 ,1, 徐瑞2,3, 李梅1, 田甲春1, 李守强1, 程建新1, 田世龙
,1, 徐瑞2,3, 李梅1, 田甲春1, 李守强1, 程建新1, 田世龙 ,1
,1Regulation Mechanism of Carvone on Seed Potato Sprouting
GE Xia ,1, XU Rui2,3, LI Mei1, TIAN JiaChun1, LI ShouQiang1, CHENG JianXin1, TIAN ShiLong
,1, XU Rui2,3, LI Mei1, TIAN JiaChun1, LI ShouQiang1, CHENG JianXin1, TIAN ShiLong ,1
,1通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-04-8接受日期:2020-09-4网络出版日期:2020-12-01
| 基金资助: |
Received:2020-04-8Accepted:2020-09-4Online:2020-12-01
作者简介 About authors
葛霞,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1658KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
葛霞, 徐瑞, 李梅, 田甲春, 李守强, 程建新, 田世龙. 香芹酮对马铃薯种薯发芽的调控机制[J]. 中国农业科学, 2020, 53(23): 4929-4939 doi:10.3864/j.issn.0578-1752.2020.23.017
GE Xia, XU Rui, LI Mei, TIAN JiaChun, LI ShouQiang, CHENG JianXin, TIAN ShiLong.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】马铃薯易于栽培,营养丰富,在膳食结构中占有独特的地位,是全球最重要、最普及的粮菜兼用作物[1]。马铃薯从田间收获后,块茎进入生长相对暂停的休眠状态,但随着生理老化和环境条件的变化,休眠被逐渐打破,块茎开始发芽[2]。发芽会加快马铃薯生理老化、失水、腐烂,从而造成品质劣变等巨大的贮藏损失[3]。特别是种薯休眠过早打破或发芽延迟,都会导致马铃薯产量的下降[4]。低温贮藏(2—4℃)常用于延长种薯的休眠期,但维持低温贮藏条件不但增加了贮藏成本,且一旦过了休眠期,即使低温也不会抑制种薯的发芽,因此,在生产实践中常采用化学方法来抑制发芽。氯苯胺灵(Chlorpropham,CIPC)是目前最常用的马铃薯抑芽剂[5],一直被用于商品薯的长期贮藏,因其抑芽作用的不可逆,不能将其用于种薯的抑芽。为了解决种薯长期贮藏发芽的问题,探索开发天然、绿色安全,既可用于种薯贮藏抑芽又能满足生产要求发芽的抑芽活性化合物已成为目前国内外的研究热点。【前人研究进展】植物精油如薄荷、茉莉、留兰香、香菜、柠檬草、香茅精油和丁香精油等及其活性成分包括香芹醇、香芹酚、橙花醇、香叶醇、香芹酮等,可抑制或调控马铃薯发芽[6,7,8,9,10]。其中香芹酮对马铃薯发芽具有显著的抑制作用[11,12],一些欧洲国家已将其作为商业化的马铃薯抑芽剂(TalentTM),且获得欧盟批准并列入欧盟农药管理条例的附录Ⅰ[11]。香芹酮在自然界中天然存在两种同分异构体,S-(+)-香芹酮是葛缕子、莳萝籽油的主要成分,R-(-)-香芹酮是留兰香、薄荷精油的主要成分,这两种同分异构体对马铃薯发芽都有抑制作用,但S-(+)-香芹酮更易被马铃薯吸收且能更快地抑制马铃薯发芽[13]。笔者课题组前期曾探讨了S-(+)-香芹酮不同处理对‘青薯9号’微型薯贮藏期间发芽调控和田间种植效果的影响,研究发现,使用S-(+)-香芹酮0.3 mL?kg-1(药剂体积/块茎重量)的处理剂量,以及距种植前进行停药4—6周的处理方式,对种薯贮藏期间芽长的调控、抑制腐烂及田间种植效果最佳[14]。【本研究切入点】虽然已有关于香芹酮及以其为主要成分的精油对马铃薯发芽抑制效果的报道,但关于S-(+)-香芹酮对块茎发芽调控的作用机制报道较少。OOSTERHAVEN等[15]研究发现S-(+)-香芹酮在抑制马铃薯发芽的同时,能促进3-羟基-3-甲基戊二酰基辅酶A还原酶(HMG-CoA还原酶)的降解,但S-(+)-香芹酮是如何通过影响HMG-CoA还原酶活性调控块茎发芽的机制尚未见报道。【拟解决的关键问题】研究S-(+)-香芹酮处理对种薯发芽调控过程中芽分生组织变化、内源激素、丙二醛(MDA)、脯氨酸(PRO)和抗氧化酶活性的影响,从激素调控和膜脂过氧化方面探讨香芹酮对种薯发芽调控的作用机制,为其在种薯上的实际应用提供理论依据。1 材料与方法
试验于2018年9月至2019年4月在甘肃省农业科学院农产品贮藏加工研究所保鲜与加工实验室进行。1.1 材料与试剂
供试马铃薯材料为‘青薯9号’微型薯,由定西市农业科学研究院提供;S-(+)-香芹酮(以下简称香芹酮,含量≥96%),购于Alfa Aesar Co., Ltd.;吲哚乙酸(IAA)、赤霉素(GA3)和脱落酸(ABA)标样、用于组织包埋切片用的石蜡均为美国Sigma公司产品;色谱纯甲醇、乙腈和乙酸为天津光复化学试剂公司生产;环保型浸蜡脱蜡透明液购于北京索莱宝科技有限公司;包埋盒底膜为江苏世泰实验器材有限公司生产;液相色谱测试用水为超纯水,其余均使用二次蒸馏水。1.2 仪器与设备
高效液相色谱仪(L-1200型、配备Agilent G1356D MWD紫外检测器,Agilent,USA);全波长酶标仪(Multiskan GO 1510型,Thermo,Vantaa Finland);紫外-可见分光光度计(Cary-100型,Agilent,USA);透明塑料密闭贮藏箱(50 cm×37 cm×27 cm,武汉市康博顺家居用品有限公司,中国);迷你风扇(9 cm×9 cm,德鑫旺工具商行,中国);数显卡尺(SF2000,桂林广陆数字测控股份有限公司,中国);烤片机(DB-B1型,常州国华电器有限公司,中国);切片机(RM2235型,Leica,德国);体视显微镜(SZX9型,Olympus Tokyo,日本);显微镜(BX51型,Olympus Tokyo,日本)。1.3 处理方法与试验设计
‘青薯9号’微型薯采收于2018年9月25日,在微型薯培育大棚内自然晾干,于10月中旬运至马铃薯恒温贮藏库,在温度12—18℃、相对湿度85%—95%的环境下,预处理2周。种薯完全木栓化后,按照不同的试验处理进行分组,每组种薯为10.0 kg。香芹酮处理在自制内循环种薯贮藏箱中进行。使用透明塑料箱、迷你风扇按图1所示组装,模拟具有内循环通风系统的马铃薯贮藏库,营造试验贮藏的微环境。吸取香芹酮3.0 mL(每kg种薯香芹酮处理剂量为0.3 mL)分散滴加在自制内循环微型薯贮藏箱底部的滤纸上,距箱底1/3处加透气隔板,然后将10.0 kg微型薯置于隔板上,密闭贮藏箱,接通风扇电源,使药剂在箱内循环20 min,同时以不做任何药剂处理为对照。每个处理3组重复。对照和处理贮藏箱分开放置,贮藏温度为(10±1)℃。贮藏期间管理方法为:密闭贮藏7 d后开盖通风,之后每2 d开盖通风一次,每次通风10 min,以保证种薯足够的氧气交换。通风后再接通风扇电源10 min,使箱内的香芹酮气体分布均匀。贮藏18周时进行6周的停药处理,停药处理方法为:将各处理微型薯装于网袋中置于通风干燥处充分散除药剂并继续贮藏至24周。贮藏期间马铃薯块茎芽长大于2 mm 时判定为发芽,发芽率为发芽块茎数占块茎总数的百分比,当发芽率达到50%时判定为萌芽[16];当块茎平均芽长在(5±0.5)mm,且发芽率大于95%时,为种薯的最佳种植时期。根据预试验结果,分别选择在贮藏0、6(对照萌芽时)、18(处理停药时)、20(处理萌芽时)和24周(处理达到适宜种植条件时)时测定对照组和处理组种薯平均芽长、失重率、吲哚乙酸(IAA)、脱落酸(ABA)、赤霉素(GA3)、MDA、PRO的含量,超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、多酚氧化酶(PPO)的活性。此外,采用上述香芹酮处理方法另外处理种薯3组,用于种薯芽分生组织的观察试验,每10个种薯装入1个小网袋,方便拿取,采用组织学分析法观察种薯不同时期芽组织的变化情况。图1
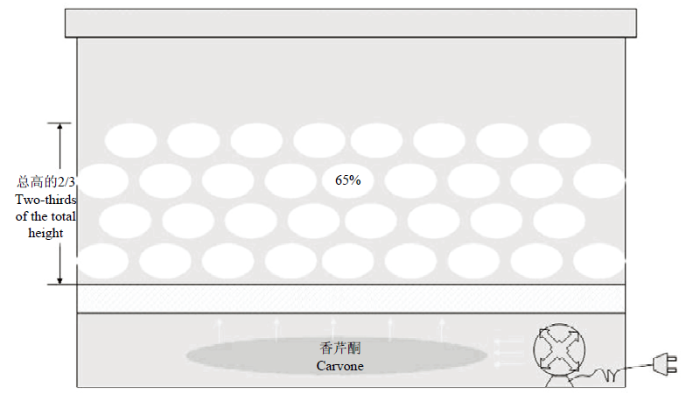 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1自制贮藏箱示意图(65%为微型薯体积占贮藏箱体积的百分数)
Fig. 1The schematic of self-made storage box (65% refers to the percentage of the volume of mini tuber potatoes to the volume of storage box)
1.4 指标测定与方法
1.4.1 平均芽长及失重率的测定 平均芽长的测定:每组随机取30个种薯进行测定,使用游标卡尺测量每个种薯顶芽的长度,测量后取其平均值。失重率的测定:每组处理固定10个种薯进行测定,分别测定小网袋中种薯的初始质量(g1)与贮藏一段时间后的质量(g2)。
$失重率(\%)=\frac{g1-g2}{g1}\times 100$
1.4.2 种薯芽分生组织的观察 分别采集未经香芹酮处理及香芹酮处理开始后1—7 d、停药后1—7 d种薯的芽分生组织若干,进行石蜡切片前处理:每次每组随机取1个小网袋的种薯,在体视镜下使用单面刀片纵切块茎的芽眼部位(以芽眼为中心,直径2—3 mm),然后将组织放入FAA固定液(10%甲醛,5%冰乙酸,50%乙醇)中,在4℃条件下保存。根据KAMENETSKY[17]描述的方法处理样品,并进行少许修正。对前处理固定好的组织更换一次新鲜的FAA固定液,然后逐步在浓度增加的乙醇(50%,70%,83%,95%和100%)中进行脱水,再用浓度逐渐增加的二甲苯(25%,50%,75%和100%)逐渐取代乙醇脱水剂,之后将组织浸入1:1(v/v)的石蜡+二甲苯混合溶液中,在42℃不断加入石蜡粒直至饱和,升温至58℃,更换融化好的纯石蜡,每4—6 h更换一次,共更换3次。设置烤片机温度70℃,将组织用融化好的石蜡在包埋盒底膜中进行包埋过夜。之后将包有组织的蜡块修整好固定在小木块上,注意芽组织的方向,调整切片厚度为7 μm,并在切片机上进行切片。将切好的切片涂布在载玻片上进行展片,然后逐步在脱蜡透明液、一系列不同浓度乙醇(100%,95%,83%,70%,50%和30%)、蒸馏水中进行脱蜡处理,之后分别在1%(w/v)番红水溶液和0.2%(w/v)固绿水溶液中染色10 min和2 min,最后使用配有相机的BX51显微镜以40—100×放大倍数对组织进行观察并拍照。
1.4.3 生化测定取样 分别在贮藏的0、6、18、20和24周,随机取对照组和处理组的种薯20个,使用打孔器取以种薯芽眼为中心,直径1 cm、长1 cm的块茎样品5 g,用锡箔纸包好后迅速用液氮冷冻,在-80℃超低温冰箱中保存备用。
1.4.4 内源激素 ABA、IAA、GA3含量的提取与测定 称取约0.1 g冷冻样品放入研钵中加液氮磨碎,加入1.0 mL甲醇/水/冰醋酸(80:20:1,V/V/V)混合溶液,4℃浸提过夜。8 000×g离心10 min,离心后取出上清液,氮吹将有机溶剂去除。用0.1 mol?L-1柠檬酸-柠檬酸钠缓冲溶液调pH至2—3,用乙酸乙酯萃取3次,合并乙酸乙酯相,氮吹至干。最后用甲醇定容至0.5 mL,使用针头式过滤器(0.22 μm)过滤于带有内衬管的样品瓶内待测。ABA、IAA、GA3的含量采用高效液相色谱法(HPLC)进行测定。色谱柱为Kromasil C18反相色谱柱(250 mm×4.6 mm,5 μm),流动相为甲醇:乙酸水溶液(1.0%)=40:60(V/V),测试条件为:紫外检测器,波长254 nm,流速0.8 mL?min-1,柱温35℃,走样时间40 min。
1.4.5 MDA和PRO含量的测定 MDA含量采用硫代巴比妥酸法[18]进行测定,PRO含量采用磺酸性茚三酮法[19]进行测定。
1.4.6 抗氧化酶SOD、CAT、POD、PPO活性的测定 SOD活性采用核黄素-NBT光化还原法[20]进行测定,CAT活性采用H2O2紫外吸收法[21]进行测定,POD活性采用愈创木酚比色法[22]进行测定,PPO活性采用邻苯二酚比色法[19]进行测定。
1.5 数据分析
数据采用Microsoft Excel 2003 软件进行分析与作图。采用SPSS Statistics 19.0软件进行差异显著性分析。2 结果
2.1 香芹酮处理对马铃薯种薯贮藏期间芽长和失重率的影响
种薯贮藏6周时,对照种薯处于萌芽状态,平均芽长为2.61 mm;贮藏18周时,对照发芽率和芽长分别为100%和32.26 mm,芽长显著高于处理芽长(0.91 mm)(P<0.05),此时香芹酮处理的种薯发芽率为0并仍处于休眠期;随后进行停药处理并继续贮藏至20周时,处理种薯处于萌芽状态,其芽长为2.16 mm;贮藏24周时,对照芽长达51.02 mm,而处理芽长仅为4.77 mm且发芽率达96.67%,说明香芹酮处理可调控种薯的发芽和芽长,使处理芽长达到适宜种植的条件(图2-a)。贮藏期间,对照和处理种薯的失重率均逐渐升高(图2-b),而处理的失重率显著低于对照(P<0.05)。在贮藏18周和24周时,处理失重率分别比对照降低了46.84% 和49.76%。说明香芹酮处理可降低马铃薯种薯贮藏期间的失重率。图2
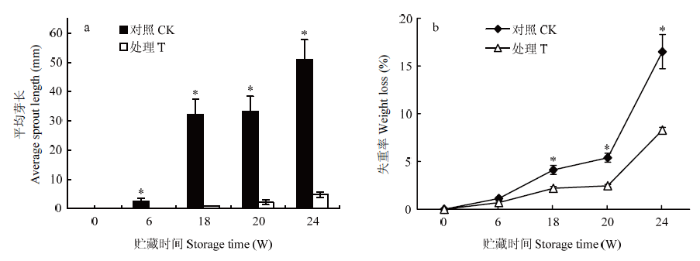 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2香芹酮处理对种薯贮藏期间平均芽长(a)和失重率(b)的影响
*代表处理间显著性差异(P<0.05)。下同
Fig. 2Effects of carvone treatment on sprout length (a) and weight loss (b) of seed potato during storage
* indicate significant differences (P<0.05). The same as below
2.2 香芹酮处理对种薯芽分生组织的影响
显微观察发现,未进行香芹酮处理的种薯芽眼分生组织保持着生长活力,但尚未开始萌动,芽尖因空气氧化而导致轻微的坏死(图3-a)。经香芹酮处理2 d后,芽分生组织末端和维管组织变黑(图3-b),并在接下来的几天芽分生组织逐渐坏死,5—7 d后整个顶端分生组织完全坏死(图3-c)。此时在种薯芽眼处肉眼可见黑色的小芽,经测量这些芽的芽长不到2 mm。图3-d为香芹酮处理停药3 d后,随着香芹酮药效的散除,在坏死的顶芽分生组织旁边腋芽开始生长。停药7 d后,在坏死的顶芽周围逐渐长出对称的腋芽(图3-e)。图3-f为处理停药14 d即贮藏20周时香芹酮处理的种薯,可肉眼观察到腋芽的萌发,每个芽眼处的腋芽生长速度一致。贮藏24周时,可见对照种薯发芽呈现明显的顶端优势(图3-g),而处理的种薯发芽不存在对照这样明显的顶端优势(图3-h)。图3
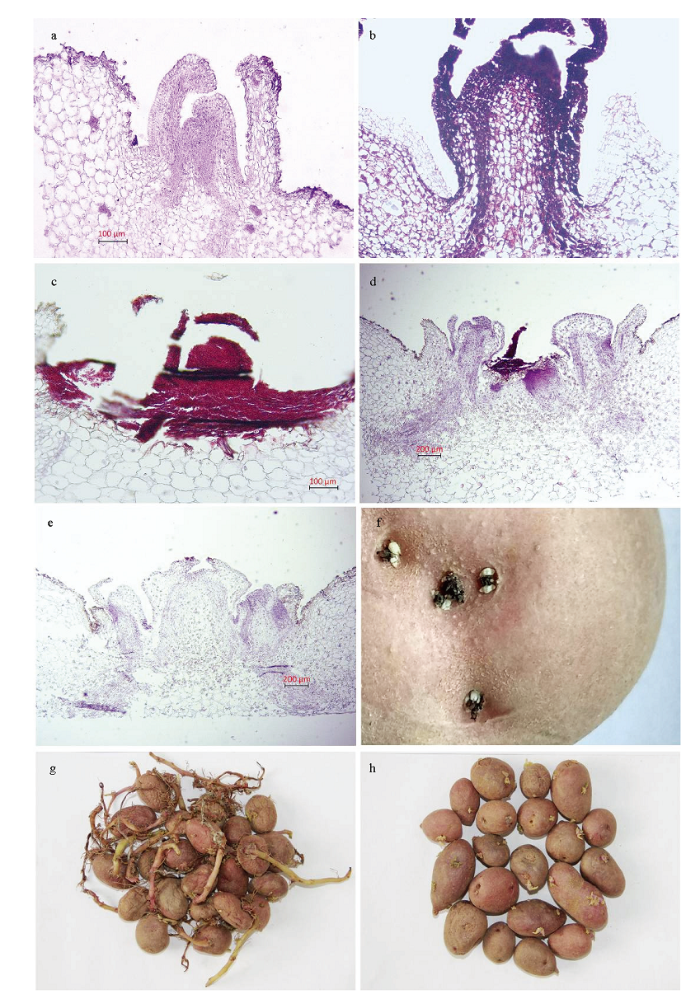 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3香芹酮处理对种薯顶芽分生组织和发芽的影响
a:未处理前种薯芽眼处分生组织;b:香芹酮处理2 d后芽的分生组织;c:香芹酮处理7 d后芽的分生组织;d:香芹酮处理停药3 d后在坏死的顶芽分生组织旁边,腋芽开始生长;e:香芹酮处理停药7 d后在坏死的顶芽周围逐渐长出对称的腋芽;f:香芹酮停药14 d即贮藏20周时香芹酮处理的种薯;g:贮藏24周时对照;h:香芹酮处理的种薯。比例尺为100 μm(a、b、c),200 μm(d、e)
Fig. 3Effects of carvone treatment on seed potato apical meristem and sprouting
a: Untreated meristem; b: Bud meristem treated with carvone 2 days after treatment; c: Bud meristem treated with carvone 7 days after treatment; d: Axillary bud began grow nearby necrotic apical meristem 3 days after carvone withdrawal; e: Axillary bud growth continues around the necrotic apical meristem 7 days after carvone withdrawal; f: Seed potatoes with carvone treatment stored for 20 weeks; g: Control seed potatoes stored for 24 weeks; h: Seed potatoes with carvone treatment stored for 24 weeks. Scale bar is 100 μm (a, b, c), 200 μm (d, e)
2.3 香芹酮处理对种薯贮藏期间内源ABA、IAA和GA3含量的影响
ABA被认为对块茎休眠有维持作用。贮藏期间,对照和香芹酮处理的种薯ABA含量随着贮藏期的延长呈整体下降趋势,且处理组ABA含量显著高于对照(P<0.05)(图4-a)。对照ABA含量在贮藏6周时相比贮藏初始下降了52.23%,说明种薯萌芽后,ABA含量迅速下降。贮藏0—18周,香芹酮处理种薯的ABA含量一直较高且变化平稳,贮藏18周时,香芹酮处理的ABA含量是对照的1.97倍(P<0.05);经停药后,香芹酮处理的ABA含量出现快速降低的趋势,与图2-a结果一致,此时处理组薯芽开始萌发。IAA对休眠打破后芽的生长有调控作用。从图4-b可以看出,贮藏期间,对照和香芹酮处理种薯IAA的含量呈现整体增加的趋势,且香芹酮处理IAA含量显著低于对照(P<0.05)。在贮藏6周和18周,香芹酮处理IAA含量比对照分别降低了40.97%和42.22%;进行香芹酮停药处理后IAA含量则呈现快速增加的趋势,贮藏20周和24周,香芹酮处理IAA的含量仅比对照分别降低17.32%和9.37%。图4-c为香芹酮处理对种薯GA3含量的影响,从图中可以看出对照和香芹酮处理种薯GA3的含量呈先增加后降低的趋势。对照在贮藏6周时种薯GA3含量达到峰值(0.83 μg?g-1 FW),而处理组GA3含量在贮藏20周时达到峰值(0.61 μg?g-1 FW),说明GA3在种薯萌发时含量较高,香芹酮处理推迟了GA3峰值的出现。块茎在休眠和萌芽过程中促进和抑制生长类植物激素之间存在着平衡关系,从图4-d看出,当(GA3+IAA)/ABA<1,即GA3+IAA<ABA时,抑制生长类激素占优势,种薯处于休眠期;当(GA3+IAA)/ABA>1,即GA3+IAA>ABA时,促进生长类激素占优势,种薯休眠被打破、开始萌芽,对照和处理组的(GA3+IAA)/ABA比值变化分别在贮藏6周和20周出现(GA3+IAA)/ABA>1的拐点,说明香芹酮处理通过改变激素水平调控种薯的发芽。图4
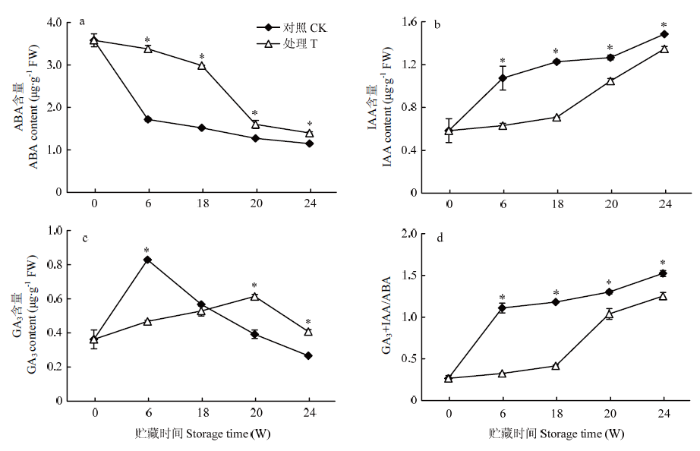 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4香芹酮处理对种薯贮藏期间内源ABA(a)、IAA(b)和GA3(c)含量以及ABA/(IAA+GA3)比值(d)的影响
Fig. 4Effect of carvone treatment on the content of endogenous IAA (a), ABA (b) and GA3 (c), and ABA/(IAA+GA3) in seed potato during storage
2.4 香芹酮处理对种薯贮藏期间MDA和PRO含量的影响
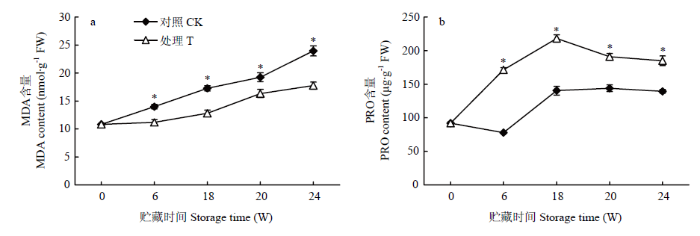
贮藏期间,对照和香芹酮处理种薯MDA含量随着贮藏时间的延长而逐渐增加(图5-a),处理种薯的MDA含量比对照低15.04%—25.90%(P<0.05),说明香芹酮处理降低了种薯中MDA的累积。从图5-b可以看出,贮藏期间,对照种薯PRO含量曲线呈先降低后增加然后趋于平缓的趋势,而香芹酮处理的种薯PRO含量呈现先升高后降低的趋势,说明种薯萌芽时PRO含量发生下降;此外,处理组种薯PRO的含量比对照显著增加了24.59%—54.45%(P<0.05),说明香芹酮处理提高了种薯中PRO的含量。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5香芹酮处理对种薯贮藏期间MDA(a)和PRO(b)含量的影响
Fig. 5Effects of carvone treatment on the content of MDA (a) and PRO (b) in seed potato during storage
2.5 香芹酮处理对种薯贮藏期间SOD、CAT、POD和PPO活性的影响
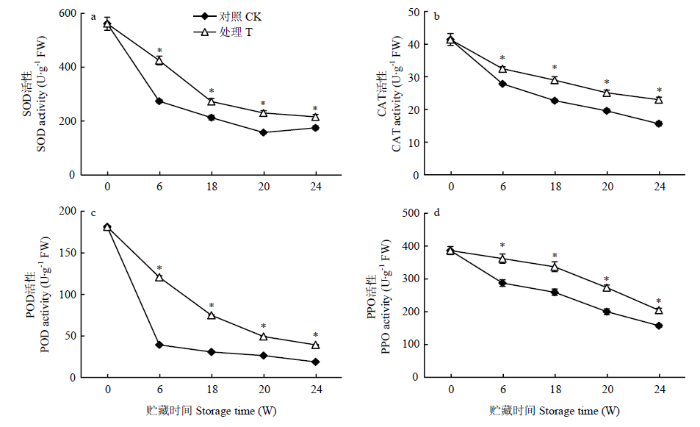
贮藏期间,对照和香芹酮处理种薯的SOD活性呈总体下降趋势(图6-a),说明随着贮藏时间的延长,块茎逐渐发生生理老化,SOD不断抵御O2-而降低了其活性。总体来说,香芹酮处理种薯的SOD活性比对照高18.86%—35.56%(P<0.05),香芹酮处理提高了种薯的SOD活性。对照和香芹酮处理种薯的CAT活性随着贮藏时间的延长而呈整体降低趋势,且香芹酮处理种薯的CAT活性比对照高14.34%—31.97%(P<0.05),说明香芹酮处理增加了种薯的CAT活性,使CAT清除H2O2能力较对照增强(图6-b)。贮藏6周时,对照和香芹酮处理种薯的POD活性分别比贮藏初始时降低了78.31%和33.47%,说明对照种薯萌芽后POD活性迅速下降,而香芹酮处理种薯的POD活性是对照的1.87—3.07倍(P<0.05),说明香芹酮处理抑制了POD活性的降低(图6-c)。贮藏期间,对照和香芹酮处理种薯的PPO活性呈现整体下降趋势,香芹酮处理种薯的PPO活性比对照高26.00%—36.41%(P<0.05),说明香芹酮处理提高了种薯的PPO活性(图6-d)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6香芹酮处理对种薯SOD(a)、CAT(b)、POD(c)和PPO(d)活性变化的影响
Fig. 6Effects of carvone treatment on the activity of SOD (a), CAT (b), POD(c) and PPO(d) in seed potato during storage
3 讨论
HMG-CoA还原酶在植物生长发育中起着至关重要的作用,HMG-CoA还原酶是甲羟戊酸途径中催化限速反应的关键酶[23]。甲羟戊酸途径是生产大量异戊二烯及其衍生物的重要途径,从胆固醇生物合成到生长控制,它是多种细胞功能的重要组成部分。在植物中,甲羟戊酸途径对许多重要次生代谢物的产生非常重要,其中包括植物激素如脱落酸、赤霉素、细胞分裂素、膜成分和光合作用所需的成分等[24]。而植物激素被认为是调节马铃薯休眠与发芽最重要的内部因素[5],通过改变特定激素水平或调节这些激素的相对含量来调节块茎的休眠,块茎组织对特定激素的敏感性也随着生理衰老过程而改变[25]。ABA是马铃薯中提取到的一种复合体物质β2抑制物的内源成分之一,在休眠块茎中含量最高,并在贮藏过程中含量下降,它是诱导和维持马铃薯块茎休眠所必需的物质,通过干扰细胞壁的松弛来抑制细胞扩张从而抑制薯芽萌发[26]。IAA是马铃薯发芽生长所必需的物质,它能促进细胞扩张和细胞分裂[27];但它对休眠没有任何影响,只在打破休眠并发芽的块茎中增加[28]。GA3具有促进种薯发芽的作用,在块茎休眠时GA3含量很少,当休眠解除、开始萌芽时含量迅速增加,在控制随后的发芽生长方面发挥重要作用[29]。OOSTERHAVEN等[15]发现,香芹酮在抑制马铃薯发芽的同时会促进HMG-CoA还原酶的降解,并且这种作用具有可逆性。因此,根据本研究结果推断,香芹酮对种薯发芽调控的机制可能是:当香芹酮作用于种薯时,它通过抑制HMG-CoA还原酶促进了ABA的合成,阻碍了IAA和GA3的合成,从而抑制了种薯的发芽;当香芹酮作用移除后,HMG-CoA还原酶活性增加,抑制了ABA含量,提高了IAA和GA3的含量,使种薯休眠被解除。此外,从香芹酮对种薯芽分生组织的影响中观察到,未经香芹酮处理的种薯由于顶端优势的作用,芽眼中最先活跃出的是顶芽,而腋芽受到抑制。香芹酮作用于种薯2 d后就开始损伤顶芽分生组织末端和维管束组织,5—7 d就可以造成顶端分生组织的坏死,这与TEPER-BAMNOLKER等[4]的研究结果一致。一方面可能是香芹酮对上述激素调控的原因使顶芽生长速度受到抑制;另一方面是由于香芹酮分子结构决定了它亲脂性的生物活性,损伤了芽分生组织的细胞膜而造成顶芽的坏死,腋芽则受到激素调控而被抑制并仍然保持着发芽活力。在停药处理3 d后,观察到香芹酮作用减弱,通过甲羟戊酸途径改变了种薯内源激素水平,从而促进了腋芽的生长。活性氧在协助种子打破休眠进而促进发芽过程中发挥着关键作用。研究发现,多种作物种子发芽都跟活性氧的产生有密切关系[30,31]。普遍认为活性氧促进种子发芽的机理为活性氧可将ABA迅速降解,并促进基因的表达而大量合成GA,使种子打破休眠而促进发芽[32,33]。发芽过程中积累的过量活性氧可直接攻击膜系统中的不饱和脂肪酸,导致膜脂过氧化。MDA是膜脂过氧化最重要的产物之一,常被用来评价细胞膜系统受伤害的程度[34],其含量的增加是块茎发生衰老的标志。本研究结果表明,香芹酮处理能显著抑制种薯中MDA的积累,叶旭等[35]也发现薄荷醇和茉莉精油在抑制马铃薯发芽的同时,可降低马铃薯中MDA的含量。此外,本研究发现香芹酮处理提高了种薯中PRO的含量,PRO是植物体内最重要的渗透调节物质之一,PRO的增加可抵御植物体因过量活性氧积累导致的膜脂过氧化而引起膜功能受损或结构破坏。此外,COLEMAN等[36]曾研究指出,块茎中PRO的含量与ABA水平呈正相关,ABA可使马铃薯中PRO的含量累积。本研究发现,香芹酮处理提高了种薯中ABA的含量,PRO的含量也相应升高,这与COLEMAN等[36]的研究结果一致。
香芹酮处理可显著提高种薯中SOD、CAT、POD和PPO的活性。其中SOD、CAT和POD作为活性氧清除酶系统中3种重要的抗氧化酶,能有效地阻止活性氧的积累,因此,香芹酮处理能够维持休眠、控制种薯的芽长和防止膜脂过氧化。此外,POD是IAA侧链氧化酶,其活性与IAA含量呈反比,因此,POD也是调控块茎休眠与萌发生理代谢的关键酶之一[16]。贮藏期间,对照和香芹酮处理种薯的PPO活性逐渐降低,这与王鹏等[37]的研究结果一致。PPO是呼吸系统的末端氧化酶,能催化酚类物质的氧化分解,杨晓玲等[38]研究证明马铃薯块茎中含有促进发芽的酚类物质。本研究发现,香芹酮处理种薯的PPO活性显著高于对照,说明香芹酮处理减少了种薯中促进发芽的酚类物质,因此抑制了薯芽的生长。香芹酮所涉及的其他作用机理有待进一步研究。
4 结论
香芹酮处理可调控种薯的发芽和芽长,降低种薯失重率和膜脂过氧化程度,延缓种薯的生理老化。香芹酮通过促进/抑制甲羟戊酸途径中的HMG-CoA还原酶调节种薯中内源激素ABA、IAA和GA3的含量,损伤顶芽分生组织和维管束组织,调控了对种薯顶芽的抑制与腋芽的萌发。此外,香芹酮处理还可通过提高种薯的SOD、CAT和PPO的活性清除过量活性氧,以减少种薯中促进发芽的酚类物质,来达到调控种薯发芽和芽长的目的。(责任编辑 赵伶俐)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1111/j.1365-2621.2010.02455.xURL [本文引用: 1]

P>In this study, we found that pressure treatments of 100 MPa applied for 5 and 10 min inhibited the sprouting of potato tubers that were stored for 3 months before the pressure treatments, for at least 6 weeks at 18 degrees C. Less intense pressure treatments of 30 and 50 MPa for 5 and 10 min did not inhibit sprouting, but hindered sprout development, evaluated by sprout length, elongation rate and mass. Pressure treatments caused a higher inhibition of sprouting, when applied to tubers after 5 months of storage, before the application of the pressure treatments. For these tubers, pressure treatments at 50 and 100 MPa for 5 and 10 min inhibited sprouting for at least 6 weeks at 18 degrees C. For the same tubers, a pressure treatment at 30 MPa for 10 min already showed some inhibitory effect on sprouting and hindered sprout development. Pressure treatments show great potential to be used, as a nonthermal and chemical-free method, to control sprouting of potato tubers.
DOI:10.1007/s10142-008-0079-6URLPMID:18317824 [本文引用: 1]

Meristem dormancy in perennial plants is a developmental process that results in repression of metabolism and growth. The cessation of dormancy results in rapid growth and should be associated with the production of nascent transcripts that encode for gene products controlling for cell division and growth. Dormancy cessation was allowed to progress normally or was chemically induced using bromoethane (BE), and microarray analysis was used to demonstrate changes in specific transcripts in response to dormancy cessation before a significant increase in cell division. Comparison of normal dormancy cessation to BE-induced dormancy cessation revealed a commonality in both up and downregulated transcripts. Many transcripts that decrease as dormancy terminates are inducible by abscisic acid particularly in the conserved BURP domain proteins, which include the RD22 class of proteins and in the storage protein patatin. Transcripts that are associated with an increase in expression encoded for proteins in the oxoglutarate-dependent oxygenase family. We conclude that BE-induced cessation of dormancy initiates transcript profiles similar to the natural processes that control dormancy.
DOI:10.1016/s1360-1385(01)02020-9URLPMID:11495763 [本文引用: 1]
DOI:10.1007/s00425-010-1154-5URL [本文引用: 2]

Sprouting of potatoes during storage, due to tuber dormancy release, is associated with weight loss and softening. Sprout-preventing chemicals, such as chlorpropham (CIPC), can negatively impact the environment and human health. Monthly thermal fogging with mint (Mentha spicata L.) essential oil (MEO) inhibited sprouting in eight potato cultivars during large-volume 6-month storage: the tubers remained firm with 38% lower weight loss after 140days of storage. The sprout-inhibitory action may be nullified: treated tubers washed with water resumed sprouting within days, with reduced apical dominance. MEO application caused local necrosis of the bud meristem, and a few weeks later, axillary bud (AX) growth was induced in the same sprouting eye. MEO components analysis showed that 73% of its content is the monoterpene R-carvone. Tubers treated with synthetic R-carvone in equivalent dose, 4.5μll−1, showed an inhibitory effect similar to that of MEO. Surprisingly, 0.5μll−1 of MEO or synthetic R-carvone catalyzed AX sprouting in the tuber. To the best of our knowledge, this is the first report of an essential oil vapor inducing early sprouting of potato tubers. R-carvone caused visible damage to the meristem membrane at sprout-inhibiting, but not sprout-inducing doses, suggesting different underlying mechanisms. After 5days’ exposure to R-carvone, its derivatives transcarveol and neo-dihydrocarveol were found in buds of tubers treated with the inhibitory dose, suggesting biodegradation. These experiments demonstrate the potential of MEO vapor as an environmentally friendly alternative to CIPC in stored potatoes and as a research tool for the control of sprouting in plants.
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.foodchem.2019.01.079URLPMID:30744843 [本文引用: 1]

Use of harmful chemicals and expensive maintenance of cold-storage conditions for controlling sprouting are among the major problems in potato storage. Here, 20 essential oils (EOs) were tested for their sprouting-inhibiting and sprouting-inducing activities. Overall, treatments of lemon grass (LG) and clove (CL) oils could induce sprouting whereas palmarosa (PR) and ajwain (AZ) oils could inhibit sprouting of potato tubers at normal-room-temperature (25+/-2 degrees C) storage. Selected-EOs treatments affected sprouting by modulation of accumulation of reducing sugars, ethylene, and expression of genes involved in tuber-sprouting such as ARF, ARP, AIP and ERF. Surprisingly, 7-days AZ-treatments could inhibit sprouting for 30-days which was mediated via damaging apical meristem. However, LG- and CL-treated tubers could produce enhanced potato yield as well. Present work clearly demonstrates that selected-EOs can be used as a promising eco-friendly approach for inducing/inhibiting sprouting of potato tubers during potato storage and those enhancing sprouting can be used for enhancing productivity.
DOI:10.1007/BF02736098URL [本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11540-019-9415-6URL [本文引用: 2]
DOI:10.1016/0926-6690(95)00005-WURL [本文引用: 1]
DOI:10.1007/BF02357935URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S0176-1617(11)80195-1URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1086/297198URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/343425a0URLPMID:1967820 [本文引用: 1]

The mevalonate pathway produces isoprenoids that are vital for diverse cellular functions, ranging from cholesterol synthesis to growth control. Several mechanisms for feedback regulation of low-density-lipoprotein receptors and of two enzymes involved in mevalonate biosynthesis ensure the production of sufficient mevalonate for several end-products. Manipulation of this regulatory system could be useful in treating certain forms of cancer as well as heart disease.
DOI:10.1080/10409239991209237URLPMID:10333388 [本文引用: 1]
DOI:10.1111/j.1365-3040.2007.01692.xURLPMID:17617825 [本文引用: 1]

To gain greater insight into the mechanism of dormancy release in the potato tuber, an investigation into physiological and biochemical changes in tuber and bud tissues during the transition from bud dormancy (immediately after harvest) to active bud growth was undertaken. Within the tuber, a rapid shift from storage metabolism (starch synthesis) to reserve mobilization within days of detachment from the mother plant suggested transition from sink to source. Over the same period, a shift in the pattern of [U-(14)C]sucrose uptake by tuber discs from diffuse to punctate accumulation was consistent with a transition from phloem unloading to phloem loading within the tuber parenchyma. There were no gross differences in metabolic capacity between resting and actively growing tuber buds as determined by [U-(14)C]glucose labelling. However, marked differences in metabolite pools were observed with large increases in starch and sucrose, and the accumulation of several organic acids in growing buds. Carboxyfluorescein labelling of tubers clearly demonstrated strong symplastic connection in actively growing buds and symplastic isolation in resting buds. It is proposed that potato tubers rapidly undergo metabolic transitions consistent with bud outgrowth; however, growth is initially prevented by substrate limitation mediated via symplastic isolation.
DOI:10.1104/pp.77.3.676URLPMID:16664118 [本文引用: 1]

The physical mechanism of seed germination and its inhibition by abscisic acid (ABA) in Brassica napus L. was investigated, using volumetric growth (= water uptake) rate (dV/dt), water conductance (L), cell wall extensibility coefficient (m), osmotic pressure ( product operator(i)), water potential (Psi(i)), turgor pressure (P), and minimum turgor for cell expansion (Y) of the intact embryo as experimental parameters. dV/dt, product operator(i), and Psi(i) were measured directly, while m, P, and Y were derived by calculation. Based on the general equation of hydraulic cell growth [dV/dt = Lm/(L + m) (Delta product operator - Y), where Delta product operator = product operator(i) - product operator of the external medium], the terms (Lm/(L + m) and product operator(i) - Y were defined as growth coefficient (k(G)) and growth potential (GP), respectively. Both k(G) and GP were estimated from curves relating dV/dt (steady state) to product operator of osmotic test solutions (polyethylene glycol 6000).During the imbibition phase (0-12 hours after sowing), k(G) remains very small while GP approaches a stable level of about 10 bar. During the subsequent growth phase of the embryo, k(G) increases about 10-fold. ABA, added before the onset of the growth phase, prevents the rise of k(G) and lowers GP. These effects are rapidly abolished when germination is induced by removal of ABA. Neither L (as judged from the kinetics of osmotic water efflux) nor the amount of extractable solutes are affected by these changes. product operator(i) and Psi(i) remain at a high level in the ABA-treated seed but drop upon induction of germination, and this adds up to a large decrease of P, indicating that water uptake of the germinating embryo is controlled by cell wall loosening rather than by changes of product operator(i) or L. ABA inhibits water uptake by preventing cell wall loosening. By calculating Y and m from the growth equation, it is further shown that cell wall loosening during germination comprises both a decrease of Y from about 10 to 0 bar and an at least 10-fold increase of m. ABA-mediated embryo dormancy is caused by a reversible inhibition of both of these changes in cell wall stability.
[本文引用: 1]
DOI:10.1111/aab.1996.129.issue-3URL [本文引用: 1]
DOI:10.1007/BF02871767URL [本文引用: 1]

At harvest, potato (Solanum tuberosum L.) tubers are dormant and will not sprout. As the period of postharvest storage is extended, tuber dormancy is broken and sprout growth commences. The loss of tuber dormancy and onset of sprout growth is accompanied by numerous biochemical changes, many of which are detrimental to the nutritional and processing qualities of potatoes. Endogenous hormones have been proposed to play a significant role in tuber dormancy regulation. The involvement of all major classes of endogenous hormones in tuber dormancy is reviewed. Based on available evidence, it is concluded that both ABA and ethylene are required for dormancy induction, but only ABA is needed to maintain bud dormancy. An increase in cytokinin sensitivity and content appear to be the principal factors leading to the loss of dormancy. Changes in endogenous IAA and GA content appear to be more closely related to the regulation of subsequent sprout growth.
DOI:10.1093/aob/mcv098URLPMID:26271119 [本文引用: 1]

BACKGROUND: Reactive oxygen species (ROS) are considered to be detrimental to seed viability. However, recent studies have demonstrated that ROS have key roles in seed germination particularly in the release of seed dormancy and embryogenesis, as well as in protection from pathogens. SCOPE: This review considers the functions of ROS in seed physiology. ROS are present in all cells and at all phases of the seed life cycle. ROS accumulation is important in breaking seed dormancy, and stimulating seed germination and protection from pathogens. However, excessive ROS accumulation can be detrimental. Therefore, knowledge of the mechanisms by which ROS influence seed physiology will provide insights that may not only allow the development of seed quality markers but also help us understand how dormancy can be broken in several recalcitrant species. CONCLUSIONS: Reactive oxygen species have a dual role in seed physiology. Understanding the relative importance of beneficial and detrimental effects of ROS provides great scope for the improvement and maintenance of seed vigour and quality, factors that may ultimately increase crop yields.
[本文引用: 1]
DOI:10.1093/jxb/erq125URLPMID:20460363 [本文引用: 1]

H(2)O(2) is known as a signal molecule in plant cells, but its role in the regulation of aqbscisic acid (ABA) and gibberellic acid (GA) metabolism and hormonal balance is not yet clear. In this study it was found that H(2)O(2) affected the regulation of ABA catabolism and GA biosynthesis during seed imbibition and thus exerted control over seed dormancy and germination. As seen by quantitative RT-PCR (QRT-PCR), H(2)O(2) up-regulated ABA catabolism genes (e.g. CYP707A genes), resulting in a decreased ABA content during imbibition. This action required the participation of nitric oxide (NO), another signal molecule. At the same time, H(2)O(2) also up-regulated GA biosynthesis, as shown by QRT-PCR. When an ABA catabolism mutant, cyp707a2, and an overexpressing plant, CYP707A2-OE, were tested, ABA content was negatively correlated with GA biosynthesis. Exogenously applied GA was able to over-ride the inhibition of germination at low concentrations of ABA, but had no obvious effect when ABA concentrations were high. It is concluded that H(2)O(2) mediates the up-regulation of ABA catabolism, probably through an NO signal, and also promotes GA biosynthesis. High concentrations of ABA inhibit GA biosynthesis but a balance of these two hormones can jointly control the dormancy and germination of Arabidopsis seeds.
[本文引用: 1]
DOI:10.1016/j.postharvbio.2016.02.002URL [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1007/BF02852813URL [本文引用: 2]
DOI:10.3321/j.issn:1000-7091.2003.01.010URL [本文引用: 1]

By analysis of the enzymes related to dormancy, it was found that activi t ies of amylase and ac t ivi ties of amylophosphorylase in dormant microtubers were much lower than these in dormant released tubers. It can be concluded hat energy was suppl ied by activating the amylase and amylophosphorylase during dormancy release. Peroxide activity was much higher in dormant microtubers than that in dormant released tubers.High active Peroxidase can reduce the concen tration of IAA. Dormancy and dormancy releasing could be regulated by peroxidase since high act iveperoxidase reduced concentration of IAA which affected tuber dormant releasing.The resul ts also sho wed polyphenol oxidase act ivity was higher in dormant microtubers than that in dormant released tubers. Polyphenol oxidase activity decreased lightly during dormancy breaking of potato microtubers. It inferred that polyphenol oxidase re gulated the respiration metabol ism during potato tuber dormancy as it was the enzyme related to respi ration.
DOI:10.3321/j.issn:1000-7091.2003.01.010URL [本文引用: 1]

By analysis of the enzymes related to dormancy, it was found that activi t ies of amylase and ac t ivi ties of amylophosphorylase in dormant microtubers were much lower than these in dormant released tubers. It can be concluded hat energy was suppl ied by activating the amylase and amylophosphorylase during dormancy release. Peroxide activity was much higher in dormant microtubers than that in dormant released tubers.High active Peroxidase can reduce the concen tration of IAA. Dormancy and dormancy releasing could be regulated by peroxidase since high act iveperoxidase reduced concentration of IAA which affected tuber dormant releasing.The resul ts also sho wed polyphenol oxidase act ivity was higher in dormant microtubers than that in dormant released tubers. Polyphenol oxidase activity decreased lightly during dormancy breaking of potato microtubers. It inferred that polyphenol oxidase re gulated the respiration metabol ism during potato tuber dormancy as it was the enzyme related to respi ration.
[本文引用: 1]
[本文引用: 1]
