 ,, 浦惠明
,, 浦惠明 ,, 高建芹, 胡茂龙, 张洁夫, 陈松江苏省农业科学院经济作物研究所/农业部长江下游棉花与油菜重点实验室,南京 210014
,, 高建芹, 胡茂龙, 张洁夫, 陈松江苏省农业科学院经济作物研究所/农业部长江下游棉花与油菜重点实验室,南京 210014Creation of High-Oleic (HO) Canola Germplasm and the Genetic and Physiological Analysis on HO Trait
LONG WeiHua ,, PU HuiMing
,, PU HuiMing ,, GAO JianQin, HU MaoLong, ZHANG JieFu, CHEN SongInstitute of the Industrial Crops, Jiangsu Academy of Agriculture Sciences/Key Lab of Cotton and Rapeseed (Nanjing) of Ministry of Agriculture, Nanjing 210014
,, GAO JianQin, HU MaoLong, ZHANG JieFu, CHEN SongInstitute of the Industrial Crops, Jiangsu Academy of Agriculture Sciences/Key Lab of Cotton and Rapeseed (Nanjing) of Ministry of Agriculture, Nanjing 210014通讯作者:
责任编辑: 李莉
收稿日期:2020-07-4接受日期:2020-09-1网络出版日期:2021-01-16
| 基金资助: |
Received:2020-07-4Accepted:2020-09-1Online:2021-01-16
作者简介 About authors
龙卫华,Tel:025-84390368;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (746KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
龙卫华, 浦惠明, 高建芹, 胡茂龙, 张洁夫, 陈松. 油菜高油酸种质的创建及高油酸性状遗传与生理特性的分析[J]. 中国农业科学, 2021, 54(2): 261-270 doi:10.3864/j.issn.0578-1752.2021.02.003
LONG WeiHua, PU HuiMing, GAO JianQin, HU MaoLong, ZHANG JieFu, CHEN Song.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】油菜是中国三大主要油料作物之一,菜籽油在中国居民日常生活中具有良好的消费基础[1]。近年来,菜籽油已成为国产植物油的第一大来源,因此,菜籽油的品质(主要是脂肪酸含量比例)对中国居民健康具有重要影响[2]。在中国油菜全面实现“双低化”(2003年)后,十八碳脂肪酸含量的改良成为目前油菜品质育种的重要目标[3]。与普通双低菜籽油相比,高油酸(high oleic,HO)菜籽油[4,5]具有四大优势:(1)较耐贮藏,货架期较长;(2)可降低低密度脂蛋白含量,减少胆固醇形成,预防人体心血管疾病;(3)甲酯化程度高,燃烧值高,更有利于生产生物柴油;(4)有利于提高菜籽油的氧化稳定性,是菜籽油不容易变质生成反式脂肪酸(或位置异构脂肪酸)和产生异味[6]。创建HO甘蓝型油菜新种质并以此培育HO油菜品种是提供高品质菜籽油的有效途径。【前人研究进展】截至目前,国内外****通过不同方法获得了HO甘蓝型油菜种质。国际上,最早用甲基磺酸乙酯(ethylmethylsulfone,EMS)诱变油菜种子并在后代筛选出高油酸突变系,油酸含量最高可达88%[7,8]。此后,有报道用叠氮化钠诱变出高油酸油菜突变体[9]。国内****也重视HO材料创制并得到HO种质。和江明等[10]最早用EMS诱变处理油菜小孢子筛选出油酸含量为80.3%的高油酸突变体材料,随后多位****通过化学诱变得到高油酸突变体[11,12,13]。而物理方法主要是采用放射性射线辐照产生变异。运用这种方法成功获得高油酸种质的共有2例,一是利用60Co射线辐照油菜干种子后连续定向选择获得最高油酸含量达93.5%(近红外法测定)的株系;二是通过航天诱变方法获得一个高油酸含量(87.22%)的突变株系[13,14]。此外,转基因方法也可以获得高油酸种质。多位****利用RNAi技术获得了油酸含量超过80%的株系,但转基因种质因需要经过严格的释放前环境评审而不能较快在育种中得到应用,而且目前还未有成功释放的先例[15,16,17,18,19,20]。综合来看,采用非转基因方法得到的高油酸种质具有可即时应用的比较优势。不同****对油菜高油酸性状的遗传模式进行了探索。品系19782/7531的高油酸性状(C18:1=78.4%)由2个突变位点控制[21]。对高油酸亲本DMS100(C18:1=77%)与普通品系衍生的DH群体进行QTL定位后发现高油酸性状主要由1个主效位点控制[11]。HO自交系Y539(C18:1=87.22%)的高油酸性状是由分别位于A05和A01染色体上的2个主效基因控制[15]。H005的高油酸性状(C18:1=83.10%)由2对具有加性和显性效应的主效基因控制[22]。HO品系SW Hickory(C18:1=78%)的高油酸性状由位于A5染色体上1个主效QTL控制[23]。HO品系N1379T(C18:1=85%)的高油酸性状则是由位于A5和C5染色体上的2个位点共同控制[24]。【本研究切入点】尽管通过不同手段得到了HO种质,但控制其HO性状的遗传模式并非一致,表明这些种质在遗传上具有不同的机制。同时,已有HO种质的生理特性也未有深入研究。21世纪初江苏省农业科学院经济作物研究所油菜研究室利用辐射方法获得油菜脂肪酸组分的变异,并结合多种育种方法,最终成功创建一个全新的HO种质B161。【拟解决的关键问题】本研究通过对B161中HO性状的遗传模式及其油酸含量变化的生理特性进行研究,推进该种质的育种利用进程,进一步改良菜籽油品质,为提供高品质菜籽油奠定基础。1 材料与方法
1.1 材料
诱变原始材料为高世代油菜自交系L13-306-171,油酸(C18:1)与亚麻酸(C18:3)含量经近红外分析含量分别约为72.21%和4.18%(后期经气相色谱分析C18:1≈68%,C18:3≈7%),其系谱见傅寿仲等[25]。常规油菜品系N15、N27和N137为3个不同遗传背景的高世代育种自交系。以上材料均由江苏省农业科学院经济作物研究所提供。1.2 油菜HO种质的诱变及选育过程
选取L13-306-171干净一致的1 000粒种子(即M0代)排列在培养皿内湿润滤纸上于25℃暗培养,每皿100粒种子,共10皿。待胚根萌动露白后送至扬州市辐照中心进行800 Gray 60Co-?射线辐照(辐照剂量参考徐华军等[26])。辐射处理后的萌动种子继续发芽后栽于大田。2004年在辐射当代(即M1代)选择优势单株套袋自交收获种子。测定脂肪酸组分后按油酸含量从高到低选择30株单株种子作为基础选择群体(即M2代)。从每个单株自交种子中分别取等量种子混合种植。随后连续3年重复上年度的方法获得M5代基础群体。在本世代选择质量较好的种子进行半粒法脂肪酸含量测定。2007年秋选取油酸含量>80%的对应半粒种子在室内发芽后栽于大田形成M6代。2008年继续选择油酸高值单株种植于大田,于2009年春季选择单株花蕾进行小孢子培养得到若干DH株系(M7代),选取油酸含量较高且亚麻酸含量较低的DH系作为候选株系。次年经全生育期观察比较后筛选最优HO株系。1.3 高油酸性状的遗传群体构建
以中选的HO种质B161(P1,C18:1=(85.36±0.50)%)和常规油菜品系N15、N27和N137亲本(P2)种植于江苏省农业科学院溧水植物科学基地油菜育种田,2014年春季杂交分别获得3个杂交组合的共6个正反交F1。2015年将各亲本自交,F1自交得到F2分离群体种子,同时F1与2个亲本分别回交得到BC1代的足量种子(BC1P1和BC1P2)。1.4 低温与常温下HO品系与常规品系的组织样品制备
将B161和N137的干净种子在培养皿中发芽后至下胚轴长约0.5 cm后,移栽至直径为15 cm且装满营养土的盆钵中,每品系6钵,每钵4株苗,置于光照培养箱(25℃ 12 h光照/15℃12 h黑暗,光照强度= 2 000 lx)内生长。于三叶期时将对应品系盆钵分成2部分:一半继续放置在原培养箱;另一半置于另一培养箱(12℃ 12 h光照/6℃ 12 h黑暗,光照强度=2 000 lx)内培养。待7 d后将盆钵取出并选取各培养箱内生长一致的菜苗,迅速洗净根系,用剪刀分离根(下胚轴以下部分)、茎(去掉最后一片叶连接点以上部分)、叶(包括去掉主叶脉后的整张叶片)和叶柄等组织,分别将两品系不同单株的相同组织混合置于牛皮纸袋中编号,烘干备用。1.5 油菜种子成熟及种子发芽过程的样品制备
于大田油菜开花后选择长势均衡、花期一致的B161和N137植株,用棉线标记主轴及倒一、倒二分枝同一天开放的花朵,每个材料标记约10株,每株标记约100朵花,一周后再标记一次作为重复。由于早期的种子含水量很大,故而自角果龄15日开始每7天取样一次,直至种子成熟。大田中角果置于冰盒中带至实验室后,迅速将幼嫩种子从角果中小心剥出,每品系每个样品至少选取100粒种子,及时烘干编号备用。发芽试验按照粮油检验发芽试验(GB/T 5520-2011)的方法进行,选取B161和N137的饱满种子自开始之日起每天于同一时间剥出发芽种子中的子叶,直至第5天成苗。取样时迅速用镊子剥取子叶部分,每品系每个样品至少选取50个子叶组织,混合后立即烘干编号备用。
1.6 取样及其脂肪酸含量测定
用于遗传分析的6个世代群体种子,按照亲本(P1、P2)、F1、BC1P1、BC1P2和F2分别取出粒数不等的种子,按照高建芹等[27]的半粒法测定种子脂肪酸含量。各个组织样品按照国标(GB/T17377-1998)的方法在安捷伦GC 6910型气相色谱仪上测定脂肪酸含量。由于根、茎、叶和叶脉组织的含油量低,因此,其干样至少保证在0.5 g以上。在加石油醚-乙醚混合液之前,样品需要充分打样磨碎。1.7 数据分析、图像绘制以及遗传模型分析
采用Microsoft Excel计算最大值、最小值、平均值、变异系数及相关系数。采用Graphpad Prism7软件进行绘图。根据作物数量性状混合遗传模型及主基因+多基因多世代联合分析方法,采用章元明等[28]发布的植物数量性状软件包(the R software package of SEgregation analysis,SEA)对3个不同遗传背景的6个世代分析群体的高油酸性状进行24个遗传模型的极大似然分析,采用最小AIC值准则筛选最适遗传模型,用最小二乘法估计相应的遗传参数,并估计主基因和多基因效应等遗传参数。2 结果
2.1 诱变群体各世代油酸与亚麻酸的含量变化及HO种质B161的获得
采用辐射诱变后发现后续世代油酸和亚麻酸含量变异范围明显增加,筛选效果明显。自诱变当代到脂肪酸含量稳定的7个世代,油酸和亚麻酸含量在定向选择过程中均经历了由集中到分散然后又集中的变化趋势(表1)。对2004年(M1代)套袋自交收的单株种子进行脂肪酸分析发现,油酸含量变幅为68.75%—78.81%,亚麻酸含量变幅为3%—8%,表明诱变产生效果。经过4个世代(M2—M5)对油酸进行极端选择后,油酸含量变幅为70.54%—86.95%,但平均值大幅升高。进一步于M6代对极端单株进行小孢子培养后获得具有65个株系的DH系群体,根据脂肪酸测定结果筛选得到油酸含量≥85%且亚麻酸含量为3%—4%的多个株系。次年经全生育期生长势及农艺性状比较后获得HO稳定品系B161(C18:1为85.4%,C18:3为2.5%)。Table 1
表1
表1诱变群体不同世代中C18:1及C18:3含量分布
Table 1
| 年度 Year | 世代 Generation | 样本数 No. of sample | C18:1 | C18:3 | ||||
|---|---|---|---|---|---|---|---|---|
| 平均值 Mean | 变幅 Range (%) | 变异系数 CV (%) | 平均值 Mean | 变幅 Range (%) | 变异系数 CV (%) | |||
| 2004 | M1 | 201 | 73.44 | 68.75—78.81 | 2.19 | 5.38 | 3.20—7.91 | 20.24 |
| 2005 | M2 | 578 | 73.97 | 56.14—84.90 | 4.50 | 6.02 | 3.52—8.97 | 32.63 |
| 2006 | M3 | 248 | 75.44 | 65.50—85.99 | 5.42 | 4.30 | 1.87—8.48 | 36.91 |
| 2007 | M4 | 217 | 78.76 | 69.36—87.19 | 9.69 | 3.92 | 1.57—14.02 | 45.04 |
| 2008 | M5 | 208 | 83.30 | 70.54—86.95 | 4.99 | 3.10 | 1.98—7.95 | 38.68 |
| 2009 | M6 | 65 | 81.23 | 73.51—85.47 | 2.36 | 4.00 | 2.54—6.38 | 14.20 |
新窗口打开|下载CSV
2.2 HO种质B161高油酸性状的遗传模式
2.2.1 遗传群体中全脂肪酸组分含量的性状变异 成功利用HO种质B161与3个常规品系构建了6个世代分离群体,其中,组合B161×N137的全脂肪酸含量情况如表2。正反交F1的油酸与亚麻酸含量值基本相当,表明HO性状不受细胞质效应的影响。3个组合衍生的6个世代分离群体油酸含量呈连续性分布,符合数量性状的特征。另外,油酸含量在3个群体中呈正态分布,表明该性状在遗传上受多基因控制。Table 2
表2
表2组合B161×N137的6个世代遗传群体脂肪酸含量统计
Table 2
| 世代 Generation | 籽粒数 No. of seeds | 脂肪酸 Fatty acid | ||||||
|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | C22:1 | ||
| P1 | 16 | 3.10±0.31 | 2.10±0.32 | 85.36±0.50 | 5.03±0.21 | 2.52±0.11 | 1.25±0.13 | 0.20±0.05 |
| P2 | 17 | 3.61±0.35 | 1.59±0.13 | 64.53±0.72 | 21.59±0.59 | 7.94±0.21 | 1.22±0.14 | 0.21±0.03 |
| F1(P1×P2) | 38 | 3.28±0.29 | 1.72±0.22 | 73.98±1.29 | 11.66±0.90 | 4.93±0.42 | 1.28±0.17 | 0.19±0.05 |
| F1(P2×P1) | 40 | 3.43±0.31 | 1.69±0.19 | 74.14±1.56 | 11.41±1.30 | 4.94±0.54 | 1.31±0.18 | 0.20±0.04 |
| BC1P1 | 95 | 3.36±0.39 | 1.86±0.20 | 79.57±4.52 | 9.30±4.07 | 3.89±1.01 | 1.33±0.16 | 0.29±0.16 |
| BC1P2 | 98 | 3.40±0.35 | 1.53±0.18 | 70.84±3.61 | 15.11±3.40 | 6.90±1.24 | 1.32±0.11 | 0.27±0.15 |
| F2 | 198 | 3.21±0.43 | 1.63±0.24 | 74.95±5.20 | 12.68±4.48 | 5.34±1.37 | 1.38±0.19 | 0.25±0.20 |
新窗口打开|下载CSV
2.2.2 菜籽油中各脂肪酸含量的相关性分析 对组合B161×N137的F2分离群体单株的脂肪酸含量进行了相关性分析(表3),结果表明,在各十八碳脂肪酸中油酸含量除了与亚油酸和亚麻酸含量具有显著负相关以外,还与棕榈酸和二十碳烯酸显著相关,而与硬脂酸含量相关较小。
Table 3
表3
表3组合B161×N137的F2群体中各脂肪酸含量的相关系数
Table 3
| 脂肪酸 Fatty acid | 棕榈酸C16:0 | 硬脂酸C18:0 | 油酸C18:1 | 亚油酸C18:2 | 亚麻酸C18:3 | 二十碳烯酸C20:1 | 芥酸C22:1 |
|---|---|---|---|---|---|---|---|
| C16:0 | 1 | -0.106 | -0.474** | 0.409** | 0.222** | -0.371** | 0.029 |
| C18:0 | 1 | 0.029 | -0.037 | -0.143* | 0.033 | -0.010 | |
| C18:1 | 1 | -0.943** | -0.541** | 0.204** | -0.062 | ||
| C18:2 | 1 | 0.303** | -0.330** | -0.007 | |||
| C18:3 | 1 | -0.092 | -0.005 | ||||
| C20:1 | 1 | 0.178* | |||||
| C22:1 | 1 |
新窗口打开|下载CSV
2.2.3 HO种质B161高油酸含量性状的遗传分析 利用SEA软件对3个遗传分析群体油酸含量数据分别计算所有的遗传模型,获得各个遗传模型的最大似然值以及AIC值。根据最小AIC的遗传模型优选准则,选取各遗传群体中3个最小AIC值的遗传模型作为备选(表4)。结果显示,油酸含量大部分符合2对主效基因的遗传模型,但不同遗传群体估算的基因互作效应略有差别。油酸含量的最佳遗传模型为2MG-A,即2对加性主基因模型。
Table 4
表4
表43个组合的候选遗传模型及其极大对数似然函数值和AIC值
Table 4
| 组合 Combination | C18:1 | ||
|---|---|---|---|
| 备选模型 Model | 极大似然值 Max-likelihood-value | AIC值 AIC value | |
| B161×N137 | 2MG-ADI | -597.9275 | 1215.855 |
| 2MG-EA | -607.3177 | 1220.635 | |
| 1MG-A | -607.3178 | 1220.636 | |
| B161×N27 | 2MG-A | -586.1464 | 1180.293 |
| 2MG-ADI | -582.3989 | 1184.798 | |
| 1MG-AD | -590.8457 | 1189.691 | |
| B161×N15 | 2MG-A | -576.5989 | 1161.198 |
| 1MG-AD | -582.3780 | 1172.756 | |
| 2MG-EAD | -583.5032 | 1173.006 | |
新窗口打开|下载CSV
对筛选得到的最佳遗传模型估计了遗传参数(表5)。结果显示,控制高油酸含量的2对主效基因在3个组合遗传群体中体现出的效应值均较为接近,每个主效基因可提高油酸含量4—6个百分点。
Table 5
表5
表5最适遗传模型下3个杂交组合中油酸含量的遗传参数估计
Table 5
| 一阶参数 Univalent parameter | 估计值 Estimate value | ||
|---|---|---|---|
| B161×N137 | B161×N27 | B161×N15 | |
| 第1对主基因的加性效应da | 5.6587 | 4.7107 | 4.3096 |
| 第2对主基因的加性效应db | 4.7263 | 6.3145 | 4.2901 |
| 第1对主基因的显性效应ha | / | / | / |
| 第2对主基因的显性效应hb | / | / | / |
新窗口打开|下载CSV
2.3 HO种质B161与常规品系苗期营养器官中油酸与亚麻酸含量的比较
不同温度下HO品系与常规品系苗期组织中油酸与亚麻酸含量具有显著变化(表6)。常规品系根、茎、叶及叶柄等组织中的油酸含量在常温和低温下没有差异;但HO品系各组织在常温下油酸含量均较常规品系高,低温使各组织油酸含量显著降低,以叶柄降幅最大。无论在低温或常温下常规品系各组织的亚麻酸含量均高于HO品系,低温使HO品系各组织的亚麻酸含量显著升高。Table 6
表6
表6不同温度下HO品系与常规品系苗期不同组织中油酸与亚麻酸的含量
Table 6
| 脂肪酸 Fatty acid | 材料 Lines | 处理 Treat | 根 Root | 茎 Stem | 叶 Leaf | 叶柄 Petiole |
|---|---|---|---|---|---|---|
| C18:1 | B161 | 常温 Normal temperature | 47.56±2.77a | 37.34±2.37a | 29.99±2.21a | 55.72±3.08a |
| 低温 Low temperature | 38.87±2.33b | 23.72±2.28b | 14.28±2.74b | 20.37±2.42b | ||
| N137 | 常温 Normal temperature | 12.67±1.90c | 15.90±1.53c | 10.48±1.52c | 7.16±0.85c | |
| 低温 Low temperature | 12.34±1.61c | 17.25±1.67c | 11.69±1.75c | 8.61±1.14c | ||
| C18:3 | B161 | 常温 Normal temperature | 18.89±1.35d | 28.17±2.26d | 30.55±1.96d | 14.41±1.43d |
| 低温 Low temperature | 25.56±2.65c | 33.31±2.22c | 38.37±2.33c | 36.92±1.27c | ||
| N137 | 常温 Normal temperature | 33.53±3.37b | 41.92±2.72b | 45.86±2.07b | 43.02±2.72b | |
| 低温 Low temperature | 49.56±3.13a | 51.91±3.45a | 52.81±1.99a | 53.75±2.38a |
新窗口打开|下载CSV
2.4 HO种质B161油酸与亚麻酸含量的变化特征
2.4.1 种子成熟过程中HO种质B161与常规品系油酸与亚麻酸的积累 HO品系B161与常规油菜品系N137在花后至种子成熟过程中油酸和亚麻酸含量的增减趋势一致,但含量具有显著差异。两品系油酸含量在此段时期内均表现为先迅速增长而后缓慢增长的趋势。B161在花后第15天油酸含量即达到约50%,至第35天达到约80%后仍继续缓慢增长;整个过程高于常规品系20个百分点。两品系亚麻酸含量在此过程中均表现为持续降低趋势,在花后15 d时两者差异不大,但在此后差距持续拉大(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1花后HO品系和常规品系种子中油酸与亚麻酸的含量变化
Fig. 1Changes of oleic acid and linolenic acid contents in seeds of HO and normal lines after pollination
2.4.2 B161与常规品系子叶中油酸与亚麻酸在发芽进程中的代谢 HO品系与常规品系子叶中油酸与亚麻酸的相对含量在发芽过程中的变化(图2)具有明显差异。在种子萌发后5 d之内,两品系子叶的油酸含量均呈持续下降趋势,下降速率接近,两品系差异值基本维持在20个百分点左右。相反的,在此进程中,两品系亚麻酸含量均呈持续上升趋势,但两品系差异经历了由小到大再变小的过程。
图2
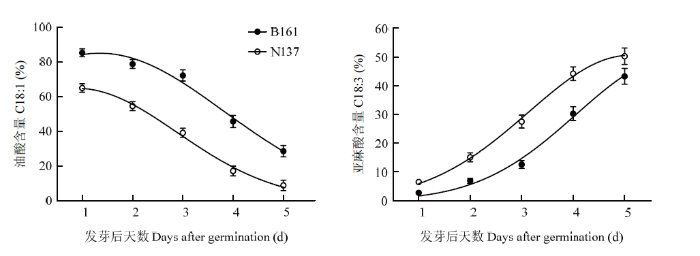 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2发芽后HO品系和常规品系子叶中油酸与亚麻酸的含量变化
Fig. 2Changes of oleic acid and linolenic acid contents in cotyledons of HO and normal lines after germination
3 讨论
诱变是创建油菜遗传变异材料的有效方法。目前,已报道的非转基因油菜HO种质均通过诱变获得,但诱变方法以及诱变对象不尽相同。大部分HO材料都是通过化学诱变油菜种子获得的[7,9,11-13,20-21,29],也有通过诱变小孢子获得[10],而利用辐射处理油菜种子得到高油酸种质的案例较少[14,15]。本研究亦是采用辐射诱变,但在辐射对象上与前人有所不同。本研究辐射对象是处于萌动露白状态的油菜种子。此种状态下油菜种子中各项生理生化活动刚刚启动,对外界环境极度敏感[30]。从理论上说辐射处理具有较高的诱变率。本研究中在早期世代(M2代)即发现了油酸含量显著升高的株系,不但说明创建思路是可行的,而且也表明突变位点位于控制HO性状的主效基因内,同时这些主效基因可能位于相对容易诱变的染色体区域。此外,由于辐射诱变可能造成油菜染色体变异,若持续采用系谱法选择可能多代自交仍不能纯合稳定,因此,本例中采用小孢子培养技术迅速固定高油酸性状,效果很好,这与LORIN等[9]使用的方法一致。本研究获得的HO品系油酸和亚麻酸含量均与现有报道中的极端值相当,表明所集成的诱变及选择方法在油菜上具有实用性,对其他的类似性状可能也具有借鉴价值。菜籽油中的油酸含量主要受遗传控制,环境影响较小[21,31]。本研究也发现HO新种质高油酸性状的遗传贡献率高,与前人结果一致。在控制该性状的基因数目上,研究结果有所不同。HU等[11]、官春云等[14]、、YANG等[23]利用QTL定位或基因克隆等方法发现高油酸性状受一个主效基因位点控制。而SCHIERHOLT等[15]和刘列钊等[21]分别利用遗传试验和分子标记筛选表明高油酸性状主要受2个主效位点控制。随后WELLS等[29]利用EMS诱变获得了一批油菜减饱和酶突变体,其不同位点突变对油酸含量的增量效应不同。ZHAO等[32]还发现在A9染色体也有一个位点控制油酸含量。这表明油菜中油酸含量的控制位点较为丰富。本研究中的高油酸新种质虽然也受2对基因控制,但其基因位点需要进一步确认。从育种实践而言,本研究创建的新种质高油酸性状遗传模式相对简单,育种利用较为容易。自2010年开始将HO种质的高油酸性状转育进入萝卜质不育系统并进行了杂交组合配置工作,品比试验发现一些组合的全生育期表现和产量与对照品种相当,表明本研究发现的高油酸种质具有育种利用潜力(电子附表1)。
脂肪酸是植物细胞质膜的主要组成成分,是细胞与环境互作的前哨,因此,脂肪酸的合成与代谢对油菜的非生物胁迫抗性至关重要[33,34,35]。油菜生育期长,无论是冬油菜还是春油菜在营养生长阶段都会受到温度影响。本研究发现,常温下HO种质营养器官以及种子中的油酸含量均高于常规品系,而亚麻酸含量则低于常规品系,表明(1)HO种质中控制油酸的基因是组成型表达,可能是油菜中2个组成型表达的BnFAD2发生了突变。(2)油菜的脂肪酸合成途径中油酸有2个去向,绝大部分进入减饱和途径生成亚油酸和亚麻酸,少部分进入加碳途径生成二十碳烯酸和芥酸[36]。基于油酸与亚麻酸之间的强烈负相关关系,亚麻酸降低是油酸升高的必然结果。也有可能是HO种质中亚麻酸合成酶也产生突变,导致该酶活性降低,不能合成更多的亚麻酸。本研究还发现,低温下HO种质油酸含量降低和亚麻酸含量增加,但均低于常规品系,这表明(1)低温能够增加突变油酸控制基因的表达,提高油酸的转化率,这与BnFAD2的特性符合[37]。(2)亚麻酸是高不饱和脂肪酸,易于氧化。其含量的增加,可能是油菜进行呼吸作用时更偏好于将亚麻酸氧化从而为其自身提供足够能量;也有可能是因为亚麻酸合成酶活性的变化所致。另外,发现HO种质与常规品系在种子成熟过程和发芽过程中的亚麻酸含量并非持续保持平行差异,说明在HO种质中除了油酸含量变化影响亚麻酸含量之外,亚麻酸本身的合成控制酶也在起作用。值得提出的是,低温下常规品系较HO种质能合成更多的高不饱和度脂肪酸,这意味着常规品系在低温下细胞膜具有更好的流动性,在表型上则可能具有更强的耐寒性。因此可以推断,在较寒冷的油菜产区,HO品系可能耐寒性较常规品系差,需要关注。
4 结论
获得双低甘蓝型油菜HO新种质B161,其高油酸性状受2个主效基因控制。B161中控制高油酸性状的基因为组成型表达,低温对其有诱导增强作用。高油酸种质B161具有育种利用潜力。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0233959URLPMID:32497146 [本文引用: 1]

Development of oilseed rape (Brassica napus L.) breeding lines producing oil characterized by high oleic and low linolenic acid content is an important goal of rapeseed breeding programs worldwide. Such kind of oil is ideal for deep frying and can also be used as a raw material for biodiesel production. By performing chemical mutagenesis using ethyl methanesulfonate, we obtained mutant winter rapeseed breeding lines that can produce oil with a high content of oleic acid (C18:1, more than 75%) and a low content of linolenic acid (C18:3, less than 3%). However, the mutant lines revealed low agricultural value as they were characterized by low seed yield, low wintering, and high content of glucosinolates in seed meal. The aim of this work was to improve the mutant lines and develop high-oleic and low-linolenic recombinants exhibiting both good oil quality and high agronomic value. The plant materials used in this study included high-oleic and low-linolenic mutant breeding lines and high-yielding domestic canola-type breeding lines of good agricultural value with high oleic acid content and extremely low glucosinolates content. Field trials were conducted in four environments, in a randomized complete block design. Phenotyping was performed for wintering, yield of seed and oil, and seed quality traits. Genotype x environment interaction was investigated with respect to the content of C18:1 and C18:3 acids in seed oil. Genotyping was done for the selection of homozygous high oleic and low linolenic lines using allele-specific CAPS markers and SNaPshot assay, respectively. Finally, new high oleic and low linolenic winter rapeseed recombinant lines were obtained for use as a starting material for the development of new varieties that may be of high value on the oil crop market.
DOI:10.1002/fsn3.560URLPMID:29564101 [本文引用: 1]

In this study, antioxidative activities of aqueous extract of Biarum bovei (BBE) in stabilizing of canola oil during storage at 20 degrees C was evaluated. For this purpose, the total phenolic (TP), flavonoids (TFC), and tocopherol content (TTC) of the extract were determined and beta-carotene bleaching system was used to assess the antioxidant efficacy of BBE. The amount of TP, TFC, and TTC in BBE indicated high antioxidant activity. So, different concentrations (0, 200, 800, and 1400 ppm) of BBE and butylatedhydroxyanisole (BHA; 100 ppm), were added to canola oil for 60 days at 20 degrees C. Peroxide value (PV), carbonyl value (CV), Total polar compounds (TPC), acid value (AV), iodine values (IV), and conjugated dienes (CD) were employed to evaluate the BBE effect on canola oil stabilizing. Results indicated that 1,400 ppm of BBE exhibited stronger antioxidant activity in canola oil than BHA.
[本文引用: 2]
[本文引用: 1]
[P].
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
DOI:10.1007/s00122-006-0315-1URLPMID:16767448 [本文引用: 4]

The quality of canola oil is determined by its constituent fatty acids such as oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3). Most canola cultivars normally produce oil with about 55-65% oleic acid and 8-12% linolenic acid. High concentrations of linolenic acid lead to oil instability and off-type flavor, while high levels of oleic acid increase oxidative stability and nutritional value of oil. Therefore, development of canola cultivars with increased oleic acid and reduced linolenic acid is highly desirable for canola oil quality. In this study, we have mapped one locus that has a major effect and one locus that has a minor effect for high oleic acid and two loci that have major effects for low linolenic acid in a doubled haploid population. The major locus for high C18:1 was proven to be the fatty acid desaturase-2 (fad2) gene and it is located on the linkage group N5; the minor locus is located on N1. One major QTL for C18:3 is the fatty acid desaturase-3 gene of the genome C (fad3c) and it is located on N14. The second major QTL resides on N4 and is the fad3a gene of the A genome. We have sequenced genomic clones of the fad2 and fad3c genes amplified from an EMS-induced mutant and a wild-type canola cultivar. A comparison of the mutant and wild-type allele sequences of the fad2 and fad3c genes revealed single nucleotide mutations in each of the genes. Detailed sequence analyses suggested mechanisms by which both the mutations can cause altered fatty acid content. Based on the sequence differences between the mutant and wild-type alleles, two single nucleotide polymorphism (SNP) markers, corresponding to the fad2 and fad3c gene mutations, were developed. These markers will be highly useful for direct selection of desirable fad2 and fad3c alleles during marker-assisted trait introgression and breeding of canola with high oleic and low linolenic acid.
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
URL [本文引用: 3]
URL [本文引用: 3]
URL [本文引用: 4]
URL [本文引用: 4]
URLPMID:11171263 [本文引用: 1]

Genetic engineering methods have been used successfully to modify the fatty acid profile of elite Australian germplasm of Brassica napus and B. juncea. Co-suppression plasmids carrying oleate desaturase genes from each species have been constructed and transferred into Australian elite breeding lines of B. napus and B. juncea using Agrobacterium tumifaciens plant-transformation techniques. Modifications to existing Brassica transformation protocols and the use of an intron-interrupted hygromycin-resistance gene as the selectable marker have resulted in improved transformation efficiencies. Silencing of the endogenous oleate desaturase genes has resulted in substantial increases in oleic acid levels, up to 89% in B. napus and 73% in B. juncea.
URLPMID:17167203 [本文引用: 1]

A seed-specific Fad2 gene expression cassette, which is free-marker gene and with sense and antisense structure, was constructed by using the promoter and terminator of rape seed storage protein cruciferin gene. Transgenic rape plants without selection marker genes were obtained by Agrobacterium-mediated transformation. The oleic acid content of transgenic plant seeds is 83.9%, which is nearly the same as that of Brassica napus with double Fad2 mutation (85%). The result of RT-PCR analysis shows that the raising of oleic acid content may be due to the degradation of Fad2 mRNA induced by co-transformation of sense-antisense Fad2 gene. These transgenic plants with high oleic acid trait grew normally and without the disadvantageous agronomic traits such as weak cold resistance, tardy development, death of buds and low rate of seed setting caused by Fad2 inactivation in mutant Brassica napus plants. This work would serve as a good base for breeding of more lines with high oleic acid content.
URLPMID:17167203 [本文引用: 1]

A seed-specific Fad2 gene expression cassette, which is free-marker gene and with sense and antisense structure, was constructed by using the promoter and terminator of rape seed storage protein cruciferin gene. Transgenic rape plants without selection marker genes were obtained by Agrobacterium-mediated transformation. The oleic acid content of transgenic plant seeds is 83.9%, which is nearly the same as that of Brassica napus with double Fad2 mutation (85%). The result of RT-PCR analysis shows that the raising of oleic acid content may be due to the degradation of Fad2 mRNA induced by co-transformation of sense-antisense Fad2 gene. These transgenic plants with high oleic acid trait grew normally and without the disadvantageous agronomic traits such as weak cold resistance, tardy development, death of buds and low rate of seed setting caused by Fad2 inactivation in mutant Brassica napus plants. This work would serve as a good base for breeding of more lines with high oleic acid content.
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 2]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00122-012-1863-1URLPMID:22534790 [本文引用: 2]

Modification of oleic acid (C18:1) and linolenic acid (C18:3) contents in seeds is one of the major goals for quality breeding after removal of erucic acid in oilseed rape (Brassica napus). The fatty acid desaturase genes FAD2 and FAD3 have been shown as the major genes for the control of C18:1 and C18:3 contents. However, the genome structure and locus distributions of the two gene families in amphidiploid B. napus are still not completely understood to date. In the present study, all copies of FAD2 and FAD3 genes in the A- and C-genome of B. napus and its two diploid progenitor species, Brassica rapa and Brassica oleracea, were identified through bioinformatic analysis and extensive molecular cloning. Two FAD2 genes exist in B. rapa and B. oleracea, and four copies of FAD2 genes exist in B. napus. Three and six copies of FAD3 genes were identified in diploid species and amphidiploid species, respectively. The genetic control of high C18:1 and low C18:3 contents in a double haploid population was investigated through mapping of the quantitative trait loci (QTL) for the traits and the molecular cloning of the underlying genes. One major QTL of BnaA.FAD2.a located on A5 chromosome was responsible for the high C18:1 content. A deleted mutation in the BnaA.FAD2.a locus was uncovered, which represented a previously unidentified allele for the high oleic variation in B. napus species. Two major QTLs on A4 and C4 chromosomes were found to be responsible for the low C18:3 content in the DH population as well as in SW Hickory. Furthermore, several single base pair changes in BnaA.FAD3.b and BnaC.FAD3.b were identified to cause the phenotype of low C18:3 content. Based on the results of genetic mapping and identified sequences, allele-specific markers were developed for FAD2 and FAD3 genes. Particularly, single-nucleotide amplified polymorphisms markers for FAD3 alleles were demonstrated to be a reliable type of SNP markers for unambiguous identification of genotypes with different content of C18:3 in amphidiploid B. napus.
DOI:10.3389/fgene.2018.00399URLPMID:30294343 [本文引用: 1]

Rapeseed (Brassica napus L.) is a vital oil crop worldwide. High oleic acid content is a desirable quality trait for rapeseed oil, which makes it more beneficial to human health. However, many germplasm resources with high oleic acid content in rapeseed have not been evaluated with regard to their genotypes, making it difficult to select the best strains with this trait for the breeding of high oleic acid rapeseed variety. This work was to explore the gene-regulation mechanism of this trait using a new super-high oleic acid content ( approximately 85%) line N1379T as genetic material. In this study, the sequences of four homologous fatty acid desaturase (BnFAD2) genes were compared between super-high ( approximately 85%, N1379T) and normal ( approximately 63%) oleic acid content lines. Results showed that there were two single-nucleotide polymorphisms (SNPs) in BnFAD2-1 and BnFAD2-2, respectively, which led to the amino acid changes (E106K and G303E) in the corresponding proteins. Functional analysis of both genes in yeast confirmed that these SNPs were loss-of-function mutations, thus limiting the conversion of oleic acid to linoleic acid and resulting in the considerable accumulation of oleic acid. Moreover, two specific cleaved amplified polymorphic sequences (CAPS) markers for the two SNPs were developed to identify genotypes of each line in the F2 and BC1 populations. Furthermore, these two mutant loci of BnFAD2-1 and BnFAD2-2 genes were positively associated with elevated oleic acid levels and had a similar effect with regard to the increase of oleic acid content. Taken together, these two novel SNPs in two different BnFAD2 genes jointly regulated the high oleic acid trait in this special germplasm. The study provided insight into the genetic regulation involved in oleic acid accumulation and highlighted the use of new alleles of BnFAD2-1 and BnFAD2-2 in breeding high oleic acid rapeseed varieties.
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]

The dormant seeds of three varieties of double-low rape were treated with 0,80,100,120,140 and 160 krad of γ-ray.The dose-effect curves of survival rate,yield,length of pod,rate of bearing pods and oil content in rapeseed fit the equation:y= Exp (-Ax-Bx2).The dose-effect curves of field germination rate,seed bearing rate,number of seeds per pod and chromosome aberration rate fit the equation:y = A + Bx.The dose-effect curve of morphological malformation rate fit the equation:y = AeBx The content's of erucic acid,linolenic acid,glucosino-lates and protein in rapeseed,changed irregularly with the increase of doses of gamma irradiation.Close correlation were found among the effects of irradiation on morphological,cytological and biochemical characters.The radiosensitivities of rape varieties were apparently different.
URL [本文引用: 1]

The dormant seeds of three varieties of double-low rape were treated with 0,80,100,120,140 and 160 krad of γ-ray.The dose-effect curves of survival rate,yield,length of pod,rate of bearing pods and oil content in rapeseed fit the equation:y= Exp (-Ax-Bx2).The dose-effect curves of field germination rate,seed bearing rate,number of seeds per pod and chromosome aberration rate fit the equation:y = A + Bx.The dose-effect curve of morphological malformation rate fit the equation:y = AeBx The content's of erucic acid,linolenic acid,glucosino-lates and protein in rapeseed,changed irregularly with the increase of doses of gamma irradiation.Close correlation were found among the effects of irradiation on morphological,cytological and biochemical characters.The radiosensitivities of rape varieties were apparently different.
URL [本文引用: 1]

运用Agilent 6890N GC对具有不同脂肪酸组分的油菜样品,采用半粒、单粒和混合样3种测试方法进行分析,建立适合于油菜籽各脂肪酸组分含量的定量测试程序.结果表明:在本研究设定的分析条件下,二阶程序升温条件下对油菜7种主要脂肪酸的检测效果好于恒温条件,而测试效率则恒温条件高于二阶程序升温条件下.重复性和再现性试验结果显示:在二阶程序升温和恒温条件下半粒种子、单粒种子和混合样3种方法测试的脂肪酸含量大于5%的组分和小于5%的组分重复间绝对误差、相对误差均小于国家标准,表明这3种方法重复性、再现性好,能满足不同品质材料、不同检测要求的全脂肪酸组分的定量分析需要.
URL [本文引用: 1]

运用Agilent 6890N GC对具有不同脂肪酸组分的油菜样品,采用半粒、单粒和混合样3种测试方法进行分析,建立适合于油菜籽各脂肪酸组分含量的定量测试程序.结果表明:在本研究设定的分析条件下,二阶程序升温条件下对油菜7种主要脂肪酸的检测效果好于恒温条件,而测试效率则恒温条件高于二阶程序升温条件下.重复性和再现性试验结果显示:在二阶程序升温和恒温条件下半粒种子、单粒种子和混合样3种方法测试的脂肪酸含量大于5%的组分和小于5%的组分重复间绝对误差、相对误差均小于国家标准,表明这3种方法重复性、再现性好,能满足不同品质材料、不同检测要求的全脂肪酸组分的定量分析需要.
[本文引用: 1]
DOI:10.1007/s11032-013-9954-5URLPMID:24489479 [本文引用: 2]

Many important plant species have polyploidy in their recent ancestry, complicating inferences about the genetic basis of trait variation. Although the principal locus controlling the proportion of polyunsaturated fatty acids (PUFAs) in seeds of Arabidopsis thaliana is known (fatty acid desaturase 2; FAD2), commercial cultivars of a related crop, oilseed rape (Brassica napus), with very low PUFA content have yet to be developed. We showed that a cultivar of oilseed rape with lower than usual PUFA content has non-functional alleles at three of the four orthologous FAD2 loci. To explore the genetic basis further, we developed an ethyl methanesulphonate mutagenised population, JBnaCAB_E, and used it to identify lines that also carried mutations in the remaining functional copy. This confirmed the hypothesised basis of variation, resulting in an allelic series of mutant lines showing a spectrum of PUFA contents of seed oil. Several lines had PUFA content of ~6 % and oleic acid content of ~84 %, achieving a long-standing industry objective: very high oleic, very low PUFA rapeseed without the use of genetic modification technology. The population contains a high rate of mutations and represents an important resource for research in B. napus.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
URLPMID:31037811 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.plantsci.2015.11.003URLPMID:26795146 [本文引用: 1]

The demand for plant-derived oils has increased substantially over the last decade, and is sure to keep growing. While there has been a surge in research efforts to produce plants with improved oil content and quality, in most cases the enhancements have been small. To add further complexity to this situation, substantial differences in seed oil traits among years and field locations have indicated that plant lipid biosynthesis is also influenced to a large extent by multiple environmental factors such as temperature, drought, light availability and soil nutrients. On the molecular and biochemical levels, the expression and/or activities of fatty acid desaturases, as well as diacylglycerol acyltransferase 1, have been found to be affected by abiotic factors, suggesting that they play a role in the lipid content and compositional changes seen under abiotic stress conditions. Unfortunately, while only a very small number of strategies have been developed as of yet to minimize these environmental effects on the production of storage lipids, it is clear that this feat will be of the utmost importance for developing superior oil crops with the capability to perform in a consistent manner in field conditions in the future.
[本文引用: 1]
[本文引用: 1]
