 ,1, 刘英杰1,2, 董勇浩1, 刘金燕1, 李炜1, 徐蓬军1, 臧云1, 任广伟
,1, 刘英杰1,2, 董勇浩1, 刘金燕1, 李炜1, 徐蓬军1, 臧云1, 任广伟 ,1
,1Effects of CMV-Infected Tobacco on the Performance, Feeding and Host Selection Behavior of Myzus persicae
CHEN Xi ,1, LIU YingJie1,2, DONG YongHao1, LIU JinYan1, LI Wei1, XU PengJun1, ZANG Yun1, REN GuangWei
,1, LIU YingJie1,2, DONG YongHao1, LIU JinYan1, LI Wei1, XU PengJun1, ZANG Yun1, REN GuangWei ,1
,1通讯作者:
责任编辑: 岳梅
收稿日期:2020-07-1接受日期:2020-08-14网络出版日期:2021-04-16
| 基金资助: |
Received:2020-07-1Accepted:2020-08-14Online:2021-04-16
作者简介 About authors
陈茜,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (571KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈茜, 刘英杰, 董勇浩, 刘金燕, 李炜, 徐蓬军, 臧云, 任广伟. 黄瓜花叶病毒侵染烟草对烟蚜生长发育、取食和选择行为的影响[J]. 中国农业科学, 2021, 54(8): 1673-1683 doi:10.3864/j.issn.0578-1752.2021.08.008
CHEN Xi, LIU YingJie, DONG YongHao, LIU JinYan, LI Wei, XU PengJun, ZANG Yun, REN GuangWei.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】黄瓜花叶病毒(cucumber mosaic virus,CMV)寄主范围广,可危害茄科、葫芦科、十字花科等多种农作物,在世界范围内广泛传播流行。由CMV造成的病害在我国多个烟区广泛分布,常导致烟草产量和品质损失严重[1]。CMV可以通过蚜虫以非持久性方式进行传播,蚜虫的迁飞与CMV病害的传播与流行密切相关。感染CMV的寄主植株的生理生化变化可影响介体蚜虫的生物学特性,CMV的2b基因在蚜虫传播CMV的过程中发挥重要的作用[2,3]。探明寄主植物-介体蚜虫-CMV的互作关系,明确CMV 2b基因在病毒传播中的作用,对制定科学有效的CMV防控策略具有重要意义。【前人研究进展】植物病毒可以通过改变寄主植物的叶片形态、代谢特征和植物对生物、非生物胁迫的反应来增强自身适应性,并可能会干扰介体昆虫与植物相互作用的所有阶段以增强病毒的传播[4,5]。在介体昆虫的寄主定位选择阶段,病毒侵染可改变寄主植物的生理生化特性,如叶片颜色、挥发性物质的释放等,从而间接改变介体蚜虫的行为,影响植物对介体蚜虫的吸引力和偏好性[6]。而在介体昆虫成功定位寄主后,植物病毒还可通过改变寄主植物的营养物质、次生代谢产物等方式改变蚜虫在感病植物上的取食行为和生长发育[7,8]。例如番木瓜环斑病毒(papaya ringspot virus,PRSV)侵染西葫芦后,感病叶片内的必需氨基酸苏氨酸、精氨酸和赖氨酸,非必需氨基酸甘氨酸和半胱氨酸以及可溶性碳水化合物浓度,相较于健康植株均显著提高;在PRSV侵染植株上取食的瓜蚜(Aphis gossypii)繁殖力、寿命及种群内禀增长率等均较健康植株显著提高[9]。不同病毒侵染寄主植物对其介体昆虫也表现出不同的影响,如芜菁花叶病毒(turnip mosaic virus,TuMV)和马铃薯Y病毒(potato virus Y,PVY)侵染寄主后均表现出对介体昆虫有利的影响[10,11],但LI等[12]研究发现,大豆花叶病毒(soybean mosaic virus,SMV)侵染大豆后,在大豆植株上取食的大豆蚜(Aphis glycines)各龄期的虫体质量都显著降低,发育历期显著延长,生殖力降低,种群增长速率降低。此外,病毒的扩散传播也与介体昆虫的取食行为密切相关。介体昆虫对植物病毒的获取和传播发生在介体昆虫的取食过程中,其在感病植株和健康植株叶片上的取食行为也存在着差异[13,14,15]。在持久性病毒马铃薯卷叶病毒(potato leafroll virus,PLRV)侵染的马铃薯叶片上,蚜虫口针刺探过程中E1波的出现显著提前,持续刺吸时间增长,即带毒叶片更利于蚜虫在寄主韧皮部刺吸,从而有利于蚜虫的获毒[16]。介体对非持久性病毒的传毒效率与pd波的数量呈正相关性,与蚜虫口针最后一次离开细胞到停止刺探的时间呈负相关性[17]。在非持久性病毒PVY侵染的烟草叶片上取食的烟蚜(Myzus persicae),与健康烟草上的烟蚜相比,其在感病叶片上的刺探次数增加、刺探频率提高,且每次刺探发生持续时间较短,有利于蚜虫口针和病毒粒子的结合[18,19]。GUO等[2]研究发现,CMV 2b基因参与病毒与寄主植物和烟蚜的互作,它可通过改变寄主植物的生理变化来调控烟蚜的取食和选择行为,CMV的侵染可导致寄主植物组织中产生更高浓度的活性氧(H2O2),蚜虫在CMV侵染的植物上比在CMV 2b基因缺失突变体(CMVΔ2b)侵染的植物上的带毒量更大,蚜虫也更频繁地离开最初的取食部位,从而促进病毒传播。【本研究切入点】CMV、寄主植物与介体蚜虫的互作关系已有大量研究,其中CMV侵染烟草对介体蚜虫影响的相关研究主要集中在繁殖特性和寄主选择行为等方面,较少关注烟草营养成分的变化,CMV、CMVΔ2b侵染烟草后对介体烟蚜的生长发育、取食行为和寄主选择行为有何影响尚未明确。【拟解决的关键问题】通过构建CMV 2b基因缺失突变体,研究CMV侵染烟草对介体烟蚜生长发育、选择行为和取食行为的影响,并结合寄主植物内总糖和游离氨基酸含量变化,分析CMV 2b基因在互作关系中的作用,以期为黄瓜花叶病毒病的防控提供理论依据。1 材料与方法
试验于2019年在中国农业科学院烟草研究所烟草病虫害监测与综合治理重点实验室完成。1.1 试验材料
供试昆虫:烟蚜由中国农业科学院烟草研究所植物保护研究中心提供,挑选来自同一母体的若蚜,用健康烟苗在人工气候箱中繁殖多代。饲养条件为光周期14L﹕10D、温度(25±1)℃、相对湿度65%±5%。供试寄主:烤烟品种K326(Nicotiana tabacum ‘K326’),在人工气候室中将烟苗培养至6—7叶期备用。培养条件同上。
供试病毒:CMV侵染性克隆质粒pCB301-Fny1、pCB301-Fny2、pCB301-Fny3由南京农业大学植物保护学院陶小荣教授提供[20]。该侵染性克隆是在植物表达载体pCB301-2x35S-HDVRZ-NOS(NCBI载体序列登录号:JN029690)的基础上构建的CMV-Fny 3条基因组的侵染性克隆。对pCB301-Fny2上与2a非重叠部分的CMV 2b基因(91 bp)缺失处理,构建CMVΔ2b突变体,由TaKaRa公司完成,获得pCB301-Fny2-2b- del质粒及菌种。通过农杆菌转染法获得CMV、CMVΔ2b毒源。缺失的序列片段为:GGCCTCTCGTTT AGAGTTATCGGCGGAAGACCATGATTTTGACGATACAGATTGGTTCGCCGGTAACGAATGGGCGGAA
GGTGCTTTCTGA。
1.2 试验方法
1.2.1 摩擦接种 取毒源烟株中部叶片,电子天平称重,在灭菌的研钵中将烟草叶片充分研磨,按1﹕10配比加入PBS缓冲液(pH 7.2),于冰上迅速研磨成浆液,过滤掉叶片残渣留取清液。选取待接种烟株下部对称位第二、三片叶,在叶面倾撒一层石英砂,移液枪吸取200 μL接种液均匀点在叶面上,使用灭菌棉棒沿叶脉走向轻轻摩擦接种。1.2.2 烟草叶片中总糖含量检测 取CMV、CMVΔ2b接毒和模拟接毒后第15天烟草植株,收集除接毒叶片外的全株其余叶片,冲洗叶片表面污物后用电子天平称量计鲜重(fresh weight,FW),将叶片置于液氮中充分研磨后转移至冷冻干燥机中冻干处理,计干重(dry weight,DW)。取冻干后的烟叶样品利用蒽酮比色法测定总糖含量,测定方法参照植物总糖含量试剂盒(品牌:cominbio,货号:ZT-2-Y)说明书。CMV、CMVΔ2b接毒和模拟接毒处理各设3组生物学重复。
1.2.3 烟草叶片中游离氨基酸含量检测 样品的获取方法同1.2.2。准确称取均匀性一致的试样(精确到0.0001 g)于50 mL离心管中,加入20 mL 0.01 mol·L-1的盐酸,涡旋混匀,超声提取30 min,冷却定容过膜后使用高速氨基酸分析仪(日立L-8900)上机检测,对不同寄主植物叶片内18种氨基酸含量进行检测。CMV、CMVΔ2b接毒和模拟接毒处理各设3组生物学重复。
1.2.4 烟蚜的寄主选择行为测试 烟蚜对不同处理 烟株的选择性试验通过Y型嗅觉仪进行,供试烟株分别为侵染15 d的CMV、CMVΔ2b侵染烟株和同时期健康烟株,供试烟蚜为2—3日龄成蚜。Y型管进气口处分别接入两个空气流量计用于控制空气流速(150—200 mL·min-1),试验时打开日光灯保证光源均匀。试验时在Y型管出气口处放置1头成蚜(饥饿处理1 h),观察记录其选择行为,10 min内烟蚜进入某一气味臂约1/2处开始计时且停留30 s以上,则记为供试蚜虫对该侧烟株有选择行为;若未进入任意一臂或者停留时间未达30 s就返回出气口方向,则记为无选择,每测试5头蚜虫交换气味管位置。每组试验设100次重复。
1.2.5 烟草上的烟蚜取食行为测试 利用刺吸电位仪DC-EPG(Giga-8)(荷兰,瓦赫宁根大学及研究中心)监测烟蚜在不同处理烟株上的取食行为。寄主植物分别为CMV、CMVΔ2b接毒和模拟接毒后第15天的烟草。取大小一致的烟蚜成虫,将昆虫电极用导电银胶粘于烟蚜前胸背板,饥饿处理1 h后分别放在寄主植物心叶下一片叶的叶背面,植物电极插入土壤中使其形成回路,关闭法拉第电笼,持续监测6 h。各处理重复试验25次,选取有效重复15次以上进行数据统计分析。
按照ALVAREZ等的方法对蚜虫的EPG波形进行解析,通过EPG Stylet+a软件对烟蚜的取食波形进行判定并标记[16,21],分析np、C、pd、E1、E2、G波及EPG测试过程中的其他相关取食行为参数。
1.2.6 烟草上的烟蚜生长发育记录 参考臧连生等[22]的方法,用直径为3.5 cm的培养皿和小夹子制作蚜虫微虫笼,用于单头烟蚜的单株培养。用干净毛笔将成蚜轻轻接到CMV侵染10 d的烟株心叶下两片叶的叶背面,待24 h后移除成蚜和多余若蚜,仅保留1头新生若蚜,用微虫笼夹住叶片固定蚜虫活动范围,编号后进行蚜虫生长发育记录。若蚜时期,每天上午和下午分分别记录烟蚜存活情况和龄期,并及时清除蜕皮,待其羽化为成蚜后,每天上午调查其存活情况及产蚜量,每次调查时移除新生若蚜,持续记录直至成蚜全部死亡。共设置CMV、CMVΔ2b侵染和健康烟草3个处理,重复50次。
1.3 数据处理
利用IBM SPSS Statistics 23 软件进行数据统计分析。采用LSD法比较不同处理间烟蚜的EPG参数、烟草叶片中总糖含量和氨基酸含量的差异显著性,采用卡方检验比较不同处理间烟蚜的选择行为差异。利用Twosex-MSchart软件[23,24],通过Bootstrap程序(Bootstrap次数为100 000)分别计算不同寄主烟株上烟蚜生命表参数和种群参数的方差和标准误,分析各处理之间的差异显著性。2 结果
2.1 CMV侵染对烟草叶片中总糖和游离氨基酸含量的影响
健康、CMV侵染和CMVΔ2b侵染的烟草叶片中总糖含量分别为229.88、144.01、198.05 mg·g-1,健康烟草中的总糖含量显著高于CMV和 CMVΔ2b侵染烟草(P<0.05);健康、CMV侵染和CMVΔ2b侵染的烟草叶片中游离氨基酸含量分别为6.14、12.35、5.48 mg·g-1,CMV侵染烟草中的游离氨基酸含量显著高于健康烟草和CMVΔ2b侵染烟草(P<0.05)(图1)。图1
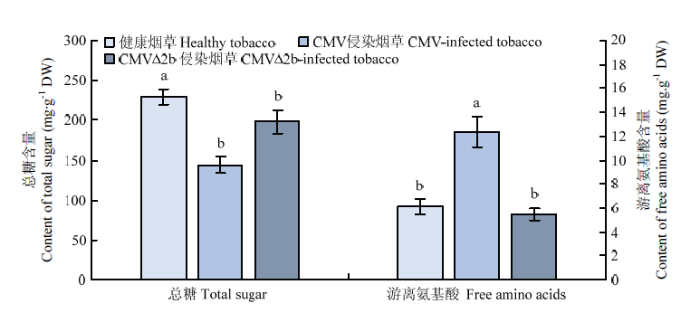 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同烟草叶片中总糖和游离氨基酸含量
图中数据为平均值±标准误。柱上不同小写字母表示该参数经单因素方差分析法检验结果差异显著(P<0.05,LSD)
Fig. 1Contents of total sugar and free amino acids in different tobacco leaves
Data in the figure are mean±SE. Different lowercase letters on the bars indicate the parameter has a significant difference at 0.05 level (LSD test after one-way ANOVA)
不同处理烟草体内18种游离氨基酸含量也具有一定差异(表1)。CMVΔ2b侵染烟草中的苏氨酸、谷氨酸、甘氨酸、酪氨酸、组氨酸、精氨酸和脯氨酸含量显著高于其他处理烟草,缬氨酸和赖氨酸含量显著高于健康烟草,而CMV侵染烟草中的天冬氨酸含量显著低于其他处理烟草,胱氨酸含量显著高于CMVΔ2b侵染烟草。
Table 1
表1
表1不同烟草叶片中游离氨基酸含量
Table 1
| 游离氨基酸 Free amino acid | 健康烟草 Healthy tobacco | CMV侵染烟草 CMV-infected tobacco | CMVΔ2b 侵染烟草 CMVΔ2b-infected tobacco |
|---|---|---|---|
| 天冬氨酸Aspartic acid | 1170.00±126.62a | 576.67±61.19b | 1230.00±92.37a |
| 苏氨酸Threonine | 786.67±91.34b | 750.00±109.70b | 2723.33±440.54a |
| 丝氨酸Serine | 636.67±126.67a | 590.00±70.24a | 930.00±104.40a |
| 谷氨酸Glutamic acid | 1960.00±292.63b | 1886.67±144.95b | 3030.00±135.77a |
| 甘氨酸Glycine | 10.00±0b | 20.00±5.77b | 70.00±10.00a |
| 丙氨酸Alanine | 253.33±8.82a | 246.67±26.03a | 263.33±12.02a |
| 缬氨酸Valine | 263.33±12.02b | 306.67±26.03ab | 356.67±29.63a |
| 蛋氨酸Methionine | 16.67±8.82a | 6.67±6.67a | 23.33±3.33a |
| 异亮氨酸Isoleucine | 53.33±3.33a | 60.00±5.78a | 50.00±3.77a |
| 亮氨酸Leucine | 70.00±5.77a | 76.67±8.82a | 76.67±8.82a |
| 酪氨酸Tyrosine | 63.33±3.33c | 96.67±6.67b | 133.33±12.02a |
| 苯丙氨酸Phenylalanine | 203.33±26.03a | 226.67±24.04a | 263.33±21.86a |
| 赖氨酸Lysine | 66.67±3.33b | 76.67±8.82ab | 100.00±11.54a |
| 组氨酸Histidine | 36.67±3.33b | 50.00±5.77b | 133.33±21.86a |
| 精氨酸Arginine | 26.67±6.67b | 26.67±3.33b | 56.67±6.67a |
| 脯氨酸Proline | 460.00±40.41b | 373.00±67.41b | 2763.33±316.88a |
| 色氨酸Tryptophane | 33.33±3.33a | 80.00±5.77a | 133.33±48.42a |
| 胱氨酸Cystine | 30.00±0ab | 33.33±6.67a | 16.67±3.33b |
新窗口打开|下载CSV
2.2 CMV侵染烟草对烟蚜寄主选择行为的影响
健康烟草与CMV侵染烟草相比,烟蚜对健康烟草有更强的选择偏好(χ2=30.224,P<0.001);CMV侵染烟草与CMVΔ2b侵染烟草相比,烟蚜对CMVΔ2b侵染烟草具有更强的选择偏好(χ2=21.062,P<0.001);烟蚜对健康烟草与CMVΔ2b侵染烟草的选择行为无显著差异(χ2=0.276,P=0.599)(图2)。图2
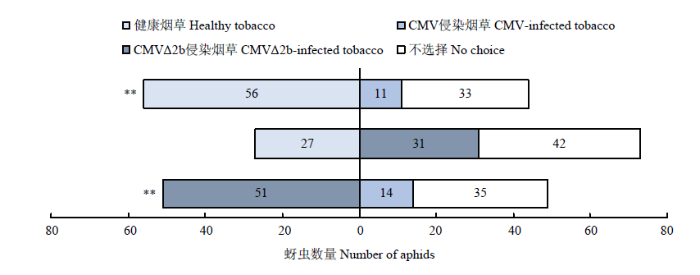 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2烟蚜对健康烟草和CMV、CMVΔ2b侵染烟草的选择趋向
**表示烟蚜对不同寄主烟株的选择行为有极显著差异(P<0.01,卡方检验)
Fig. 2Preference of M. persicae on healthy, CMV-infected and CMVΔ2b-infected tobacco
** represents extremely significant differences in the selection results of aphids on different host tobacco plants (P<0.01, Chi-square test)
2.3 CMV侵染烟草对烟蚜取食行为的影响
采用EPG技术对烟蚜在不同寄主烟草上的取食行为进行了监测,选取包含np、C、pd、E1、E2、G波共6个波形在内的19个相关参数进行统计分析(表2)。Table 2
表2
表2不同烟草寄主上的烟蚜取食EPG参数
Table 2
| EPG参数 EPG parameter | 健康烟草 Healthy tobacco (n=15) | CMV侵染烟草 CMV-infected tobacco (n=15) | CMVΔ2b侵染烟草 CMVΔ2b-infected tobacco (n=15) | |
|---|---|---|---|---|
| 1 | 第一次刺探发生时间Time to 1st probe from start of EPG (min) | 0.64±0.16a | 0.75±0.42a | 0.76±0.17a |
| 2 | 第一次E1波前刺探次数Number of probes to the 1st E1 | 20.40±6.69a | 14.00±2.60a | 15.33±4.05a |
| 3 | pd次数Number of probes | 197.73±9.53ab | 217.93±50.51a | 184.27±11.28b |
| 4 | np次数Number of np | 39.40±7.97a | 48.00±7.57a | 46.07±6.17a |
| 5 | np 持续时长Total duration of np (min) | 52.69±8.52a | 63.43±9.67a | 61.79±8.56a |
| 6 | 口针第一次到达韧皮部的时间Time to phloem from the start of EPG (min) | 87.72±31.03a | 67.05±8.22a | 79.89±14.70a |
| 7 | E1次数Number of E1 | 19.53±2.77a | 16.60±3.23a | 19.13±2.81a |
| 8 | E1持续总时长Total duration of E1 (min) | 58.31±6.58a | 69.64±8.09a | 62.00±6.83a |
| 9 | E1持续总时长/总记录时间Total duration of E1/total record time (%) | 16.20±1.83a | 16.56±2.25a | 17.22±1.90a |
| 10 | E2的次数Number of E2 | 13.67±2.37a | 9.27±2.45a | 14.73±2.72a |
| 11 | E2持续时长>10 min次数Number of sustained E2 (>10 min) | 0.80±0.24a | 0.33±0.16a | 1.00±0.29a |
| 12 | E2持续总时长Total duration of E2 (min) | 41.70±9.55ab | 19.15±5.30b | 42.93±8.81a |
| 13 | E2持续总时长/记录总时间Total duration of E2/total record time (%) | 11.59±2.65ab | 5.32±1.47b | 11.92±2.45a |
| 14 | E1+E2持续总时长Total duration of E (min) | 100.03±13.87a | 78.79±11.65a | 104.92±11.91a |
| 15 | 最长E2波时间Duration of the longest E2 (min) | 15.50±4.16a | 6.11±1.65b | 15.54±3.33a |
| 16 | G波持续总时间Duration of G (min) | 24.13±0.00a | 24.52±20.08a | 13.57±2.73b |
| 17 | 出现G波的蚜虫百分比Percentage of aphids showing waveform G (%) | 6.67b | 20.00a | 6.67b |
| 18 | C波持续总时长Total duration of C (min) | 208.51±10.37a | 213.59±11.85a | 191.85±10.95a |
| 19 | C波持续总时长/记录总时间Total duration of C/total record time (%) | 57.92±2.88a | 59.33±3.29a | 53.29±1.78a |
新窗口打开|下载CSV
健康、CMV侵染和CMVΔ2b侵染烟草的3个处理中,烟蚜的第一次刺探发生时间、非刺探波(np波)次数和持续时间均无显著差异。在烟蚜刺探路径阶段,烟蚜在CMV侵染烟草上的刺探发生次数(pd波)显著高于CMVΔ2b侵染烟草(表2,参数3)。烟蚜的韧皮部取食阶段,烟蚜在CMVΔ2b侵染烟草上韧皮部的取食时长(E2波)显著高于CMV侵染烟草(表2,参数12和13)。烟蚜在3种寄主烟草上均有木质部吸食的发生(G波),其中烟蚜在CMV侵染烟草上木质部吸食的发生概率显著高于其他寄主烟株(表2,参数17)。
2.4 CMV侵染烟草对烟蚜生命参数的影响
由图3可知,CMV侵染烟草提高了烟蚜的若蚜死亡率,2龄若蚜死亡率及若蚜期死亡率均显著高于健康烟草和CMVΔ2b侵染烟草(P<0.05)。1、3、4龄若蚜死亡率在CMV、CMVΔ2b侵染烟草和健康烟草3个处理间无显著差异。图3
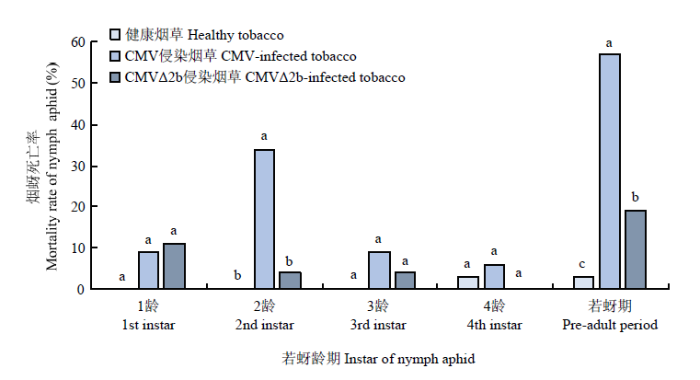 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同烟草寄主上的烟蚜若蚜死亡率
图中数据为平均数±标准误。柱上不同小写字母表示经Bootstrap程序检验差异显著(P<0.05)
Fig. 3Mortality rate of nymph aphids on different tobacco hosts
Data in the figure are mean±SE. Different lowercase letters on the bars indicate significant difference at 0.05 level (Bootstrap program)
由表3、表4可知,各处理烟草上烟蚜4龄若蚜发育历期、成虫期、成虫繁殖前期和繁殖期均无显著差异,但CMV侵染烟草上烟蚜的1龄、2龄、3龄若蚜历期、若蚜历期、总繁殖前期均显著高于健康烟草和CMVΔ2b侵染烟草,CMV侵染烟草上烟蚜的寿命显著低于健康烟草和CMVΔ2b侵染烟草(P<0.05)。健康烟草上烟蚜的繁殖力显著高于CMV侵染烟草(P<0.05),健康烟草与CMVΔ2b侵染烟草上烟蚜的繁殖力无显著差异。
由表5可知,烟蚜在CMV、CMVΔ2b侵染烟草和健康烟草上的种群参数亦有一定差异。其中,烟蚜的平均世代周期(T)无显著差异,但健康烟草上烟蚜的内禀增长率(r)和周限增长率(λ)均显著高于CMV和CMVΔ2b侵染烟草(P<0.05)。健康烟草和CMVΔ2b侵染烟草上烟蚜的净生殖率(R0)显著高于CMV侵染烟草,健康烟草上烟蚜的总繁殖率GRR显著高于CMVΔ2b侵染烟草。
Table 3
表3
表3不同烟草寄主上烟蚜的发育历期及寿命
Table 3
| 寄主植物 Host plant | 1龄若蚜 1st instar | 2龄若蚜 2nd instar | 3龄若蚜 3rd instar | 4龄若蚜 4th instar | 若蚜历期 Pre-adult period | 成虫期 Adult period | 寿命 Adult longevity |
|---|---|---|---|---|---|---|---|
| 健康烟草 Healthy tobacco | 1.52±0.10b | 1.61±0.11b | 1.55±0.10b | 2.16±0.14a | 6.81±0.16b | 15.66±1.52a | 22.03±1.54a |
| CMV侵染烟草 CMV-infected tobacco | 2.19±0.20a | 2.80±0.21a | 2.71±0.25a | 2.00±0.22a | 9.40±0.58a | 11.47±1.68a | 12.49±1.49b |
| CMVΔ2b侵染烟草 CMVΔ2b-infected tobacco | 1.67±0.12b | 1.83±0.17b | 1.45±0.13b | 2.32±0.15a | 7.32±0.24b | 16.09±1.77a | 20.26±1.94a |
新窗口打开|下载CSV
Table 4
表4
表4不同烟草寄主上烟蚜的繁殖历期及繁殖力
Table 4
| 寄主植物 Host plant | 成虫繁殖前期 Adult pre-reproductive period (d) | 总繁殖前期 Total pre-reproductive period (d) | 繁殖期 Reproductive days (d) | 繁殖力(单雌产蚜量) Fecundity (number of nymphs produced by one female aphid) |
|---|---|---|---|---|
| 健康烟草 Healthy tobacco | 0.88±0.16a | 7.69±0.21b | 10.66±1.19a | 24.66±3.44a |
| CMV侵染烟草 CMV-infected tobacco | 1.17±0.35a | 10.33±0.88a | 9.25±1.40a | 12.13±2.93b |
| CMVΔ2b侵染烟草 CMVΔ2b-infected tobacco | 0.81±0.19a | 8.00±0.32b | 10.86±1.17a | 19.32±2.86ab |
新窗口打开|下载CSV
Table 5
表5
表5不同烟草寄主上烟蚜的种群参数
Table 5
| 寄主植物 Host plant | 内禀增长率 Intrinsic rate of increase (r, d-1) | 周限增长率 Finite rate of increase (λ, d-1) | 净生殖率 Net reproductive rate (R0, offspring) | 平均世代周期 Mean generation time (T, d) | 总繁殖率 Gross reproduction rate (GRR) |
|---|---|---|---|---|---|
| 健康烟草 Healthy tobacco | 0.25±0.01a | 1.28±0.02a | 23.91±3.39a | 12.95±0.32a | 34.73±2.95a |
| CMV侵染烟草 CMV-infected tobacco | 0.11±0.02c | 1.11±0.02c | 5.20±1.58b | 15.65±1.36a | 27.78±7.18ab |
| CMVΔ2b侵染烟草 CMVΔ2b-infected tobacco | 0.20±0.01b | 1.22±0.02b | 15.74±2.69a | 13.59±0.49a | 27.43±2.24b |
新窗口打开|下载CSV
3 讨论
植物病毒的传播与介体昆虫的取食与定殖密切相关,在取食之前,介体昆虫主要依靠视觉和嗅觉选择目标植物[25,26]。相较于健康植物,病毒侵染后的寄主植物释放的挥发物发生改变且对介体昆虫的选择行为产生影响[3,8,27-28]。本研究发现,烟蚜对健康烟草和CMVΔ2b侵染烟草有着更强的选择趋向。而MAUCK等[8]研究发现,CMV侵染西葫芦后,与健康植株相比,带毒植株释放了更多的挥发性物质,吸引了桃蚜(M. persicae)和瓜蚜前来定殖取食,且蚜虫在获毒后会向健康植株迁移。WU等[29]发现CMV 2b蛋白与拟南芥JAZ蛋白(茉莉酸信号的关键抑制因子)通过物理作用结合以阻止JAZ降解,从而减弱茉莉酸信号以增强寄主对介体蚜虫的吸引力。但也有研究表明,CMV的侵染虽然改变了寄主植物的挥发物释放,但并不能增加寄主植物对蚜虫的吸引力[3]。以上研究结果之间的差异可能与寄主植物种类或病毒侵染阶段不同有关。植物病毒可以通过改变寄主植物营养成分的变化影响介体昆虫的生长发育和取食行为,从而影响介体对病毒的传播[7,12,30-31]。研究表明,蚜虫在摄取并分解食物中的氨基酸后在体内重新合成游离氨基酸,蚜虫可以通过自我调控适应氨基酸匮乏的取食状况[32]。PVY的侵染导致烟草体内可溶性糖和氨基酸含量的增加,促使有翅蚜的产生和扩散,利于PVY的传播[33]。从烟蚜在不同寄主上的取食行为来看,烟蚜在CMV侵染烟草上的刺探行为发生次数最多,并且花费较少的时间在韧皮部进食阶段(E2波),这表明相较于CMVΔ2b侵染烟草和健康烟草,CMV侵染后烟草叶片的营养品质和物理结构并不适宜烟蚜取食。与健康烟草和CMVΔ2b侵染烟草相比,CMV侵染叶片中总糖含量降低,游离氨基酸的含量显著升高,表明烟蚜偏好取食总糖含量高但氨基酸含量低的叶片组织,这与MAUCK等的研究结果相吻合[27]。非持久性病毒的传播与蚜虫在取食过程中的刺探行为密切相关[34,35],病毒的获取和接种都发生在pd波阶段,刺探行为的增加会提高非持久性病毒的传播效率。相较于CMVΔ2b侵染烟草,CMV侵染烟草上烟蚜的刺探行为(pd波)发生频率较高,GUO等[2]研究表明,蚜虫在CMV侵染烟草上比在CMVΔ2b烟草上更易获毒。因此推测在寄主植物、介体蚜虫与病毒的互作关系中,CMV 2b基因可通过提高烟蚜的刺探频率来促进病毒的传播。此外,G波的发生代表着蚜虫在木质部的主动吸食,CMV侵染烟草上的烟蚜在木质部吸食(G波)的发生概率高于健康烟草和CMVΔ2b侵染的烟草,这表明CMV侵染烟草上的烟蚜更容易发生木质部进食以获取无机盐和水分。
在植物病毒、介体昆虫和寄主植物的互作关系中,寄主植物体内的病毒含量影响寄主植物自身的防御水平以及介体昆虫的繁殖力[36]。王佳等[37]研究发现,CMV侵染的烟草抑制了烟蚜的种群增长率,降低烟草对烟蚜的适合度,促使蚜虫选择新寄主。本研究发现,相较于健康烟草,CMV的侵染导致烟蚜若蚜历期延长、寿命缩短、繁殖力降低,并造成烟蚜在2龄若蚜时的大量死亡,提高了整个若蚜期的死亡率;通过对烟蚜种群参数进行分析,发现健康烟草有利于烟蚜生长发育和繁殖,CMV侵染烟草不适于烟蚜生长发育和繁殖,这与CMV侵染烟草导致氨基酸和总糖含量及其比例发生变化有关,同时在试验中发现感染CMV的烟草叶片革质化,这也不适于烟蚜取食。相较于CMV侵染烟株,烟蚜在CMVΔ2b侵染烟株和健康烟株上有着更好的适应性。有研究表明CMV 2b基因参与寄主与介体蚜虫互作,间接影响蚜虫对CMV的传播,病毒的其他蛋白也会与CMV 2b蛋白相互作用,共同调控寄主植物对蚜虫的抗性[38,39],这也为在生产实践中通过调控CMV 2b基因来控制蚜虫传播CMV提供了理论依据,为减少CMV在田间的流行提供了防控新思路。
对于持久性传播的病毒,如PLRV,病毒的侵染提高了寄主植物“营养品质”,蚜虫优先定殖和取食受侵染的植物,种群快速增长,最终蚜虫从受侵染寄主植株上向新寄主转移,有利于病毒传播[40]。对于非持久性传播的CMV,本研究发现该病毒的侵染降低了寄主烟株的“营养品质”,不适于烟蚜取食,从而降低烟蚜繁殖力并造成烟蚜大量死亡。MAUCK等[8,41]的研究表明,CMV侵染西葫芦导致叶片“营养品质”下降,蚜虫种群数量显著降低,且在受侵染西葫芦上的烟蚜更容易被寄生蜂寄生。而同样通过蚜虫非持久性传播的PVY在侵染烟草后,虽然提高了感病烟草上烟蚜的繁殖力,但有翅蚜产生高峰期提前,也同样促进了烟蚜的扩散和传毒[33]。由此可见,两种传播机制的病毒虽然介体的传毒方式不同,但侵染寄主植物后,可通过调控寄主植物代谢或介体昆虫的生长发育、取食行为、选择行为等策略,进一步影响病毒的传播与流行。
4 结论
CMV侵染烟草后,降低了介体烟蚜的寄主偏好性。CMV 2b基因的存在提高了烟蚜在烟草上的刺探频率,不利于烟蚜在韧皮部的取食。CMV的侵染改变了烟草中总糖和游离氨基酸等营养物质的组成,降低了烟蚜的寄主适合度。CMV的侵染对烟蚜的生长发育产生不利影响,延长了烟蚜的若蚜历期、提高了若蚜的死亡率,降低了烟蚜寿命和繁殖力,促使蚜虫寻找新的合适寄主,从而促进了病毒的传播。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
URLPMID:30381318 [本文引用: 3]
DOI:10.1186/s12985-017-0754-0URLPMID:28468686 [本文引用: 3]

BACKGROUND: Aphids, including the generalist herbivore Myzus persicae, transmit cucumber mosaic virus (CMV). CMV (strain Fny) infection affects M. persicae feeding behavior and performance on tobacco (Nicotiana tabacum), Arabidopsis thaliana and cucurbits in varying ways. In Arabidopsis and cucurbits, CMV decreases host quality and inhibits prolonged feeding by aphids, which may enhance virus transmission rates. CMV-infected cucurbits also emit deceptive, aphid-attracting volatiles, which may favor virus acquisition. In contrast, aphids on CMV-infected tobacco (cv. Xanthi) exhibit increased survival and reproduction. This may not increase transmission but might increase virus and vector persistence within plant communities. The CMV 2b counter-defense protein diminishes resistance to aphid infestation in CMV-infected tobacco plants. We hypothesised that in tobacco CMV and its 2b protein might also alter the emission of volatile organic compounds that would influence aphid behavior. RESULTS: Analysis of headspace volatiles emitted from tobacco plants showed that CMV infection both increased the total quantity and altered the blend produced. Furthermore, experiments with a CMV 2b gene deletion mutant (CMV2b) showed that the 2b counter-defense protein influences volatile emission. Free choice bioassays were conducted where wingless M. persicae could choose to settle on infected or mock-inoculated plants under a normal day/night regime or in continual darkness. Settling was recorded at 15 min, 1 h and 24 h post-release. Statistical analysis indicated that aphids showed no marked preference to settle on mock-inoculated versus infected plants, except for a marginally greater settlement of aphids on mock-inoculated over CMV-infected plants under normal illumination. CONCLUSIONS: CMV infection of tobacco plants induced quantitative and qualitative changes in host volatile emission and these changes depended in part on the activity of the 2b counter-defense protein. However, CMV-induced alterations in tobacco plant volatile emission did not have marked effects on the settling of aphids on infected versus mock-inoculated plants even though CMV-infected plants are higher quality hosts for M. persicae.
[本文引用: 1]
URLPMID:25375140 [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOI:10.4161/cib.3.6.13094URLPMID:21331245 [本文引用: 4]

Plant chemicals mediating interactions with insect herbivores seem a likely target for manipulation by insectvectored plant pathogens. Yet, little is currently known about the chemical ecology of insect-vectored diseases or their effects on the ecology of vector and nonvector insects. We recently reported that a widespread plant pathogen, Cucumber mosaic virus (CMV), greatly reduces the quality of host-plants (squash) for aphid vectors, but that aphids are nevertheless attracted to the odors of infected plants-which exhibit elevated emissions of a volatile blend otherwise similar to the odor of healthy plants. This finding suggests that exaggerating existing host-location cues can be a viable vector attraction strategy for pathogens that otherwise reduce host quality for vectors. Here we report additional data regarding the effects of CMV infection on plant interactions with a common nonvector herbivore, the squash bug, Anasa tristis, which is a pest in this system. We found that adult A. tristis females preferred to oviposit on healthy plants in the field, and that healthy plants supported higher populations of nymphs. Collectively, our recent findings suggest that CMV-induced changes in host plant chemistry influence the behavior of both vector and non-vector herbivores, with significant implications both for disease spread and for broader community-level interactions.
DOI:10.1038/s41598-019-39256-5URLPMID:30792431 [本文引用: 1]

The association of plant viruses with their vectors has significant implications for virus transmission and spread. Only a few studies, with even fewer pathosystems, have explored non-persistent (NP) virus-vector interactions that are presumed to be transient. We studied how a NP virus, Papaya ringspot virus (PRSV) influenced the behavior and biology of its vector, the melon aphid (Aphis gossypii Glover) and the non-vector, silverleaf whitefly (Bemisia tabaci Gennadius). We also assessed whether the fitness effects on aphids are modulated through changes in the host plant, squash (Cucurbita pepo L.) nutrient profile. The overall performance of A. gossypii was substantially higher on PRSV-infected plants, along with increased arrestment on PRSV-infected than non-infected plants. No such PRSV-modulated fitness effects were observed with B. tabaci. PRSV-infected plants had increased concentrations of free essential amino acids: threonine, arginine and lysine; non-essential amino acids: glycine and homocysteine; and soluble carbohydrates: galactose, raffinose and cellobiose. In general, PRSV encouraged long-term feeding and enhanced fitness of A. gossypii through host plant nutrient enrichment. These findings provide evidence for a NP virus mediated positive fitness effects on its vector, with no spillover fitness benefits to the non-vector within the same feeding guild.
URLPMID:24372679 [本文引用: 1]
[本文引用: 1]
URLPMID:29945216 [本文引用: 2]
DOI:10.1111/j.1364-3703.2004.00240.xURLPMID:20565624 [本文引用: 1]

SUMMARY Aphids are the most common vector of plant viruses. Mechanisms of transmission are best understood by considering the routes of virus movement in the aphid (circulative versus non-circulative) and the sites of retention or target tissues (e.g. stylets, salivary glands). Capsid proteins are a primary, but not necessarily sole, viral determinant of transmission. A summary is presented of the taxonomic affiliations of the aphid transmitted viruses, including 8 families, 18 genera, and taxonomically unassigned viruses.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:24329574 [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:32523545 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/s12985-016-0524-4URLPMID:27103351 [本文引用: 1]

BACKGROUND: Cucumber mosaic virus (CMV) causes appreciable losses in vegetables, ornamentals and agricultural crops. The green peach aphid, Myzus persicae Sulzer (Aphididae) is one of the most efficient vectors for CMV. The transmission ecology of aphid-vectored CMV has been well investigated. However, the detailed description of the dynamic change in the plant-CMV-aphid interaction associated with plant defense and virus epidemics is not well known. RESULTS: In this report, we investigated the relationship of virus titer with plant defense of salicylic acid (SA) and jasmonic acid (JA) during the different infection time and their interaction with aphids in CMV-infected tobacco plants. Our results showed that aphid performance changed with virus titer and plant defense on CMV-inoculated plants. At first, plant defense was low and aphid number increased gradually. The plant defense of SA signaling pathway was induced when virus titer was at a high level, and aphid performance was correspondingly reduced. Additionally, the winged aphids were increased. CONCLUSION: Our results showed that aphid performance was reduced due to the induced plant defense mediated by Cucumber mosaic virus titer. Additionally, some wingless aphids became to winged aphids. In this way CMV could be transmitted with the migration of winged aphids. We should take measures to prevent aphids in the early stage of their occurrence in the field to prevent virus outbreak.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/mpp.12892URLPMID:31777194 [本文引用: 1]

Cucumber mosaic virus (CMV), which is vectored by aphids, has a tripartite RNA genome encoding five proteins. In tobacco (Nicotiana tabacum), a subgroup IA CMV strain, Fny-CMV, increases plant susceptibility to aphid infestation but a viral mutant unable to express the 2b protein (Fny-CMV2b) induces aphid resistance. We hypothesized that in tobacco, one or more of the four other Fny-CMV gene products (the 1a or 2a replication proteins, the movement protein, or the coat protein) are potential aphid resistance elicitors, whilst the 2b protein counteracts induction of aphid resistance. Mutation of the Fny-CMV 2b protein indicated that inhibition of virus-induced resistance to aphids (Myzus persicae) depends on amino acid sequences known to control nucleus-to-cytoplasm shuttling. LS-CMV (subgroup II) also increased susceptibility to aphid infestation but the LS-CMV2b mutant did not induce aphid resistance. Using reassortant viruses comprising different combinations of LS and Fny genomic RNAs, we showed that Fny-CMV RNA 1 but not LS-CMV RNA 1 conditions aphid resistance in tobacco, suggesting that the Fny-CMV 1a protein triggers resistance. However, the 2b proteins of both strains suppress aphid resistance, suggesting that the ability of 2b proteins to inhibit aphid resistance is conserved among divergent CMV strains.
[本文引用: 1]
DOI:10.1038/srep10963URLPMID:26043237 [本文引用: 1]

Plant viruses can profoundly alter the phenotypes of their host plants, with potentially far-reaching implications for ecology. Yet few studies have explored the indirect, host-mediated, effects of plant viruses on non-vector insects. We examined how infection of Cucurbita pepo plants by Cucumber mosaic virus (CMV) impacted the susceptibility of aphids (Myzus persicae) to attack by the parasitoid wasp Aphidius colemani. In semi-natural foraging assays, we observed higher rates of aphid parasitism on infected plants compared to healthy plants. Subsequent experiments revealed that this difference is not explained by different attack rates on plants differing in infection status, but rather by the fact that parasitoid larvae successfully complete their development more often when aphid hosts feed on infected plants. This suggests that the reduced nutritional quality of infected plants as host for aphids--documented in previous studies--compromises their ability to mount effective defenses against parasitism. Furthermore, our current findings indicate that the aphid diet during parasitoid development (rather than prior to wasp oviposition) is a key factor influencing resistance. These findings complement our previous work showing that CMV-induced changes in host plant chemistry alter patterns of aphid recruitment and dispersal in ways conducive to virus transmission.
