 ,, 崔会婷, 王珍, 张铁军, 龙瑞才, 杨青川, 康俊梅
,, 崔会婷, 王珍, 张铁军, 龙瑞才, 杨青川, 康俊梅 ,中国农业科学院北京畜牧兽医研究所,北京 100193
,中国农业科学院北京畜牧兽医研究所,北京 100193Cloning and Function Analysis of MsNST in Lignin and Cellulose Biosynthesis Pathway from Alfalfa
JIANG Xu ,, CUI HuiTing, WANG Zhen, ZHANG TieJun, LONG RuiCai, YANG QingChuan, KANG JunMei
,, CUI HuiTing, WANG Zhen, ZHANG TieJun, LONG RuiCai, YANG QingChuan, KANG JunMei ,Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193
,Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193通讯作者:
责任编辑: 林鉴非
收稿日期:2019-10-8接受日期:2019-12-26网络出版日期:2020-09-16
| 基金资助: |
Received:2019-10-8Accepted:2019-12-26Online:2020-09-16
作者简介 About authors
蒋旭,Tel:010-62816357;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (5370KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
蒋旭, 崔会婷, 王珍, 张铁军, 龙瑞才, 杨青川, 康俊梅. 紫花苜蓿MsNST的克隆及对木质素与纤维素合成的功能分析[J]. 中国农业科学, 2020, 53(18): 3818-3832 doi:10.3864/j.issn.0578-1752.2020.18.016
JIANG Xu, CUI HuiTing, WANG Zhen, ZHANG TieJun, LONG RuiCai, YANG QingChuan, KANG JunMei.
0 引言
【研究意义】紫花苜蓿是世界上最重要的豆科牧草之一,其产量和品质一直是苜蓿产业发展所关注的热点。影响苜蓿品质的主要因素有纤维素、木质素和粗蛋白含量以及能量等,其中木质素和纤维素含量是影响苜蓿消化率和营养价值的重要因素之一。木质素和纤维素是组成植物细胞壁的主要成分,占植物总生物量的绝大部分,对植物正常生长发育非常重要。对牧草而言,纤维素与木质素含量的多少决定牧草消化率和营养价值的高低,牧草木质化程度越高,其消化率越低,营养价值也越低[1,2]。因此,研究苜蓿细胞壁生物合成途径中关键基因的生物学功能,为揭示苜蓿纤维素与木质素合成的分子调控机制奠定基础,并为利用基因工程的方法改善紫花苜蓿品质提供参考。【前人研究进展】纤维素与木质素是由单体聚合而成的大分子有机物质,从单体的合成到聚合均有一系列基因参与完成,从而使植物细胞壁物质合成通路具有多基因调控的特性(图1)。通过对拟南芥等植物木质素、纤维素生物合成机制的研究表明,植物NST转录因子是次生细胞壁物质合成的总开关,对细胞壁生物合成起到关键的调控作用[3,4,5]。拟南芥中存在3个NST同源基因,NST1和NST2、NST3之间功能存在冗余,共同调控次生壁发育,NST1和NST2基因调节拟南芥花药内壁细胞次生壁发育,并对花药正常开裂起到关键作用[6];NST1、NST3调控木质部与束间纤维细胞次生壁的合成[4,5];ZHONG等研究拟南芥三突变体nst1nst2nst3,结果表明突变体茎木质部与纤维细胞次生壁完全缺失,造成植株倒伏[7],暗示NST对木质素和纤维素合成具有重要的调节作用。研究表明,NST转录因子主要通过激活靶基因启动子区SNBE元件(Secondary wall NAC binding element)调控基因表达[8]。降低拟南芥NST1和NST3表达量,次生壁物质合成关键基因的表达量受到了不同程度的下调[4,5]。例如,nst1nst3双突变体中4-香豆酸辅酶A连接酶基因(4CL1),咖啡酰辅酶连接酶基因(COMT1),纤维素合酶亚基基因(CesA7, CesA8)等参与次生壁木质素与纤维素物质合成基因表达量显著下调[5]。单子叶植物玉米中过表达ZmNST3/4后能够激活一系列纤维素与木质素相关合成基因CesA4、CesA9、4CL、HCT的表达,从而调控玉米次生壁纤维素与木质素合成[9]。植物次生细胞壁物质合成也会受到激素信号的诱导[10]。赤霉素(gibberellin,GA)是调控植物生长发育的重要激素之一,GA通过诱导植物体内酚类化合物等次生代谢来调节木质素的合成[11]。研究表明,GA信号通路的主要调控方式是通过诱导DELLA蛋白降解完成的[12]。水稻中GA通过解除DELLA蛋白OsSLR1对NAC29/31、MYB103L等次生壁转录调控因子的抑制作用,从而激活NAC-MYB次生壁合成通路,促进纤维素合成[13,14]。水杨酸(salicylicacid,SA)是植物内源激素,对长春花幼苗外源施加水杨酸能够增加PAL、POD酶活性,导致细胞壁木质化程度提高,增强植物的抗逆性,以抵御不良环境胁迫[15]。生产实践中用多效唑(paclobutrazol,PCB)作为一种植物生长调节剂来增加茎秆木质素含量,增强机械强度,防止倒伏[16]。小麦苗期用PCB处理,小麦株高降低,基部茎秆细胞壁加厚,木质素与纤维含量上升,增强了小麦的抗逆性[17]。由此可见,外源激素和植物生长调节剂可诱导木质素和纤维素合成相关基因的表达,对促进植物细胞壁合成有重要的调节作用。【本研究切入点】NST转录因子已在拟南芥、玉米和杨树等植物中开展大量研究,结果表明NST通过激活植物次生壁木质素和纤维素合成途径中一系列相关基因的表达,从而对木质素和纤维素合成起重要的调节作用。紫花苜蓿是优质饲草,木质素和纤维素含量是影响苜蓿消化率和营养价值的主要因素之一,然而苜蓿中关于NST转录因子调控木质素与纤维素合成的分子机制鲜有报道。【拟解决的关键问题】本研究以紫花苜蓿为研究对象,克隆了MsNST转录因子,分析了其在外源激素诱导下的表达模式;通过在拟南芥和苜蓿中超表达MsNST,解析了该基因对苜蓿细胞壁中纤维素和木质素合成以及总糖含量的影响,为揭示细胞壁物质合成的分子机制奠定基础。图1
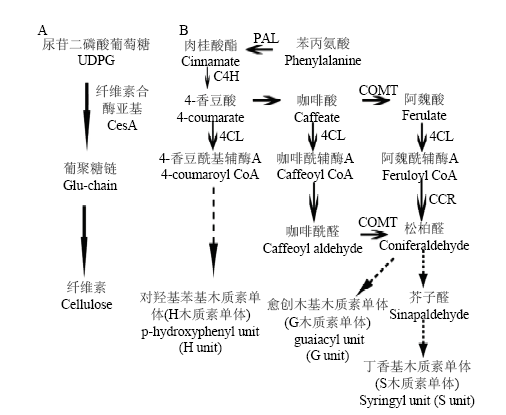 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1纤维素与木质素合成途径示意图
A.纤维素合成示意图;B.木质素单体合成示意图,CesA:纤维素合酶亚基 PAL:苯丙氨酸解氨酶,C4H:肉桂酸4-羟化酶,4CL:4香豆酸辅酶A连接酶, COMT:咖啡酰辅酶连接酶,CCR:肉桂酸辅酶A还原酶
Fig. 1Cellulose and lignin biosynthesis pathway
A.Schematic diagram of cellulose synthesis; B. Schematic diagram of lignin monomer synthesis. CesA, Cellulose Synthase. PAL: phenylalanine ammonia-lyase, C4H: Cinnamate-4-hydrolylase. 4CL: 4-coumarate coenzyme A ligase, COMT: caffeic acid 3-O-methyltransferase. CCR: cinnamyl coenzyme A reductase
1 材料与方法
1.1 供试材料与培养
紫花苜蓿中苜1号(Medicago sativa L. Zhongmu No.1)、拟南芥(Col生态型)由中国农业科学北京畜牧兽医研究所饲草遗传育种实验室保存。紫花苜蓿种子置于铺有双层滤纸的培养皿中发芽3d,移栽到1/2 Hoagland营养液中置于植物生长间培养(16 h光照 / 8 h黑暗,温度25℃,湿度60%—70%)。培养30d进行胁迫处理,分别以20 μmol GA3、250 μmol SA、10 μmol PCB处理12 h,每隔4 h取全部茎段,立即投入液氮冷冻,保存在-80℃冰箱,不处理为对照,每个处理3个生物学重复。拟南芥种子经过1%升汞消毒,灭菌水清洗7次,播种于1/2 MS固体培养基上,在光照培养箱中培养2周(16 h光照 / 8 h黑暗,22℃,湿度60%—70%),然后移栽到土壤(营养土﹕蛭石=1﹕1)中生长7周用于后续试验。转基因紫花苜蓿通过扦插扩繁观察表型并进行指标测定。本研究始于2018年开始,所有试验均在中国农业科学北京畜牧兽医研究所饲草遗传育种实验室完成。1.2 MsNST的克隆
紫花苜蓿总RNA提取参考植物总RNA提取试剂盒说明书操作(Promega,上海普洛麦格生物技术有限公司)。取1μg总RNA,利用反转录试剂盒(Takara,北京六合通生物技术有限公司)反转录为cDNA。由蒺藜苜蓿MtNST1全长CDS序列(GenBank: GU144511.1)为参考,分别在5′UTR与3′UTR区设计一对引物MsNST-f/r (表1),以紫花苜蓿cDNA为模板克隆MsNST,通过测序获取MsNST全长CDs序列(北京天一辉远生物技术有限公司)。Table 1
表1
表1研究所用的引物序列
Table 1
| 引物 Primer | 核酸序列(5′-3′) Nucleotide sequence (5′-3′) | 功能 Function | 文献Reference |
|---|---|---|---|
| MsNST-f | TCAACTTTTTGGGTCCCTTGTG | MsNST克隆 Cloning of MsNST | |
| MsNST-r | TCACCACATGCTATCACCATTG | ||
| actin-2s | CAAAAGATGGCAGATGCTGAGGAT | 内参基因 Reference gene Actin | |
| actin-2a | CATGACACCAGTATGACGAGGTCG | ||
| qNST-f | TCATCTCAAAACCCTAGACAGCCC | 实时定量PCR qRT-PCR | |
| qNST-r | GTAATTTGCTTCATAATTCTCTTCCTTG | ||
| Ntest-f | GCACAATCCCACTATCCTTCG | 转基因拟南芥阳性鉴定Identification of transgenic lines | |
| Ntest-r | AGTTTTTTGATTTCACGGGTTGGGG | ||
| W-NST-F | CATTTGGAGAGAACACGGGGGACTCTAGAATGCCTGATAACATGAGTATAT | Pbi121-MsNST超表达载体构建 Construction of Pbi121-MsNST overexpression vector | |
| W-NST-R | AACATAAGGGACTGACCACCCGGGGATCCTCACCACATGCTTATCAC CATT | ||
| Atact-f | GCAACATACGACGAAATCAAGAA | qRT-PCR内参基因 Actin gene for qRT-PCR | [18] [19] [20] [21] |
| Atact-r | CGACACGAGAACTGTAACCCC | ||
| AtPAL1-f | ATGGAGATTAACGGGGCACAC | 木质素与纤维素合成相关基因的表达分析 Expression analysis of lignin and cellulose biosynthesis related to genes | |
| AtPAL1-r | GTACCGCCGAGAACACCGCC | ||
| At4CL1-f | GATTTGAGCTCGATAAGAGTGGTG | ||
| At4CL1-r | ATTTGCTAGTTTTGCCCTCA | ||
| AtCESA4-f | ATTCTGGGTGATTGGCGG | ||
| AtCESA4-r | AATAATGAGAGTTGTCGGAGGG | ||
| AtCESA7-f | TTCTTGCCTACTGTATCCTTCC | ||
| AtCESA7-r | GCTAACTCCGCTCCATCTCA | ||
| AtCESA8-f | CATCCCAACGCTATCAAACCTA | ||
| AtCESA8-r | CTGAGACACCTCCAATAACCCA | ||
| MsCESA3-f | TCGATGGGCTTTAGGTTCAG | ||
| MsCESA3-r | TGAGAAGAGGAATGGAAGTG | ||
| MsCESA6-f | CCCTCTTCATATCCATCGCAG | ||
| MsCESA6-r | CACCTCCAATCACCCAAAAC | ||
| MsCESA7-f | GATGAAGCAAGACAACCACTG | ||
| MsCESA7-r | CTGGGTTCATAAGTCTGTATCGG | ||
| MsPAL-f | ATGAGGTGAAGCGTATGGTG | ||
| MsPAL-r | CATCCCTAGCAGATTCAGACAG | ||
| Ms4CL-f | TTCACGTCCTTGCCTCATCA | ||
| Ms4CL-r | CCAAGTTTGTTGAGACCGGAGG | ||
| MsCOMT-f | AAAGTGATTGTGGCAGAATGCA | ||
| MsCOMT-r | TTTTGTGGCCAGGCTTGAA |
新窗口打开|下载CSV
1.3 生物信息学分析
利用NCBI的ORF finder(1.4 GA3、SA、PCB诱导下MsNST表达分析
分别取GA3、SA、PCB诱导下各处理的样品提取总RNA,反转录为cDNA。依据基因的序列设计1对引物qNST-f/r(表1),以紫花苜蓿β-Actin 2为内参,通过qRT-PCR检测各个时间点MsNST基因的表达水平。1.5 过表达载体PBI121-MsNST的构建与遗传转化
利用W-NST-F/R为上下游引物扩增带有特殊同源臂的PCR产物,将PCR产物与PBI121过表达载体连接,并转入GV3101农杆菌感受态细胞,采用花序浸染法对拟南芥进行遗传转化。另外,将PBI121- MsNST质粒转入农杆菌EHA105中,以紫花苜蓿叶片为外植体,采用根癌农杆菌侵染法进行转化[22]。1.6 拟南芥中过表达MsNST转基因植株的表型分析
为了筛选阳性转基因拟南芥植株,将T0代拟南芥种子用1%升汞消毒后种在含kan(50 mg·L-1)的1/2 MS固体培养基上,筛选kan抗性拟南芥转基因植株,设计一对引物Ntest-f/r(表1)进行转基因植株鉴定。为了探索过表达MsNST对拟南芥下胚轴发育的影响,将T2代拟南芥种子消毒后,播种在1/2 MS固体平板上,分别在避光和正常光照条件下生长,挑选20株长势一致的拟南芥株系,其中10株置于同一个平板上,每隔1d统计每株下胚轴长度,共统计5d;另外10株置于培养箱中培养到7周龄,分别对株高、鲜重进行统计。同时,将7周龄拟南芥茎基部第一节用刀片截成0.5cm的茎段,放入75%酒精中真空抽气10min,室温放置5 h固定,将材料转入包埋剂中室温放置过夜。用冷冻切片机(莱卡Y470)将茎部横切,切片厚度为50μm,置于载玻片上,加一滴1%间苯三酚于切片上室温染色2min,然后再加一滴40%浓盐酸进行显色,甘油封片后在光学显微镜(Olympus FV500,日本)下观察拍照。1.7 纤维素与木质素合成相关基因的表达分析
分别取过表达MsNST的紫花苜蓿与拟南芥转基因植株茎组织提取总RNA,反转录为cDNA。依据基因序列设计1对引物qNST-f/r(表1),以β-Actin为内参基因,利用qRT-PCR分析转基因株系中MsNST的相对表达水平。选取表达量最高的株系检测与纤维素和木质素合成相关合成基因的表达量变化,其中紫花苜蓿分别为MsCesA3,MsCesA6,MsCesA7,MsPAL,Ms4CL,MsCoMT,拟南芥为AtPAL1、At4CL1、AtCesA4、AtCesA7、AtCesA8(引物见表1),每个反应3个生物学重复。1.8 总糖、结晶纤维素和木质素含量测定
取生长7周龄转基因与野生型拟南芥各10株,分别置于牛皮纸袋放入鼓风干燥箱烘干至恒重(80℃),利用组织研磨仪将样品粉碎(振动频率为70Hz)。分别称取约300 mg粉末,参考Foster方法进行粗细胞壁分离(cell wall resider,CWR),测定木质素含量[23]。称取100 mg CWR置于试管中,加入72 % 浓硫酸,冰浴30 min,12 000 r/min离心,将上清液稀释10倍,采用植物结晶纤维素检测试剂盒(Solarbio, 北京索莱宝科技有限公司)测定纤维素的含量。分别称取5 mg CWR,用72 %浓硫酸室温水解20 min,用蒸馏水将硫酸浓度稀释到4 %,加热到121℃水解1 h,利用蒽酮比色法测定总糖含量,以上试验均为3个生物学重复。2 结果
2.1 MsNST的克隆及生物信息学分析
同源克隆获得MsNST的cDNA序列为1 121bp,最大开放阅读框为945bp,编码314个氨基酸(图2);Blast结果表明,该基因与其它物种的NST同源,故将该基因命名为MsNST;ExASy在线预测MsNST蛋白分子量为36.25 kD,理论等电点为6.84;MsNST全部氨基酸的平均亲水系数为-0.986,表明其为亲水性蛋白质(图3-A);TMHMM预测该蛋白无跨膜结构域(图3-B)。该蛋白的二级结构以无规则卷曲为主,占总体的60.83 %,α螺旋占17.52 %,β折叠占4.14 %,转角为17.52 %(图3-C)。以拟南芥NAC1蛋白为模板模拟蛋白三维结构并建模,结果表明MsNST可能是通过形成中心对称的二聚体结构行使功能(图3-D);通过Cell-PLoc 2.0软件预测,该蛋白定位在细胞核上(图3-E)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2紫花苜蓿MsNST的克隆
A:MsNST的PCR扩增;B:MsNST的核酸与氨基酸序列
Fig. 2Cloning of MsNST from alfalfa
A: PCR amplification of MsNST; B: Nucleic acid and deduced amino acid sequences of MsNST
图3
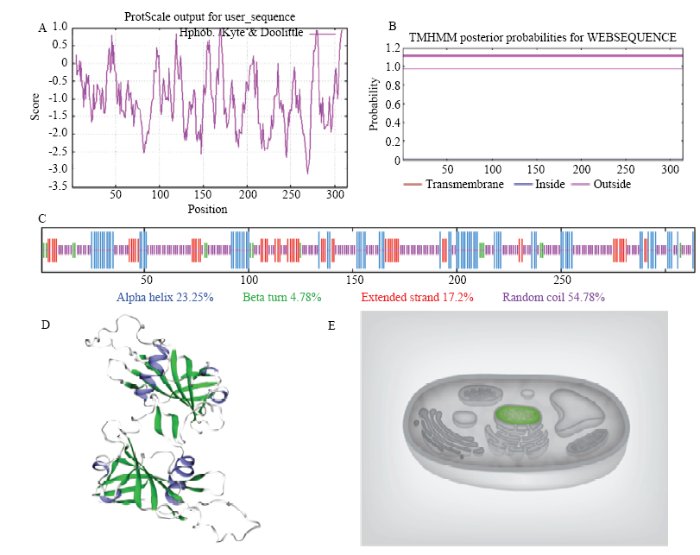 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3MsNST生物信息学分析
A:MsNST的亲水性;B:MsNST无跨膜结构;C:MsNST的二级结构;D:MsNST三级结构预测与建模;E:MsNST亚细胞定位预测
Fig. 3Bioinformatics analysis of MsNST
A: Hydrophilicity of MsNST protein predicted by Protscale; B: Non-transmamrane domain for MsNST protein predicted by TMHMM; C: Secondary structure of MsNST predicted by SOPMA; D: Prediction and construction of MsNST tertiary structure by SWISS-MODEL; E: Prediction of MsNST subcellular localization by Cell-Ploc
MsNST与拟南芥3个NST氨基酸序列进行多重比对表明,MsNST与拟南芥AtNST1的相似性较高为55.9%,与AtNST2和AtNST3的相似性分别为51.6%、49%。MsNST的N端序列保守,具有NAC 类转录因子特有的5个保守亚结构域[24],而C端序列不保守(图4)。进化树分析表明,MsNST与豆科模式植物蒺藜苜蓿MtNST1的亲缘关系最近,不同物种进化树分为单子叶与双子叶2大类,MsNST分布在双子叶植物分支中,表明NST转录因子在双子叶和单子叶植物中存在进化差异(图5)。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4MsNST和AtNST1,2,3的氨基酸序列比对
黑色区域为全部氨基酸序列相同,灰色为50%以上序列相同,划横线区为NAC保守结构域,方框中氨基酸为形成二聚体的保守序列
Fig. 4Amino acid alignment of MsNST and AtNST1, 2, and 3
Homology level was highlighted by shading in color: black for 100%, grey for ≥50% identity. Conservative domain of NAC was underlined in black, and the amino acid in the box is a conservative sequence for formation of dimer
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同植物NST基因的系统进化树
图中0.05为遗传距离,分叉处数值为bootstrap校正值,括号里为基因名称
Fig. 5Phylogenetic analysis of NSTs from different plant species
The number 0.05 represents the evolution distance,the number above branches represent the bootstrap value. The Numbers in parentheses are gene locus names
2.2 不同诱导条件下MsNST的表达分析
植物激素对植物生长发育和形态建成具有重要的调节作用。为了揭示施加外源激素对MsNST表达的调控作用,本研究分别用赤霉素、多效唑和水杨酸处理紫花苜蓿,分析MsNST受激素诱导后的表达特性,结果表明,GA3处理会诱导MsNST表达量上调,在诱导12h时表达量达到最高水平,为对照的2.19倍(图6-A)。施加PCB诱导结果表明,在PCB诱导初期,MsNST基因表达量下调在4h时达到最低水平,是未处理的0.72倍,随后表达量上调,在诱导12h达到最高表达水平,为对照的3.65倍(图6-B)。施加SA诱导初期,在4h时表达量显著降低为对照的0.49倍,随后表达量上调,在12h时达到最高水平是对照的3.67倍(图6-C)。图6
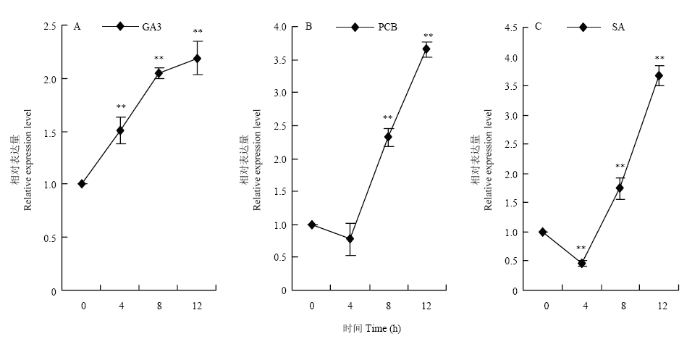 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6利用qRT-PCR检测不同激素处理对苜蓿茎MsNST表达的影响
GA3,SA,PCB分别代表赤霉素、水杨酸和多效唑Bar表示标准差
Fig. 6Effects of various treatments on MsNST expression in alfalfa stems using qRT-PCR
MsNST under GA3 (gibberellin3), SA (salicylic acid), PCB (paclobutrazol) treatment. Error bars represent standard error of the mean
2.3 拟南芥中过表达MsNST的功能分析
为验证MsNST的功能,通过kan抗性筛选与PCR鉴定过表达MsNST拟南芥阳性株系(图7-B)。随机挑选6个过表达的转基因株系与野生型相比株高出现不同程度的矮化(图7-A),其中35S::MsNST-Line4中MsNST表达量最高,是对照的3.47倍(图7-C)。观测Line4和WT植株下胚轴的生长情况发现,光照条件下35S::MsNST-Line4与WT的下胚轴长度变化不明显;黑暗条件下,35S::MsNST-Line4下胚轴生长受到抑制,萌发5天时35S::MsNST-Line4下胚轴长度为9.02 mm,显著短于对照组(11.39mm)(图7-D)。对生长7周龄的转基因和野生型拟南芥株高等指标进行测定,转基因拟南芥株高平均值为27.62cm,与对照相比株高降低了10.4%;转基因拟南芥鲜重降低(1.12g),比对照降低了20%(图7-E)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转基因拟南芥鉴定与表型分析
A:5周龄野生型与转基因拟南芥植株,bar=2cm;B:转基因拟南芥阳性鉴定;C:不同株系转基因拟南芥相对表达量分析;D:过表达MsNST基因抑制拟南芥下胚轴生长,n=30,误差为±SD下同,“*” 和“**”分别代表t检验P<0.05和<0.01。下同;E:7周野生型拟南芥与转基因拟南芥株高,鲜重统计分析
Fig. 7Identification and phenotypic analysis of transgenic Arabidopsis thaliana
A: Phenotype of five-week old transgenic Arabidopsis in green house,bars=2cm; B: Ampification of MsNST gene from transgenic Arabidopsis; C: The expression level of MsNST in transgenic Arabidopsis; D: Over expression MsNST result in growth inhibition of Arabidopsis hypocotyls. n=30,error bars replace ± standard deviation. “*” or “**” on behalf of P value < 0.05 or <0.01 by stutent t test analysis; E: Statistics analysis of plant height and fresh weight of transgenic Arabidopsis thaliana and WT
2.4 过表达MsNST对木质素、纤维素及总糖含量的影响
通过对过表达拟南芥35S::MsNST-Line4和WT植株从下数第一节茎段横切,利用间苯三酚-盐酸对细胞壁中木质素染色,显微镜观测发现35S::MsNST-Line4的束间纤维(interfascicular fiber,IF)细胞壁厚度增加(图8-A),统计分析表明Line4束间纤维细胞壁平均厚度为1.58μm,WT细胞壁厚度为0.92μm,与WT相比过表达MsNST纤维细胞壁厚度增加了71.7%(图8-B),暗示MsNST对束间纤维细胞壁的合成具有一定调节作用。对过表达Line4株系的木质素和纤维素含量测定表明,转基因植株细胞壁木质素含量相比WT提高了11.7%(图8-C);Line4株系结晶纤维素含量为239.15 mg·g-1,是WT的1.13倍(图8-D);总糖含量为355.55mg·g-1,比WT提高了7%(图8-E)。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8过表达MsNST对细胞壁厚度及木质素、纤维素和总糖含量的影响
A:间苯三酚-盐酸对细胞壁中木质素染色;B:Image J软件对细胞壁厚度统计;C:拟南芥茎木质素含量;D:拟南芥茎纤维素含量测定;E:拟南芥茎总糖含量测定,n=10,使用t检验做显著性分析“*”代表P<0.05,“**”代表P<0.01;bar为±SD
Fig. 8Effects of overexpression MsNST on cell wall thickness, lignin, cellulose and total sugar content in transgenic Arabidopsis thaliana
A: Stem cell walls of Arabidopsis stain by phloroglucinol-hydrochloric acid; B: Statistical analysis of interfascicular fiber cell wall thickness; C: Determination of lignin content of transgenic Arabidopsis stem; D: Determination of cellulose content in transgenic Arabidopsis stem; E: Determination of total sugar content in transgenic Arabidopsis stem
2.5 过表达MsNST拟南芥中纤维素和木质素合成关键基因的表达分析
为了揭示MsNST对次生壁纤维素和木质素合成关键基因的调控作用,利用qRT-PCR检测Line4株系中次生壁纤维素合成关键基因AtCesA4、AtCesA7、AtCesA8和木质素合成相关基因AtPAL1、At4CL1和AtCOMT的表达量水平,结果显示过表达MsNST提高了次生壁纤维素合酶亚基基因AtCesA4,7,8的表达水平,其中AtCesA7表达量上调最为明显,是野生型的2.81倍;木质素合成关键基因AtPAL1, At4CL1和AtCOMT的表达水平也提高,分别是对照的1.64倍,1.77倍和2.28倍(图9)。图9
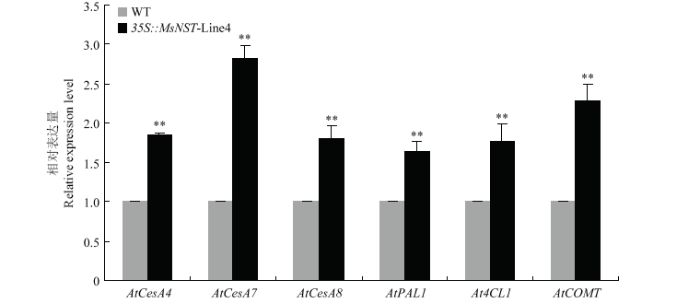 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9过表达MsNST基因拟南芥中纤维素与木质素合成相关基因的表达分析
Fig. 9Expression analysis of genes related to cellulose and lignin synthesis in overexpression MsNST transgenic Arabidopsis thaliana
2.6 过表达MsNST紫花苜蓿的鉴定与木质素和纤维素合成相关基因的表达分析
经抗性筛选获得抗kan的紫花苜蓿株系,经鉴定获得的4株过表达转基因株系,其中OE1表达量最高,是对照的2.7倍(图10-A, B)。生长1个月的OE1植株高度为23.45cm,低于对照组26.32cm(图10-C)。从顶端往下测定6个节间长度的结果表明,从第二个节间开始明显短于对照组(图10-D)。qRT-PCR分别检测各转基因株系中MsNST的表达量,结果表明过表达株系OE1中MsNST表达量显著上调,是对照组的2.74倍。对纤维素与木质素合成关键基因的表达分析表明,纤维素合成关键基因MsCesA6和MsCesA7表达量上调表达,分别为对照的1.45倍和1.43倍(图10-E)。木质素合成关键基因MsPAL、Ms4CL和MsCOMT也得到不同程度上调,其中MsPAL表达量最大,是对照的1.5倍(图10-E)。图10
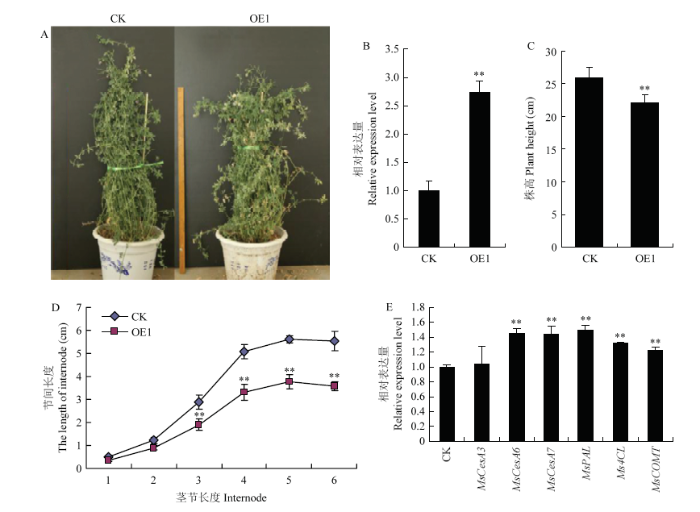 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10过表达MsNST紫花苜蓿的获取及其木质素和纤维素合成相关基因的表达分析
A:过表达转基因紫花苜蓿表型;B:过表达转基因紫花苜蓿相对表达量分析;C:过表达转基因紫花苜蓿与对照的株高;D:过表达转基因紫花苜蓿与对照组的顶部6节间高度;E:转基因紫花苜蓿纤维素与木质素合成相关基因相对表达量分析
Fig. 10Identification of transgenic alfalfa and analysis of genes related to cellulose and lignin synthesis of transgenic lines
A: The phenotype of overexpression alfalfa; B: Analysis of MsNST expression level of transgenic alfalfa and control; C: The plant height of overexpressing alfalfa compare with control; D: The length of six stem internodes from top; E: Relative expression levels of genes related to cellulose and lignin synthesis in transgenic alfalfa (OE1)
3 讨论
NAC家族的NST转录因子是植物特有的转录因子,调控植物次生壁物质合成[3-5, 7, 9, 25]。OOKA在分析拟南芥和水稻NAC转录因子的保守性时,发现N端含有A、B、C、D、E 5个保守的结构域[24]。本研究将MsNST氨基酸序列与拟南芥3个同源基因进行相似性比对,结果表明在N端也具有5个典型的NAC保守结构域,与Ooka的分析结果一致;然而MsNST氨基酸序列的C端不保守,可能是导致该基因的功能具有多样性的主要原因(图3)。系统进化树分析显示,不同植物的NST分为单子叶和双子叶两个分枝,暗示不同物种之间存在一定程度的进化;但是MsNST与豆科模式植物蒺藜苜蓿NST1的同源性最近,表明其可能与MtNST1具有相似的功能。前人研究表明NAC类转录因子大都通过两个反向平行的β折叠片和2个盐桥作用形成同源或异源二聚体,起到转录激活或抑制作用,其中N端保守的精氨酸(R)和谷氨酸(E)位点对盐桥形成起到关键作用[26]。对MsNST蛋白序列分析表明,N端保守序列中具有参与盐桥形成的精氨酸和谷氨酸保守位点,三级结构预测也表明其能够通过两个β折叠形成同源二聚体结构,表明MsNST很可能通过形成同源二聚体结构对靶基因起作用。GA是植物内源激素,在植物次生壁发育中起到重要作用。研究表明,烟草内源GA3含量增加会提高茎的木质化程度[27]。拟南芥和白桦外源施加GA也显著增加茎中木质部的面积占比[28,29]。此外,水稻OsSLR1是一种受GA信号诱导裂解的DELLA阻遏蛋白,可以直接与OsNAC相互作用,抑制NAC-MYB级联反应,调控次生壁合成基因表达[13]。以上研究结果与本研究中GA3诱导MsNST表达水平升高相符合(图5-A),表明在紫花苜蓿中GA3具有激活MsNST表达的作用。生产中常用植物生长调节剂多效唑(PCB)降低顶端优势,增加壁厚,提高植株耐逆性。施加PCB能够显著提高茎秆中PAL和CAD等酶活性,增加植物次生壁厚度,促进茎秆木质素积累,增强植物茎秆的抗倒伏能力[17,30-31]。研究表明PCB抑制植物体内GA的生物合成[32],本研究施加PCB短期内(0—4h),MsNST表达量受到PCB影响而降低,可能是PCB抑制了植物体内的GA合成,降低MsNST表达。PCB处理4—8h时MsNST表达量显著提高,这与施加PCB能够提高植株茎中细胞壁厚度与积累木质素相符合。然而,PCB导致次生壁NST类转录因子激活的分子调控机制还未见报道,笔者猜想PCB可能还存在其他途径直接或间接影响MsNST表达,具体分子调控机制还需要进一步的研究。SA是植物响应生物胁迫的应激反应激素,由苯丙氨酸氨裂解酶(PAL)催化合成的肉桂酸盐经苯甲酸盐合成[33]。外施SA 12h提高了MsNST的表达水平,表明SA能够通过诱导MsNST表达促进细胞壁积累木质素和纤维素,增加植物对病原菌的抵抗能力[15, 34-35],但是SA诱导初期MsNST表达量出现明显下调的现象还没有明确的研究结果。
拟南芥、玉米和杨树等植物中,过表达NST会造成纤维细胞次生壁增厚,细胞壁中纤维素与木质素含量显著增加[5, 9, 25, 36]。此外,拟南芥nst1nst3和截形苜蓿mtnst1突变体中,由于NST的突变,造成植物茎纤维细胞次生壁缺失,木质素与纤维素含量显著降低[4-5, 37]。本研究在拟南芥与紫花苜蓿过表达MsNST,导致转基因植株矮化,各节间高度受到抑制。此外,拟南芥花序茎束间纤维细胞壁增厚,细胞壁结晶纤维素与木质素含量显著增加,表明MsNST与其它植物中NST转录因子的功能相似,在调控纤维素与木质素生物合成过程中起到重要作用。笔者还发现MsNST影响黑暗条件下拟南芥下胚轴发育,暗示MsNST可能在拟南芥下胚轴发育时参与细胞壁物质合成。研究表明,许多纤维素和木质素合成相关基因表达受到NST的影响,从而影响次生壁纤维素与木质素合成,例如AtCesA4、AtCesA7和AtCesA8是组装拟南芥次生壁CSC必须的亚基,对植物次生壁纤维素合成起到关键作用[38,39,40,41]。研究表明,拟南芥MYB46及其同源基因MYB83是SND1/NST3转录因子的直接靶基因,MYB46通过反应顺式调控元件(SMRE)直接结合CesAs的启动子,形成保守的NACs-MYBs-CesAs纤维素合成基因调控网络[42]。木质素合成调控机制还未明确,但是拟南芥PAL1、4CL1、COMT等木质素合成过程中重要酶基因表达都受到NST转录因子的影响[3,4,5]。豆科模式植物蒺藜苜蓿与单子叶玉米中NST突变导致纤维素和木质素合成关键基因表达量下调[9, 37]。本研究对过表达MsNST拟南芥和紫花苜蓿转基因株系的纤维素与木质素合成基因定量分析表明,MsNST对纤维素和木质素合成相关基因的表达具有一定的调节作用,从而促进纤维素与木质素合成。本研究初步揭示了紫花苜蓿MsNST转录因子在细胞壁纤维素和木质素合成中的正调控作用。
4 结论
本研究克隆了紫花苜蓿木质素和纤维素合成过程中的关键基因(MsNST),该基因在细胞壁木质素和纤维素合成中起到正向调控作用,激活下游基因表达。MsNST基因隶属NAC转录因子家族,并具有该家族的特有的NAC保守结构域。MsNST受到外源激素GA3、PCB和SA诱导表达。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-313X.2008.03633.xURLPMID:18657234 [本文引用: 3]

Three distinct pattern elements of the silique are thought to contribute to its dehiscence: a separation layer, cells with a secondary wall adjacent to the separation layer, and a valve endocarp layer with secondary wall. However, the role of the secondary wall has not been proven, and the factors that regulate its formation in siliques remain to be characterized. We show here that secondary wall formation in siliques is necessary for dehiscence, and that two plant-specific transcription factors, NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 and 3 (NST1 and NST3), regulate its formation in siliques of Arabidopsis. The promoters of the NST1 and NST3 genes were active in the valve endocarp layer and in cells surrounding vascular vessels in the replum, and NST1 promoter activity only was faintly detectable at valve margins. In nst1 mutants, specific loss of secondary walls was evident at valve margins, while nst1 nst3 double mutants lacked secondary walls in all parts of the siliques, with the exception of vascular vessels. These siliques were similarly indehiscent. The promoters of two tissue-identity genes, INDEHISCENT (IND) and SHATTERPROOF2 (SHP2), were as active in the nst1 nst3 mutant as in the wild-type. Moreover, the ectopic secondary wall formation that occurs in the fruitfull (ful) mutant was absent in the ful nst1 double mutant. We propose that secondary walls in valve margins are required for dehiscence, and that NST1 and NST3 regulate their formation in siliques in a partially redundant manner after the establishment of tissue identity.
DOI:10.1007/s00425-007-0498-yURL [本文引用: 5]

Secondary walls are the major component of wood, and studies of the mechanisms regulating secondary wall synthesis is important for understanding the process of wood formation. We have previously shown that the NAC domain transcription factor SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1) is a key regulator of secondary wall synthesis in fibers of Arabidopsis thaliana stems and dominant repression of SND1 leads to a reduction in secondary wall thickening in fibers. However, T-DNA knockout of the SND1 gene did not cause an alteration in secondary wall thickness, suggesting that other SND1 homologs may compensate for the loss of SND1 expression. Here, we studied the effects of simultaneous inhibition of SND1 and its homolog, NAC SECONDARY WALL THICKENING PROMOTING FACTOR1 (NST1), on secondary wall synthesis in fibers. We show that simultaneous RNA interference (RNAi) inhibition of the expression of both SND1 and NST1 genes results in loss of secondary wall formation in fibers of stems. The fiber cells in the stems of SND1/NST1–RNAi plants lack all three major secondary wall components, including cellulose, xylan, and lignin, which is accompanied by a severe reduction in the expression of genes involved in their biosynthesis. In addition, inhibition of SND1 and NST1 leads to down-regulation of several fiber-associated transcription factor genes. Double T-DNA knockout mutations of SND1 and NST1 genes cause the same effects, as does simultaneous RNAi inhibition of SND1 and NST1. Our results provide first line evidence demonstrating that SND1 and NST1 function redundantly in the regulation of secondary wall synthesis in fibers.
DOI:10.1105/tpc.106.047043URL [本文引用: 8]
[本文引用: 1]
DOI:10.4161/15592324.2014.989746URLPMID:25751728 [本文引用: 2]

Transcriptional regulation of secondary wall biosynthesis in Arabidopsis thaliana has been shown to be mediated by a group of secondary wall NAC master switches, including NST1, NST2, SND1 and VND1 to VND7. It has been shown that VND1 to VND7 regulate secondary wall biosynthesis in vessels, NST1 and NST2 function redundantly in anther endothecium, and SND1 and NST1 are required for secondary wall thickening in fibers of stems. However, it is unknown whether NST2 is involved in regulating secondary wall biosynthesis in fibers of stems. In this report, we demonstrated that similar to SND1, NST2 together with NST1 were highly expressed in interfascicular fibers and xylary fibers but not in vessels of stems. Although simultaneous mutations of SND1 and NST1 have been shown to result in a significant impairment of secondary wall thickening in fibers, a small amount of secondary walls was deposited in fibers during the late stage of stem development. In contrast, simultaneous mutations of SND1, NST1 and NST2 led to a complete loss of secondary wall thickening in fibers. These results demonstrate that NST2 together with SND1 and NST1 regulate secondary wall biosynthesis in fibers of stems.
DOI:10.1093/mp/ssq062URLPMID:20935069 [本文引用: 1]

We report the genome-wide analysis of direct target genes of SND1 and VND7, two Arabidopsis thaliana NAC domain transcription factors that are master regulators of secondary wall biosynthesis in fibers and vessels, respectively. Systematic mapping of the SND1 binding sequence using electrophoretic mobility shift assay and transactivation analysis demonstrated that SND1 together with other secondary wall NACs (SWNs), including VND6, VND7, NST1, and NST2, bind to an imperfect palindromic 19-bp consensus sequence designated as secondary wall NAC binding element (SNBE), (T/A)NN(C/T) (T/C/G)TNNNNNNNA(A/C)GN(A/C/T) (A/T), in the promoters of their direct targets. Genome-wide analysis of direct targets of SND1 and VND7 revealed that they directly activate the expression of not only downstream transcription factors, but also a number of non-transcription factor genes involved in secondary wall biosynthesis, cell wall modification, and programmed cell death, the promoters of which all contain multiple SNBE sites. SND1 and VND7 directly regulate the expression of a set of common targets but each of them also preferentially induces a distinct set of direct targets, which is likely attributed to their differential activation strength toward SNBE sites. Complementation study showed that the SWNs were able to rescue the secondary wall defect in the snd1 nst1 mutant, indicating that they are functionally interchangeable. Together, our results provide important insight into the complex transcriptional program and the evolutionary mechanism underlying secondary wall biosynthesis, cell wall modification, and programmed cell death in secondary wall-containing cell types.
DOI:10.1016/j.plantsci.2017.03.012URLPMID:29241570 [本文引用: 4]

Secondary walls are the most abundant biomass produced by plants, and they consist mainly of lignin, cellulose and hemicellulose. Understanding how secondary wall biosynthesis is regulated could potentially provide genetic tools for engineering biomass components, especially in maize and Sorghum bicolor. Although many works have focused on secondary wall biosynthesis in dicotyledons, little has been reported for these monocotyledons. In this study, we cloned two NAC transcriptional factor genes, ZmNST3 and ZmNST4, and analyzed their functions in maize secondary wall formation process. ZmNST3 and ZmNST4 were expressed specifically in secondary wall-forming cells, expression of ZmNST3/4 can restore the pendent phenotype of Arabidopsis nst1nst3 double mutant. ZmNST3/4-overexpressing Arabidopsis and maize displayed a thickened secondary wall in the stem, and knockdown maize showed defective secondary wall deposition. ZmNST3/4 could regulate the expression of ZmMYB109/128/149. Our results revealed that ZmNST3/4 are master switches of the maize secondary wall biosynthesis process and provides new evidence that the secondary wall regulatory pathway is conserved in different plant species.
[本文引用: 1]
[本文引用: 1]
DOI:10.3389/fpls.2018.01391URLPMID:30294339 [本文引用: 1]

Light intensity and hormones (gibberellins; GAs) alter plant growth and development. A fine regulation triggered by light and GAs induces changes in stem cell walls (CW). Cross-talk between light-stimulated and GAs-induced processes as well as the phenolic compounds metabolism leads to modifications in lignin formation and deposition on cell walls. How these factors (light and GAs) promote changes in lignin content and composition. In addition, structural changes were evaluated in the stem anatomy of tobacco plants. GA3 was sprayed onto the leaves and paclobutrazol (PAC), a GA biosynthesis inhibitor, via soil, at different irradiance levels. Fluorescence microscopy techniques were applied to detect lignin, and electron microscopy (SEM and TEM) was used to obtain details on cell wall structure. Furthermore, determination of total lignin and monomer contents were analyzed. Both light and GAs induces increased lignin content and CW thickening as well as greater number of fiber-like cells but not tracheary elements. The assays demonstrate that light exerts a role in lignification under GA3 supplementation. In addition, the existence of an exclusive response mechanism to light was detected, that GAs are not able to replace.
DOI:10.1080/15592324.2018.1445933URLPMID:29485381 [本文引用: 1]

DELLA proteins act as negative regulators in gibberellin (GA) signal transduction. GA-induced DELLA degradation is a central regulatory system in GA signaling pathway. Intensive studies have revealed the degradation mechanism of DELLA and the functions of DELLA as a transcriptional regulator. Meanwhile, recent studies suggest the existence of a DELLA-independent GA signaling pathway. In this review, we summarized the DELLA-independent GA signaling pathway together with the well-analyzed DELLA-dependent pathway.
DOI:10.1105/tpc.15.00015URLPMID:26002868 [本文引用: 2]

Cellulose, which can be converted into numerous industrial products, has important impacts on the global economy. It has long been known that cellulose synthesis in plants is tightly regulated by various phytohormones. However, the underlying mechanism of cellulose synthesis regulation remains elusive. Here, we show that in rice (Oryza sativa), gibberellin (GA) signals promote cellulose synthesis by relieving the interaction between SLENDER RICE1 (SLR1), a DELLA repressor of GA signaling, and NACs, the top-layer transcription factors for secondary wall formation. Mutations in GA-related genes and physiological treatments altered the transcription of CELLULOSE SYNTHASE genes (CESAs) and the cellulose level. Multiple experiments demonstrated that transcription factors NAC29/31 and MYB61 are CESA regulators in rice; NAC29/31 directly regulates MYB61, which in turn activates CESA expression. This hierarchical regulation pathway is blocked by SLR1-NAC29/31 interactions. Based on the results of anatomical analysis and GA content examination in developing rice internodes, this signaling cascade was found to be modulated by varied endogenous GA levels and to be required for internode development. Genetic and gene expression analyses were further performed in Arabidopsis thaliana GA-related mutants. Altogether, our findings reveal a conserved mechanism by which GA regulates secondary wall cellulose synthesis in land plants and provide a strategy for manipulating cellulose production and plant growth.
DOI:10.1007/s11103-015-0376-0URLPMID:26350403 [本文引用: 1]

Although the main genes in rice involved in the biosynthesis of secondary wall components have been characterized, the molecular mechanism underlying coordinated regulation of genes expression is not clear. In this study, we reported a new rice variety, cef1, showed the culm easily fragile (CEF) without other concomitant phenotypes. The CEF1 gene encodes a MYB family transcription factor OsMYB103L, was cloned based on map-based approach. Bioinformatics analyses indicated that CEF1 belongs to the R2R3-MYB subfamily and highly similar to Arabidopsis AtMYB103. Expression pattern analysis indicated that CEF1 is mainly expressed in internodes and panicles. Biochemical assays demonstrated that OsMYB103L is a nuclear protein and shows high transcriptional activation activity at C-terminus. OsMYB103L mediates cellulose biosynthesis and secondary walls formation mainly through directly binding the CESA4, CESA7, CESA9 and BC1 promoters and regulating their expression. OsMYB103L may also function as a master switch to regulate the expression of several downstream TFs, which involved in secondary cell wall biosynthesis. Furthermore, OsMYB103L physically interacts with SLENDER RICE1 (SLR1), a DELLA repressor of GA signaling, and involved in GA-mediated regulation of cellulose synthesis pathway. Our findings revealed that OsMYB103L plays an important role in GA-regulating secondary cell wall synthesis, and the manipulation of this gene provide a new strategy to help the straw decay in soil.
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 2]
URL [本文引用: 2]
DOI:10.1111/pbi.12842URLPMID:28944540

Cellulose is an abundant biopolymer and a prominent constituent of plant cell walls. Cellulose is also a central component to plant morphogenesis and contributes the bulk of a plant's biomass. While cellulose synthase (CesA) genes were identified over two decades ago, genetic manipulation of this family to enhance cellulose production has remained difficult. In this study, we show that increasing the expression levels of the three primary cell wall AtCesA6-like genes (AtCesA2, AtCesA5, AtCesA6), but not AtCesA3, AtCesA9 or secondary cell wall AtCesA7, can promote the expression of major primary wall CesA genes to accelerate primary wall CesA complex (cellulose synthase complexes, CSCs) particle movement for acquiring long microfibrils and consequently increasing cellulose production in Arabidopsis transgenic lines, as compared with wild-type. The overexpression transgenic lines displayed changes in expression of genes related to cell growth and proliferation, perhaps explaining the enhanced growth of the transgenic seedlings. Notably, overexpression of the three AtCesA6-like genes also enhanced secondary cell wall deposition that led to improved mechanical strength and higher biomass production in transgenic mature plants. Hence, we propose that overexpression of certain AtCesA genes can provide a biotechnological approach to increase cellulose synthesis and biomass accumulation in transgenic plants.
DOI:10.1104/pp.103.026484URLPMID:14612585

Lignin, one of the most abundant terrestrial biopolymers, is indispensable for plant structure and defense. With the availability of the full genome sequence, large collections of insertion mutants, and functional genomics tools, Arabidopsis constitutes an excellent model system to profoundly unravel the monolignol biosynthetic pathway. In a genome-wide bioinformatics survey of the Arabidopsis genome, 34 candidate genes were annotated that encode genes homologous to the 10 presently known enzymes of the monolignol biosynthesis pathway, nine of which have not been described before. By combining evolutionary analysis of these 10 gene families with in silico promoter analysis and expression data (from a reverse transcription-polymerase chain reaction analysis on an extensive tissue panel, mining of expressed sequence tags from publicly available resources, and assembling expression data from literature), 12 genes could be pinpointed as the most likely candidates for a role in vascular lignification. Furthermore, a possible novel link was detected between the presence of the AC regulatory promoter element and the biosynthesis of G lignin during vascular development. Together, these data describe the full complement of monolignol biosynthesis genes in Arabidopsis, provide a unified nomenclature, and serve as a basis for further functional studies.
DOI:10.1371/journal.pone.0103808URLPMID:25084115

Abiotic stress represents a serious threat affecting both plant fitness and productivity. One of the promptest responses that plants trigger following abiotic stress is the differential expression of key genes, which enable to face the adverse conditions. It is accepted and shown that the cell wall senses and broadcasts the stress signal to the interior of the cell, by triggering a cascade of reactions leading to resistance. Therefore the study of wall-related genes is particularly relevant to understand the metabolic remodeling triggered by plants in response to exogenous stresses. Despite the agricultural and economical relevance of alfalfa (Medicago sativa L.), no study, to our knowledge, has addressed specifically the wall-related gene expression changes in response to exogenous stresses in this important crop, by monitoring the dynamics of wall biosynthetic gene expression. We here identify and analyze the expression profiles of nine cellulose synthases, together with other wall-related genes, in stems of alfalfa plants subjected to different abiotic stresses (cold, heat, salt stress) at various time points (e.g. 0, 24, 72 and 96 h). We identify 2 main responses for specific groups of genes, i.e. a salt/heat-induced and a cold/heat-repressed group of genes. Prior to this analysis we identified appropriate reference genes for expression analyses in alfalfa, by evaluating the stability of 10 candidates across different tissues (namely leaves, stems, roots), under the different abiotic stresses and time points chosen. The results obtained confirm an active role played by the cell wall in response to exogenous stimuli and constitute a step forward in delineating the complex pathways regulating the response of plants to abiotic stresses.
DOI:10.1111/j.1469-8137.2010.03621.xURLPMID:21251001

* Downregulation of hydroxycinnamoyl CoA: shikimate hydroxycinnamoyl transferase (HCT) in alfalfa (Medicago sativa) reduces lignin levels and improves forage quality and saccharification efficiency for bioethanol production. However, the plants have reduced stature. It was previously reported that HCT-down-regulated Arabidopsis have impaired auxin transport, but this has recently been disproved. * To address the basis for the phenotypes of lignin-modified alfalfa, we measured auxin transport, profiled a range of metabolites including flavonoids and hormones, and performed in depth transcriptome analyses. * Auxin transport is unaffected in HCT antisense alfalfa despite increased flavonoid biosynthesis. The plants show increased cytokinin and reduced auxin levels, and gibberellin levels and sensitivity are both reduced. Levels of salicylic, jasmonic and abscisic acids are elevated, associated with massive upregulation of pathogenesis and abiotic stress-related genes and enhanced tolerance to fungal infection and drought. * We suggest that HCT downregulated alfalfa plants exhibit constitutive activation of defense responses, triggered by release of bioactive cell wall fragments and production of hydrogen peroxide as a result of impaired secondary cell wall integrity.
DOI:10.1007/978-1-4939-1658-0URLPMID:25568905 [本文引用: 1]
[本文引用: 1]
DOI:10.1093/dnares/10.6.239URLPMID:15029955 [本文引用: 2]

The NAC domain was originally characterized from consensus sequences from petunia NAM and from Arabidopsis ATAF1, ATAF2, and CUC2. Genes containing the NAC domain (NAC family genes) are plant-specific transcriptional regulators and are expressed in various developmental stages and tissues. We performed a comprehensive analysis of NAC family genes in Oryza sativa (a monocot) and Arabidopsis thaliana (a dicot). We found 75 predicted NAC proteins in full-length cDNA data sets of O. sativa (28,469 clones) and 105 in putative genes (28,581 sequences) from the A. thaliana genome. NAC domains from both predicted and known NAC family proteins were classified into two groups and 18 subgroups by sequence similarity. There were a few differences in amino acid sequences in the NAC domains between O. sativa and A. thaliana. In addition, we found 13 common sequence motifs from transcriptional activation regions in the C-terminal regions of predicted NAC proteins. These motifs probably diverged having correlations with NAC domain structures. We discuss the relationship between the structure and function of the NAC family proteins in light of our results and the published data. Our results will aid further functional analysis of NAC family genes.
DOI:10.1093/treephys/tpz004URLPMID:30806711 [本文引用: 2]

Wood fibers form thick secondary cell wall (SCW) in xylem tissues to give mechanical support to trees. NAC SECONDARY WALL THICKENING PROMOTING FACTOR3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1 (NST3/SND1) and NST1 were identified as master regulators of SCW formation in xylem fiber cells in the model plant Arabidopsis thaliana. In Populus species, four NST/SND orthologs have been conserved and coordinately control SCW formation in wood fibers and phloem fibers. However, it remains to be elucidated whether SCW formation in other xylem cells, such as ray parenchyma cells and vessel elements, is regulated by NST/SND orthologs in poplar. We knocked out all NST/SND genes in hybrid aspen using the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 nuclease (Cas9) system and investigated the detailed histological appearance of stem tissues in the knockout mutants. Observation by light microscopy and transmission electron microscopy showed that SCW was severely suppressed in wood fibers, phloem fibers and xylem ray parenchyma cells in the knockout mutants. Although almost all wood fibers lacked SCW, some fiber cells formed thick cell walls. The irregularly cell wall-forming fibers retained primary wall and SCW, and were mainly located in the vicinity of vessel elements. Field emission-scanning electron microscope observation showed that there were no apparent differences in the structural features of pits such as the shape and size between irregularly SCW-forming wood fibers in the knockout mutants and normal wood fibers in wild-type. Cell wall components such as cellulose, hemicellulose and lignin were deposited in the cell wall of irregularly SCW-forming wood fibers in quadruple mutants. Our results indicate that four NST/SND orthologs are master switches for SCW formation in wood fibers, xylem ray parenchyma cells and phloem fibers in poplar, while SCW is still formed in limited wood fibers, which are located at the region adjacent to vessel elements in the knockout mutants.
DOI:10.1016/j.plantsci.2005.05.035URL [本文引用: 1]
DOI:10.1104/pp.103.036988URLPMID:15122040 [本文引用: 1]

Gibberellins (GAs) are involved in regulation of many aspects during plant development. To investigate the impact of altered GA levels on plant growth and metabolism, transgenic tobacco (Nicotiana tabacum) plants have been engineered to express either a GA20-oxidase (AtGA20-ox) or a GA2-oxidase (AtGA2-ox) gene from Arabidopsis under control of the cauliflower mosaic virus 35S promoter. Resulting plants were characterized by elongated or stunted shoot growth, respectively, indicating changes in the content of bioactive GAs. In accordance with the effect on plant growth, biomass production was increased or decreased in AtGA20-ox or AtGA2-ox plants, respectively, and was found to be positively correlated with the rate of photosynthesis as determined at the whole plant level. Differences in dry matter accumulation were most likely due to changes in lignin deposition as indicated by histochemical staining and quantitative measurements. Altered lignification of transgenic plants was paralleled by up- or down-regulation of the expression of lignin biosynthetic genes. Short-term GA3 feeding of excised petioles induced lignin formation in the absence of a transcriptional activation of pathway-specific genes. Thus, short-term GA treatment mediates lignin deposition most likely by polymerization of preformed monomers, whereas long-term effects on lignification involve elevated production of precursors by transcriptional stimulation of the biosynthetic pathway. Interestingly, analysis of stem cross sections revealed a differential effect of GA on the formation of xylem and pith cells. The number of lignified vessels was increased in AtGA20-ox plants pointing to a stimulation of xylem formation while the number of pith cells declined indicating a negative regulation.
DOI:10.1105/tpc.111.084020URL [本文引用: 1]

Secondary growth of the vasculature results in the thickening of plant structures and continuously produces xylem tissue, the major biological carbon sink. Little is known about the developmental control of this quantitative trait, which displays two distinct phases in Arabidopsis thaliana hypocotyls. The later phase of accelerated xylem expansion resembles the secondary growth of trees and is triggered upon flowering by an unknown, shoot-derived signal. We found that flowering-dependent hypocotyl xylem expansion is a general feature of herbaceous plants with a rosette growth habit. Flowering induction is sufficient to trigger xylem expansion in Arabidopsis. By contrast, neither flower formation nor elongation of the main inflorescence is required. Xylem expansion also does not depend on any particular flowering time pathway or absolute age. Through analyses of natural genetic variation, we found that ERECTA acts locally to restrict xylem expansion downstream of the gibberellin (GA) pathway. Investigations of mutant and transgenic plants indicate that GA and its signaling pathway are both necessary and sufficient to directly trigger enhanced xylogenesis. Impaired GA signaling did not affect xylem expansion systemically, suggesting that it acts downstream of the mobile cue. By contrast, the GA effect was graft transmissible, suggesting that GA itself is the mobile shoot-derived signal.
DOI:10.3390/ijms160922960URLPMID:26404260 [本文引用: 1]

Gibberellin (GA) is a key signal molecule inducing differentiation of tracheary elements, fibers, and xylogenesis. However the molecular mechanisms underlying the effect of GA on xylem elongation and secondary wall development in tree species remain to be determined. In this study, Betula platyphylla (birch) seeds were treated with 300 ppm GA(3) and/or 300 ppm paclobutrazol (PAC), seed germination was recorded, and transverse sections of hypocotyls were stained with toluidine blue; the two-month-old seedlings were treated with 50 muM GA(3) and/or 50 muM PAC, transverse sections of seedling stems were stained using phloroglucinol-HCl, and secondary wall biosynthesis related genes expression was analyzed by real-time quantitative PCR. Results indicated that germination percentage, energy and time of seeds, hypocotyl height and seedling fresh weight were enhanced by GA(3), and reduced by PAC; the xylem development was wider in GA(3)-treated plants than in the control; the expression of NAC and MYB transcription factors, CESA, PAL, and GA oxidase was up-regulated during GA(3) treatment, suggesting their role in GA(3)-induced xylem development in the birch. Our results suggest that GA(3) induces the expression of secondary wall biosynthesis related genes to trigger xylogenesis in the birch plants.
DOI:10.1007/s10725-017-0342-8URL [本文引用: 1]
DOI:10.1007/s11356-018-2965-3URLPMID:30121770 [本文引用: 1]

Lodging is a major constraint contributing to poor grain yield and quality of wheat (Triticum aestivum L.) worldwide. The use of plant growth regulators is becoming a foremost agro-chemical approach for minimizing the risk of lodging in cereal crops. The present study was conducted to examine the effects of the paclobutrazol application on culm physical strength, lignin content, and lodging resistance of wheat. Wheat seeds were soaked in paclobutrazol at the concentrations of 0 (CK, as control), 200 (PB1), 300 (PB2), and 400 (PB3) mg L(-1). Our results showed that paclobutrazol resulted in a dose-dependent decrease of plant height, internode length, and center of gravity height. Paclobutrazol treatments evidently increased the culm diameter, culm filling degree, and wall thickness of basal internodes, resulting in greater stalk-breaking strength and lodging resistance index (CLRI), where their maximum values were obtained with PB1 treatment. In addition, the activities of lignin-related enzymes were improved by paclobutrazol, particularly at low concentration, which increased the lignin accumulation of the basal internodes of wheat, subsequently improving the capability of stalk lodging resistance. Moreover, the correlation analysis revealed significant correlations between stem diameter, culm filling degree, and lignin with stalk bending strength and CLRI. The paclobutrazol concentration >/= 300 mg L(-1) (PB2 and PB3 treatments) showed inhibitive effects on various culm morphological traits. These results suggest that not only the plant height, but also the lignin contents and physical strength of internodes are closely related with the lodging resistance of wheat, and reduction in plant height along with improved culm morphological characteristics and higher lignin accumulation in basal internodes could effectively relieve the risk of lodging.
DOI:10.1105/tpc.5.8.887URLPMID:8400871 [本文引用: 1]

Three independent recessive mutations at the SPINDLY (SPY) locus of Arabidopsis confer resistance to the gibberellin (GA) biosynthesis inhibitor paclobutrazol. Relative to wild type, spy mutants exhibit longer hypocotyls, leaves that are a lighter green color, increased stem elongation, early flowering, parthenocarpy, and partial male sterility. All of these phenotypes are also observed when wild-type Arabidopsis plants are repeatedly treated with gibberellin A3 (GA3). The spy-1 allele is partially epistatic to the ga1-2 mutation, which causes GA deficiency. In addition, the spy-1 mutation can simultaneously suppress the effects of the ga1-2 mutation and paclobutrazol treatment, which inhibit different steps in the GA biosynthesis pathway. This observation suggests that spy-1 activates a basal level of GA signal transduction that is independent of GA. Furthermore, results from GA3 dose-response experiments suggest that GA3 and spy-1 interact in an additive manner. These results are consistent with models in which the SPY gene product regulates a portion of the GA signal transduction pathway.
[本文引用: 1]
DOI:10.1016/j.plantsci.2017.06.014URLPMID:28818383 [本文引用: 1]

In addition to playing a key role in the response to environmental changes, cell walls are also considered as a valuable feedstock for cellulosic ethanol. Here we explored the effects of the stress-response hormones, salicylic acid and methyl jasmonate, on cell wall biosynthesis and biomass digestibility in Brachypodium distachyon, a species recently considered as a suitable model for biomass conversion. We found that in response to salicylic acid or methyl jasmonate treatment, plant growth was reduced coupled with significant changes in cell wall composition. Cellulose content increased in response to methyl jasmonate whereas a reduction in lignin content was found after salicylic acid application. Moreover, hemicellulose composition was altered and increases in caffeic acid, ferulic acid and p-coumaric acid content were detected in response to both treatments. The hormonal profile and the expression pattern of genes involved in cell wall biosynthesis were also modified. Biomass digestibility was reduced in leaf tissue after salicylic acid treatment and was negatively correlated with ferulic acid and p-coumaric acid content. The results obtained here aid in our understanding of cell wall dynamics in response to stress and will enable the development of new strategies to improve cell wall digestibility in bioenergy feedstock.
URL [本文引用: 1]

【目的】探讨苯并噻二唑(BTH)、水杨酸(SA)和纳米硅(SiO2)对甜瓜幼苗白粉病抗性的诱导作用及与木质素、HRGP含量的关系。【方法】以抗白粉病甜瓜品种‘银帝’和感病品种‘卡拉克赛’为材料,用BTH、SA和SiO2溶液分别预处理,5 d后接种白粉病菌并分4次调查处理植株的发病情况、测定叶片细胞壁中木质素和羟脯氨酸糖蛋白(HRGP)含量。【结果】(1)BTH和SA处理显著降低了甜瓜幼苗的白粉病病情指数,尤以BTH效果为好,抗病品种发病较感病品种轻,SiO2只在发病初期显著降低病情指数。(2)白粉菌接种和BTH、SA处理对甜瓜叶片木质素及HRGP含量增加具有显著的系统诱导作用,且细胞壁中HRGP积累与木质素沉积在时间进程和强度上表现出明显的同步性,抗病品种的增加程度大于感病品种,SiO2无显著诱导效果。【结论】HRGP的积累和细胞壁的木质化与甜瓜对白粉病的抗性反应有关,是寄主-病菌互作的重要生化机制之一。
URL [本文引用: 1]

【目的】探讨苯并噻二唑(BTH)、水杨酸(SA)和纳米硅(SiO2)对甜瓜幼苗白粉病抗性的诱导作用及与木质素、HRGP含量的关系。【方法】以抗白粉病甜瓜品种‘银帝’和感病品种‘卡拉克赛’为材料,用BTH、SA和SiO2溶液分别预处理,5 d后接种白粉病菌并分4次调查处理植株的发病情况、测定叶片细胞壁中木质素和羟脯氨酸糖蛋白(HRGP)含量。【结果】(1)BTH和SA处理显著降低了甜瓜幼苗的白粉病病情指数,尤以BTH效果为好,抗病品种发病较感病品种轻,SiO2只在发病初期显著降低病情指数。(2)白粉菌接种和BTH、SA处理对甜瓜叶片木质素及HRGP含量增加具有显著的系统诱导作用,且细胞壁中HRGP积累与木质素沉积在时间进程和强度上表现出明显的同步性,抗病品种的增加程度大于感病品种,SiO2无显著诱导效果。【结论】HRGP的积累和细胞壁的木质化与甜瓜对白粉病的抗性反应有关,是寄主-病菌互作的重要生化机制之一。
DOI:10.1105/tpc.18.00315URLPMID:30242037 [本文引用: 1]

Secondary cell walls (SCWs) are formed in some specific types of plant cells, providing plants with mechanical strength. During plant growth and development, formation of secondary cell walls is regulated by various developmental and environmental signals. The underlying molecular mechanisms are poorly understood. In this study, we analyzed the blue light receptor cryptochrome1 (cry1) mutant of Arabidopsis thaliana for its SCW phenotypes. During inflorescence stem growth, SCW thickening in the vasculature was significantly affected by blue light. cry1 plants displayed a decline of SCW thickening in fiber cells, while CRY1 overexpression led to enhanced SCW formation. Transcriptome analysis indicated that the reduced SCW thickening was associated with repression of the NST1-directed transcription regulatory networks. Further analyses revealed that the expression of MYC2/MYC4 that is induced by blue light activates the transcriptional network underlying SCW thickening. The activation is caused by direct binding of MYC2/MYC4 to the NST1 promoter. This study demonstrates that SCW thickening in fiber cells is regulated by a blue light signal that is mediated through MYC2/MYC4 activation of NST1-directed SCW formation in Arabidopsis.
DOI:10.1111/j.1365-313X.2010.04223.xURLPMID:20408998 [本文引用: 2]

To identify genes controlling secondary cell wall biosynthesis in the model legume Medicago truncatula, we screened a Tnt1 retrotransposon insertion mutant population for plants with altered patterns of lignin autofluorescence. From more than 9000 R1 plants screened, four independent lines were identified with a total lack of lignin in the interfascicular region. The mutants also showed loss of lignin in phloem fibers, reduced lignin in vascular elements, failure in anther dehiscence and absence of phenolic autofluorescence in stomatal guard cell walls. Microarray and PCR analyses confirmed that the mutations were caused by the insertion of Tnt1 in a gene annotated as encoding a NAM (no apical meristem)-like protein (here designated Medicago truncatula NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1, MtNST1). MtNST1 is the only family member in Medicago, but has three homologs (AtNST1-AtNST3) in Arabidopsis thaliana, which function in different combinations to control cell wall composition in stems and anthers. Loss of MtNST1 function resulted in reduced lignin content, associated with reduced expression of most lignin biosynthetic genes, and a smaller reduction in cell wall polysaccharide content, associated with reduced expression of putative cellulose and hemicellulose biosynthetic genes. Acid pre-treatment and cellulase digestion released significantly more sugars from cell walls of nst1 mutants compared with the wild type. We discuss the implications of these findings for the development of alfalfa (Medicago sativa) as a dedicated bioenergy crop.
DOI:10.1016/s1360-1385(02)02335-xURLPMID:12399182 [本文引用: 1]

Recent research has provided insights into how plants make cellulose - the major structural material of their cell walls and the basis of the cotton and wood fibre industries. Arabidopsis thaliana mutants impaired in cellulose production are defective in genes encoding membrane-bound glycosyltransferases, an endo-1,4-beta-glucanase and several enzymes involved in the N-glycosylation and quality-control pathways of the endoplasmic reticulum. The glycosyltransferases form the rosette terminal complexes seen in plasma membranes making cellulose. Synthesis might start by making lipoglucans, which, in turn, might form the substrate for the endo-1,4-beta-glucanase, before being elongated to form the long, crystalline microfibrils that assemble in the cell wall.
DOI:10.1093/pcp/pcf164URLPMID:12514238 [本文引用: 1]

Modern techniques of gene cloning have identified the CesA genes as encoding the probable catalytic subunits of the plant CelS, the cellulose synthase enzyme complex visualized in the plasma membrane as rosettes. At least 10 CesA isoforms exist in Arabidopsis and have been shown by mutant analyses to play distinct role/s in the cellulose synthesis process. Functional specialization within this family includes differences in gene expression, regulation and, possibly, catalytic function. Current data points towards some CesA isoforms potentially being responsible for initiation or elongation of the recently identified sterol beta-glucoside primer within different cell types, e.g. those undergoing either primary or secondary wall cellulose synthesis. Different CesA isoforms may also play distinct roles within the rosette, and there is some circumstantial evidence that CesA genes may encode the catalytic subunit of the mixed linkage glucan synthase or callose synthase. Various other proteins such as the Korrigan endocellulase, sucrose synthase, cytoskeletal components, Rac13, redox proteins and a lipid transfer protein have been implicated to be involved in synthesizing cellulose but, apart from CesAs, only Korrigan has been definitively linked with cellulose synthesis. These proteins should prove valuable in identifying additional CelS components.
DOI:10.1105/tpc.11.5.769URLPMID:10330464 [本文引用: 1]

The irregular xylem3 (irx3) mutant of Arabidopsis has a severe deficiency in secondary cell wall cellulose deposition that leads to collapsed xylem cells. The irx3 mutation has been mapped to the top arm of chromosome V near the marker nga106. Expressed sequence tag clone 75G11, which exhibits sequence similarity to cellulose synthase, was found to be tightly linked to irx3, and genomic clones containing the gene corresponding to clone 75G11 complemented the irx3 mutation. Thus, the IRX3 gene encodes a cellulose synthase component that is specifically required for the synthesis of cellulose in the secondary cell wall. The irx3 mutant allele contains a stop codon that truncates the gene product by 168 amino acids, suggesting that this allele is null. Furthermore, in contrast to radial swelling1 (rsw1) plants, irx3 plants show no increase in the accumulation of beta-1,4-linked glucose in the noncrystalline cell wall fraction. IRX3 and RSW1 fall into a distinct subgroup (Csa) of Arabidopsis genes showing homology to bacterial cellulose synthases.
DOI:10.1105/tpc.9.5.689URLPMID:9165747 [本文引用: 1]

Recessive mutations at three loci cause the collapse of mature xylem cells in inflorescence stems of Arabidopsis. These irregular xylem (irx) mutations were identified by screening plants from a mutagenized population by microscopic examination of stem sections. The xylem cell defect was associated with an up to eightfold reduction in the total amount of cellulose in mature inflorescence stems. The amounts of cell wall-associated phenolics and polysaccharides were unaffected by the mutations. Examination of the cell walls by using electron microscopy demonstrated that the decreases in cellulose content of irx lines resulted in an alteration of the spatial organization of cell wall material. This suggests that a normal pattern of cellulose deposition may be required for assembly of lignin or polysaccharides. The reduced cellulose content of the stems also resulted in a decrease in stiffness of the stem material. This is consistent with the irregular xylem phenotype and suggests that the walls of irx plants are not resistant to compressive forces. Because lignin was implicated previously as a major factor in resistance to compressive forces, these results suggest either that cellulose has a direct role in providing resistance to compressive forces or that it is required for the development of normal lignin structure. The irx plants had a slight reduction in growth rate and stature but were otherwise normal in appearance. The mutations should be useful in facilitating the identification of factors that control the synthesis and deposition of cellulose and other cell wall components.
DOI:10.1093/pcp/pcr185URLPMID:22197883 [本文引用: 1]

MYB46 and MYB83 are two functionally redundant Arabidopsis thaliana MYB transcription factors that act as master switches regulating secondary wall biosynthesis. Here, we report the identification of the transcriptional responsive elements and global analysis of the direct targets of MYB46 and MYB83. Using the estrogen-inducible direct activation system, we found that a number of previously identified MYB46 downstream transcription factors, including MYB43, MYB52, MYB54, MYB58, MYB63 and KNAT7, are direct targets of MYB46. Promoter deletion coupled with transactivation analysis of the MYB63 promoter led to the identification of a 7 bp sequence that is sufficient to be responsive to MYB46 activation, and therefore this sequence is designated as the secondary wall MYB-responsive element (SMRE). Further single nucleotide mutation together with electrophoretic mobility shift assay mapped the SMRE consensus sequence as ACC(A/T)A(A/C)(T/C). Genome-wide analysis of direct targets of MYB46 demonstrated that it directly regulates the expression of not only a number of downstream transcription factors, but also a suite of secondary wall biosynthetic genes, some of which are also directly activated by secondary wall NAC (SWN) master switches or by MYB46 direct targets. Furthermore, MYB83 was found to bind to the same SMRE consensus sequence and activate the same set of direct targets as MYB46. Our study has revealed that the transcription program regulating secondary wall biosynthesis involves a multileveled feed-forward loop regulatory structure in which MYB46/MYB83 together with their regulators SWNs and their direct targets regulate an array of downstream genes thereby activating the secondary wall biosynthetic program.
