP2-3、CsSABP2-4第13位氨基酸分别突变为丝氨酸、异亮氨酸。
克隆测序分析显示,锦橙CsSABP2-1、CsSABP2- 2、CsSABP2-3、CsSABP2-4的CDS序列长分别为804、792、819、828 bp,编码268、264、273、276个氨基酸。需指出的是,CsSABP2-1含有12个结合SA的氨基酸残基,CsSABP2-2含有10个,CsSABP2-3含有9个,CsSABP2-4含有2个(图5),暗示4个基因可能具有不同的SA结合能力。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5CsSABP2氨基酸序列比对
红色代表相同氨基酸,黑色代表不同氨基酸,黄色代表催化三联体,绿色代表SA关键结合位点,蓝色菱形表示结合SA的残基,绿色和蓝色箭头标识二级结构元素
Fig. 5Amino acid sequence comparison of CsSABP2
Red represents the same amino acid, black represents different amino acids, yellow represents catalytic triplets, green represents SA key binding sites, blue diamond indicates SA-binding residues, green and blue arrows identify the secondary structural elements
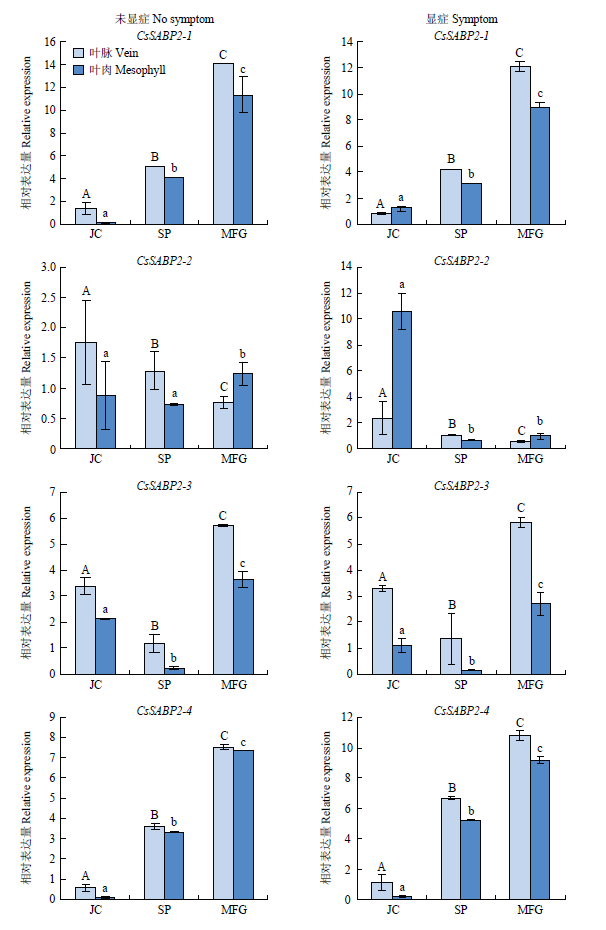
2.5 CsSABP2在不同耐病品种中响应CLas侵染的表达分析
以各品种感病前叶片为对照,分析了不同品种感病后未显症与显症叶片不同组织(叶脉、叶肉)中的基因表达量。无论显症和未显症叶片,耐病品种酸柚和马蜂柑中CsSABP2-1和 CsSABP2-4表达量均显著高于易感病品种锦橙。未显症叶片中,CsSABP2-1在3个品种叶脉中的表达量均高于叶肉;显症叶片中,CsSABP2-1在酸柚和马蜂柑的叶脉中表达水平高于叶肉,锦橙中无明显差异。对于CsSABP2-4,3个品种叶脉中基因表达水平均高于叶肉。CsSABP2-2在锦橙中表达水平高于酸柚和马蜂柑,其中,锦橙显症叶片中,CsSABP2-2在叶肉中表达量为酸柚的15.5倍。CsSABP2-3也受CLas侵染诱导表达,但与品种间耐病性无明显相关性(图6)。结果表明,相较于易感品种锦橙,CsSABP2-1、CsSABP2-4在耐病品种酸柚、马蜂柑中受CLas诱导高水平表达。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6CsSABP2在不同品种中响应CLas感染的表达
Fig. 6Gene expression of CsSABP2s in response to CLas infection in different varieties
2.6 CsSABP2响应SA和MeSA诱导的表达分析
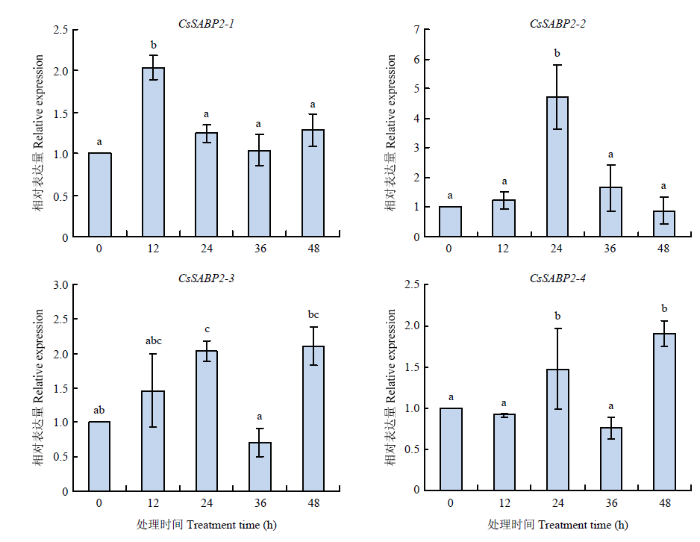
外施SA诱导4个基因的表达变化不明显。CsSABP2-1响应速度最快,在12 h即达到最高表达量,但24、36、48与0 h相比无明显差异;其次是CsSABP2-2,在24 h达到最高表达量,同样,在24、36、48 h与0 h相比无明显差异;而CsSABP2-3和CsSABP2-4在48 h才到达峰值(图7)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7SA诱导CsSABP2的表达分析
柱上不同字母表示在P<0.05水平下差异显著。
Fig. 7Analysis of CsSABP2s expression induced by SA
Different letters on the bars indicate significant difference at P<0.05 level. The same as
MeSA处理后,CsSABP2-1和 CsSABP2-4显著下调表达。而CsSABP2-2表达量急剧上升,与0 h相比,在24 h时上升至36.2倍。CsSABP2-3响应MeSA诱导变化趋势不明显。结果表明,CsSABP2-1、CsSABP2-2、CsSABP2-4在MeSA信号调控中起关键作用(图8)。
图8
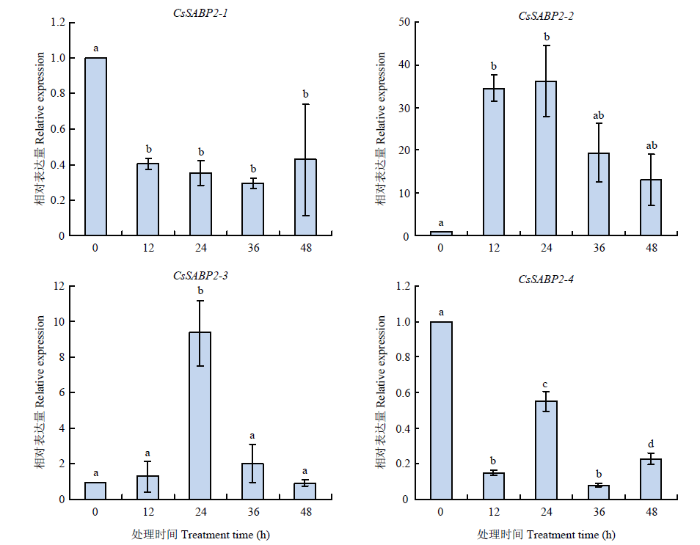 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8MeSA诱导CsSABP2的表达分析
Fig. 8Analysis of CsSABP2s expression induced by MeSA
3 讨论
由于CLas专性寄生在柑橘韧皮部,因此,本研究以叶脉和叶肉组织作为CLas侵染过程中SAR反应的侵染部位和非侵染部位,研究SAR响应CLas侵染的特征。ZOU等[18]发现与已感病锦橙相比,耐病酸柚表现出明显的延迟和较缓和的症状,对CLas生长的耐受性高于锦橙;而SHOKROLLAH等[30]发现马蜂柑对HLB的耐性也明显强于甜橙类。笔者课题组前期研究发现MeSA介导的SAR反应可能在柑橘品种耐性中起正调控作用[4,18],但在其他品种中是否有相似机制,依然不清楚。因此,本研究较详细地分析了锦橙、酸柚、马蜂柑3个品种SAR响应CLas侵染的特征。结果显示,耐病酸柚和马蜂柑中SAR响应CLas侵染明显强于易感病锦橙。这进一步暗示,增强SAR抗性反应可能是增强柑橘对HLB耐性的一条有效途径[18]。SAR Marker基因CsPR2和CsPR5在酸柚和马蜂柑侵染部位叶肉中上调表达水平显著高于叶脉,且显著高于锦橙;但是CsPR1在锦橙侵染部位叶脉中高水平表达,且显著高于酸柚和马蜂柑叶脉,而在叶肉中低水平表达。这种表达差异强烈暗示CsPR1在CLas引起的锦橙LAR中起主要作用,而CsPR2和CsPR5在CLas引起的酸柚和马蜂柑SAR中起主要作用。MeSA与柑橘对HLB的耐受性相关[18],本研究中,通过外施MeSA发现,MeSA显著上调处理端与非处理端SAR Marker基因的表达;MeSA诱导非处理端H2O2的积累显著强于SA;MeSA抑制了感病叶片中淀粉的积累。进一步表明,MeSA介导的SAR在调控柑橘HLB抗性中起着重要作用。
SABP2是MeSA信号介导SAR应答病原菌侵染的关键酶基因。本研究通过分析锦橙和酸柚感染CLas转录组筛选出6个SABP2差异表达基因,生物信息学分析表明CsSABP2-1、CsSABP2-2、CsSABP2-3和CsSABP2-4具有SABP2功能。SABP2是可溶性蛋白,与SA的亲和力很高,所以即使是在距离被侵染部位较远的组织中,当SA的浓度在0.5—9.0 μmol·L-1时,也可以产生有效的结合[31]。本研究显示,CsSABP2-1含有12个结合SA的氨基酸残基,CsSABP2-2含有10个,CsSABP2-3含有9个,CsSABP2-4含有2个。重要的是,CsSABP2-3和CsSABP2-4的SA高亲和结合位点Ala-13分别突变为Ser-13、Ile-13。同时在SA处理的基因表达分析中,发现CsSABP2-1在12 h就迅速响应SA,表达量达到最高;其次是CsSABP2-2,在24 h达到最高表达量;而CsSABP2-3和CsSABP2-4在48 h才到达峰值。上述结果表明,这4个基因具有不同的SA结合能力,CsSABP2-1结合SA的能力较强,CsSABP2-4结合SA的能力较差。SA是SABP2脂酶活性的抑制剂,SA与SABP2的结合抑制MeSA向SA的转化[20],因此,CsSABP2-1的脂酶活性可能是最弱的,而CsSABP2-4的脂酶活性可能是最强的。4个基因的SA结合能力和脂酶活性有待进一步验证。
非侵染部位MeSA积累和向SA的转化是激活SAR的关键。SABP2催化MeSA转化为 SA,这对诱导烟草 SAR是必不可少的[19,32]。在外施激素处理锦橙叶片中,CsSABP2主要响应MeSA的诱导表达。同时,4个基因在MeSA诱导条件下,呈现不同的表达谱,特别是CsSABP2-1和CsSABP2-4与CsSABP2-2呈现完全相反的诱导表达趋势。MeSA显著抑制CsSABP2-1、CsSABP2-4的表达,但明显激活了CsSABP2-2的表达。相似的,CLas感染显著上调锦橙特别是叶肉中CsSABP2-2的表达,而明显抑制CsSABP2-1、CsSABP2-4表达。在耐病品种酸柚和马蜂柑中,CLas抑制CsSABP2-2的表达,但显著激活CsSABP2-1、CsSABP2-4的表达。这些结果表明,CsSABP2-2与柑橘感病性相关,而CsSABP2-1、CsSABP2-4与柑橘耐病性相关,而且三者在应答病原菌侵染中可能存在协调调控MeSA信号传导作用。这种协调关系不仅反映在转录水平上,也可能与三者蛋白质活性有关,CsSABP2-1具有SA高亲和、低脂酶活性,CsSABP2-4具有SA低亲和、高脂酶活性,而CsSABP2-2介于两者之间。
这种SA亲和性和脂酶活性的差异可能在CsSABP2-1、CsSABP2-2、CsSABP2-4协调调控柑橘MeSA信号响应 CLas侵染中起着关键作用。另外,有研究表明,SABP2可能以形成二聚体执行功能[20],因此CsSABP2-1、CsSABP2-2、CsSABP2-4也有可能相互间形成异二聚体协调调控柑橘应答CLas。下一步可通过柑橘转基因等技术深入解析CsSABP2-1、CsSABP2-2、CsSABP2-4调控柑橘HLB抗性的功能及其协调关系。
4 结论
MeSA在介导柑橘SAR抗病反应中起正向调控作用。SA与MeSA信号转导的关键酶基因CsSABP2-1和CsSABP2-4表达水平与柑橘HLB耐性紧密相关,而CsSABP2-2表达水平与柑橘HLB感病性紧密相关。 CsSABP2-1、CsSABP2-2、CsSABP2-4在调控SA与MeSA信号转换中可能起着关键的协同作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOI:10.1146/annurev-phyto-073009-114418URLPMID:20415578 [本文引用: 1]

Huanglongbing (HLB) is the most destructive citrus pathosystem worldwide. Previously known primarily from Asia and Africa, it was introduced into the Western Hemisphere in 2004. All infected commercial citrus industries continue to decline owing to inadequate current control methods. HLB increase and regional spatial spread, related to vector populations, are rapid compared with other arboreal pathosystems. Disease dynamics result from multiple simultaneous spatial processes, suggesting that psyllid vector transmission is a continuum from local area to very long distance. Evolutionarily, HLB appears to have originated as an insect endosymbiont that has moved into plants. Lack of exposure of citrus to the pathogen prior to approximately 100 years ago did not provide sufficient time for development of resistance. A prolonged incubation period and regional dispersal make eradication nonviable. Multiple asymptomatic infections per symptomatic tree, incomplete systemic distribution within trees, and prolonged incubation period make detection difficult and greatly complicate disease control.
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
URLPMID:28637377 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:24086326 [本文引用: 1]
[本文引用: 1]
DOI:10.1094/MPMI-12-16-0257-RURLPMID:28488467 [本文引用: 1]

Pathogens from the fastidious, phloem-restricted 'Candidatus Liberibacter' species cause the devastating Huanglongbing (HLB) disease in citrus worldwide and cause diseases on many solanaceous crops and plants in the Apiaceae family. However, little is known about the pathogenic mechanisms due to the difficulty in culturing the corresponding 'Ca. Liberibacter' species. Here, we report that the citrus HLB pathogen 'Ca. L. asiaticus' uses an active salicylate hydroxylase SahA to degrade salicylic acid (SA) and suppress plant defenses. Purified SahA protein displays strong enzymatic activity to degrade SA and its derivatives. Overexpression of SahA in transgenic tobacco plants abolishes SA accumulation and hypersensitive response (HR) induced by nonhost pathogen infection. By degrading SA, 'Ca. L. asiaticus' not only enhances the susceptibility of citrus plants to both nonpathogenic and pathogenic Xanthomonas citri but also attenuates the responses of citrus plants to exogenous SA. In addition, foliar spraying of 2,1,3-benzothiadiazole and 2,6-dichloroisonicotinic acid, SA functional analogs not degradable by SahA, displays comparable (and even better) effectiveness with SA in suppressing 'Ca. L. asiaticus' population growth and HLB disease progression in infected citrus trees under field conditions. This study demonstrates one or more pathogens suppress plant defenses by degrading SA and establish clues for developing novel SA derivatives-based management approaches to control the associated plant diseases.
[本文引用: 1]
URLPMID:12897246 [本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-313X.2008.03618.xURLPMID:18643994 [本文引用: 1]

Salicylic acid-binding protein 2 (SABP2) is essential for the establishment of systemic acquired resistance (SAR) in tobacco; SABP2's methyl salicylate (MeSA) esterase activity is required in healthy systemic tissues of infected plants to release the active defense phytohormone SA from MeSA, which serves as a long-distance signal for SAR. In the current study, we characterize a new gene family from Arabidopsis thaliana encoding 18 potentially active alpha/beta fold hydrolases that share 32-57% identity with SABP2. Of 14 recombinant AtMES (MES for methyl esterase) proteins tested, five showed preference for MeSA as a substrate and displayed SA inhibition of MeSA esterase activity in vitro (AtMES1, -2, -4, -7, and -9). The two genes encoding MeSA esterases with the greatest activity, AtMES1 and -9, as well as AtMES7 were transcriptionally upregulated during infection of Arabidopsis with avirulent Pseudomonas syringae. In addition, conditional expression of AtMES1, -7, or -9 complemented SAR deficiency in SABP2-silenced tobacco, suggesting that these three members of the AtMES family are SABP2 functional homologs (orthologs). Underexpression by knockout mutation and/or RNAi-mediated silencing of multiple AtMES genes, including AtMES1, -2, -7, and -9, compromised SAR in Arabidopsis and correlated with enhanced accumulation of MeSA in the systemic tissue of SAR-induced plants. Together, the data show that several members of the AtMES gene family are functionally homologous to SABP2 and redundant for MeSA hydrolysis and probably SAR. These data suggest that MeSA is a conserved SAR signal in Arabidopsis and tobacco.
[本文引用: 1]
[本文引用: 5]
[本文引用: 2]
[本文引用: 4]
URLPMID:17916738 [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
URLPMID:27866312 [本文引用: 1]
DOI:10.1038/ng.2472URLPMID:23179022 [本文引用: 1]

Oranges are an important nutritional source for human health and have immense economic value. Here we present a comprehensive analysis of the draft genome of sweet orange (Citrus sinensis). The assembled sequence covers 87.3% of the estimated orange genome, which is relatively compact, as 20% is composed of repetitive elements. We predicted 29,445 protein-coding genes, half of which are in the heterozygous state. With additional sequencing of two more citrus species and comparative analyses of seven citrus genomes, we present evidence to suggest that sweet orange originated from a backcross hybrid between pummelo and mandarin. Focused analysis on genes involved in vitamin C metabolism showed that GalUR, encoding the rate-limiting enzyme of the galacturonate pathway, is significantly upregulated in orange fruit, and the recent expansion of this gene family may provide a genomic basis. This draft genome represents a valuable resource for understanding and improving many important citrus traits in the future.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
