 ,中国农业大学园艺学院,北京 100089
,中国农业大学园艺学院,北京 100089Cloning and Functional Analysis of CsRPL1/2 in Cucumber
SONG WeiYuan, HOU Yu, ZHAO JianYu, LIU XiaoFeng, ZHANG XiaoLan ,College of Horticulture, China Agricultural University, Beijing 100089
,College of Horticulture, China Agricultural University, Beijing 100089通讯作者:
责任编辑: 赵伶俐
收稿日期:2019-06-3接受日期:2019-07-2网络出版日期:2020-01-01
| 基金资助: |
Received:2019-06-3Accepted:2019-07-2Online:2020-01-01
作者简介 About authors
宋维源,E-mail:songwy@cau.edu.cn。

摘要
关键词:
Abstract
Keywords:
PDF (6472KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
宋维源, 侯钰, 赵剑宇, 刘小凤, 张小兰. 黄瓜CsRPL1/2的克隆及其功能分析[J]. 中国农业科学, 2020, 53(1): 148-159 doi:10.3864/j.issn.0578-1752.2020.01.014
SONG WeiYuan, HOU Yu, ZHAO JianYu, LIU XiaoFeng, ZHANG XiaoLan.
0 引言
【研究意义】黄瓜(Cucumis sativus L.)作为一种世界性蔬菜,在我国也有相当大的种植面积。2017年我国黄瓜栽培面积达1.24×106 hm2,产量达6 387.5万t,分别占全世界黄瓜栽培总面积和总产量的54.5%和77.46%(FAO数据)。黄瓜最主要的商品器官是果实,属于瓠果,由子房下位的三心皮合生雌蕊发育而来,心皮发育对于黄瓜产量和外观品质至关重要。而作为模式植物——拟南芥的果实属于角果类型,AtRPL是拟南芥果实发育调控网络中的重要基因,参与胎座框的形成[1]。因此,研究黄瓜RPL在果实生长发育中的作用对黄瓜育种具有重要意义。【前人研究进展】1984年,SCOTT等[2]运用分子生物学手段,首次发现了导致果蝇身体的一部分转换为另一部分(例如果蝇的触角转变成足或翅)的同源异形基因。随后研究发现,这些同源异形基因都编码一段具有螺旋-转角-螺旋(helix-turn-helix)特征的保守氨基酸序列,并命名为同源域(homeodomain HD)。植物中的许多HD同源基因已经被鉴定出来[3,4],其中一类特殊的同源基因家族TALE[5,6](three-amino-acid-loop- extension)通过编码转录因子在植物生长发育过程中发挥重要调控功能。TALE家族首个基因于1991年在玉米中被报道,并命名为KNT1[7](KNOTTED-1),属于KNOX(KNOTTED-like homeodomain)亚家族,另外,TALE还包含BELL(BEL1-like homeodomain)亚家族。BELL家族蛋白除了具有标志性的Homedomain结构域[5,6],还具有SKY-Domain和BELL-Domain结构域,两者合称为MID(MEINOX interacting domain),可以与KNOX蛋白的MEINOX结构域结合形成异源二聚体,进而调控下游基因的表达[8,9,10]。拟南芥中,BELL亚家族一共包含13个成员,其中BELL1(BEL1)为该家族中首个发现的基因[11]。REPLUMLESS(RPL),又名BELLRINGER(BLR)、PENNYWISE(PNY)、VAAMANA(VAN)、LARSON(LSN)同属于该家族,并且在植物生长发育多个方面行使重要功能。茎尖分生组织(SAM)是一群未分化的细胞,在植物生长过程中分化出各种侧生器官的同时也保持着自身数量稳定不变[12,13,14]。SHOOTMERISTEMLESS(STM)是分生组织的标记基因,基因功能缺失强突变体表现为缺少SAM,进而不能产生侧生器官[15,16]。RPL也被证明在分生组织保持中发挥作用,RPL功能缺失会加重stm弱突变体的表型,后续研究指出RPL与STM和BREVIPEDICELLUS(BP)都存在直接互作参与分生组织保持[17,18]。在植物株型方面,RPL突变体表现出植株矮小、果荚变短、节间不规则以及侧生器官增多的表型[17,19],表明RPL在SAM中调控侧生器官的分化。RPL同样在植物从营养生长到生殖生长这一转变过程中发挥作用。RPL(PNY)和PNF的双突变体植株不能开花,且双突中成花关键基因如LEAFY(LFY)、APETALA1(AP1)和CAULIFLOWER(CAL)的表达量下调[20,21]。在RPL(PNY)和PNF双突变体背景下过表达LFY可以使植物恢复开花,但过表达Flowering Locus T(FT)却不能,表明FT需要RPL(PNY)和PNF来行使功能[21,22]。在雌蕊发育过程中,RPL调控拟南芥胎座框形成,与FUL共同限制SHATTERPROOF 1(SHP1)、SHATTERPROOF 2(SHP2)在开裂区表达,从而形成木质层与离层,保证果实的正常开裂[1]。【本研究切入点】RPL的基因功能在拟南芥上研究较为深入,在植物生长发育过程中从器官起始到成花转变再到果实发育都发挥了重要作用。黄瓜作为重要蔬菜作物,其果实类型为瓠果,RPL在黄瓜生长发育中发挥的作用目前未知。【拟解决的关键问题】本研究利用同源序列比对的方法克隆得到黄瓜中RPL的同源基因CsRPL1/2,并利用时空表达分析、遗传转化拟南芥等方法初步探究CsRPL1/2在黄瓜生殖生长中的功能。1 材料与方法
1.1 试验材料
1.1.1 植物材料以黄瓜自交系461为试验材料。55℃温汤浸种后,于28℃暗培养催芽2 d,种子根约1 cm时播种于营养钵,待黄瓜幼苗长到三叶一心时定植于温室中。取黄瓜30 d左右苗龄植株的茎、叶、生长点,以及盛果期的雄花芽、雌花芽、开花当天的雄花、开花当天的雌花、3 mm果、1 cm果、2 cm果、3 cm果液氮速冻后,于-80℃超低温冰箱保存备用。
以拟南芥野生型Columbia(Col)为试验材料。0.03% TritionX-100/75%酒精消毒后播种于MS固体培养基上,4℃黑暗条件下低温处理4 d,转入16 h光照/8 h黑暗24℃环境中培养。约10 d待其长出真叶后移栽到土壤培养基质(花卉营养土﹕草炭﹕蛭石≈1﹕1﹕1)中,并进行后续试验。
1.1.2 试剂与菌株
提取植物总RNA试剂Trizol采购于北京华越洋生物科技有限公司;限制性内切酶XbaⅠ、KpnⅠ采购于New England Biolabs公司;Taq DNA聚合酶、同源重组酶采购于北京艾德莱生物科技有限公司;High-Fidelity DNA聚合酶采购于北京聚合美生物科技有限公司;RT-PCR试剂盒采购于北京天根生化科技有限公司;A-Tailing Enzyme、pMD19-T Vector、荧光定量试剂盒采购于TaKaRa公司;原位杂交探针合成试剂盒DIG RNA Labeling Kit(SP6/T7)采购于Roche公司;亚历山大染液采购于Solarbio公司;所用大肠杆菌感受态DH5α、根癌农杆菌感受态EHA105与植物过表达载体pSuper1300为本实验室保存。
1.2 试验方法
1.2.1 植物各部位RNA的提取与反转录根据华越洋公司提供的Trizol说明书提取黄瓜各部位RNA,利用天根公司反转录试剂盒合成单链cDNA,-80℃保存备用。
1.2.2 黄瓜CsRPL1/2克隆
将拟南芥BELL亚家族基因蛋白序列在黄瓜数据库中(http://www.cucurbitgenomics. org/)进行BLAST比对得到黄瓜BELL亚家族基因蛋白序列。用MEGA 5.2对拟南芥与黄瓜BELL亚家族基因蛋白序列进行系统发育树分析[23]。其中与拟南芥AtRPL(AT5G02030)同源性较高的为CsRPL1(Csa3G733340),同源性次之的为CsRPL2(Csa4G297540)。根据CsRPL的CDS序列,利用Primier 5.0设计克隆引物CsRPL1-F/R、CsRPL2-F/R(引物序列见表1)。用高保真聚合酶进行PCR扩增。扩增程序为95℃预变性2 min;94℃变性25 s;58℃复性30 s;68℃延伸30 s,35个循环;68℃延伸5 min。经电泳检测,琼脂糖凝胶回收产物3′端加A后连接pMD19-T载体。冰浴20 min,42℃热激2 min,转化大肠杆菌感受态DH5α。筛选阳性克隆送北京擎科新业生物技术有限公司测序。
Table 1
表1
表1引物序列
Table 1
| 引物名称 Primer name | 引物序列Primer sequence (5′-3′) |
|---|---|
| CsRPL1-F | ATGGCTGAGGGTATTGAATCCTAC |
| CsRPL1-R | TCAGCCCACAAAGTCATGTAACAGC |
| CsRPL2-F | ATGGCGGAGGGTTTTGAAGTTTAC |
| CsRPL2-R | TCAAAACCACTCATTTACAAACCCT |
| CsRPL1-1300-F | TACACCAAATCGACTCTAGAATGGCTGAGGGTATTGAATC |
| CsRPL1-1300-A | CCTTGCTCACCATGGTACCGCCCACAAAGTCATGTAACA |
| CsRPL2-1300-F | TACACCAAATCGACTCTAGAATGGCGGAGGGTTTTGAAGT |
| CsRPL2-1300-A | CCTTGCTCACCATGGTACCAAACCACTCATTTACAAACC |
| 35S-F | GTAAGGGATGACGCACAATC |
| CsRPL1-OV-R | ATTGAGAGAAGCGTTGATTTATGAG |
| CsRPL2-OV-R | TACTCAAATGTCGTAACCACTGCTT |
| UBI-F | CACCAAGCCCAAGAAGATC |
| UBI-R | TAAACCTAATCACCACCAGC |
| Actin-F | CCTTCGTCTTGATCTTGCGG |
| Actin-R | AGCGATGGCTGGAACAGAAC |
| CsRPL1-qPCR-F | CCGCTCACAATGGAAAACCC |
| CsRPL1-qPCR-R | AGGATACATTCAAGGGCTCG |
| CsRPL2-qPCR-F | TACGCCTCCTTTCGTTCCACT |
| CsRPL2-qPCR-R | TTGTCGTCGGAGATGTTAGGG |
| CsRPL1-SP6 | GATTTAGGTGACACTATAGAATGCTACCCGCTCACAATGGAAAAC |
| CsRPL1-T7 | TGTAATACGACTCACTATAGGGGTCCACCCATCACATACCCA |
| CsRPL2-SP6 | GATTTAGGTGACACTATAGAATGCTCTTCTTCTTTTTTTTCACCCG |
| CsRPL2-T7 | TGTAATACGACTCACTATAGGGCTTCCAAGGAGTCAAAAACGG |
新窗口打开|下载CSV
1.2.3 黄瓜CsRPL1/2蛋白保守域分析
在http://gsds.cbi.pku.edu.cn/网站分析CsRPL1/2的基因结构。用MEGA 5.2中的Clustal W对包括CsRPL在内的13种RPL同源基因的蛋白序列进行比对[23]。CsRPL1/2及同源蛋白3个域的氨基酸保守性利用在线工具(http://weblogo.berkeley.edu/logo.cgi)进行分析。
1.2.4 黄瓜CsRPL1/2过表达载体构建及遗传转化拟南芥
用XbaⅠ、KpnⅠ限制性内切酶对pSuper1300植物双元表达载体进行双酶切。以CsRPL1-pMD19-T、CsRPL2-pMD19-T质粒为模板,分别利用引物CsRPL1- 1300-F/R、CsRPL2-1300-F/R为基因的CDS序列添加同源臂接头。利用同源重组试剂盒对已切开的载体与带有同源臂接头的基因片段进行同源重组,转化大肠杆菌感受态DH5α,筛选阳性克隆送北京擎科新业生物技术有限公司测序。提取测序正确的CsRPL1-1300、CsRPL2-1300菌液质粒,冻融法转化至根癌农杆菌EHA105感受态中,PCR鉴定后将携带质粒的农杆菌通过农杆菌蘸花法遗传转化拟南芥。
1.2.5 转基因拟南芥阳性植株筛选与鉴定
收获农杆菌侵染后的拟南芥T0代植株种子,消毒后播种于含25 mg∙L-1潮霉素的MS固体培养基上培养筛选。7—10 d后,将根生长较长且已长出真叶的阳性苗移栽到土壤培养基中。1—2周后提取拟南芥叶片DNA,利用引物35S-F、CsRPL1-OV-R、CsRPL2-OV-R进行PCR鉴定,其中35S-F为载体35S启动子上特异片段,CsRPL1-OV-R、CsRPL2-OV-R分别为CsRPL1、CsRPL2 CDS序列上特异片段。对阳性转基因拟南芥植株进行单株收种,再次播种后即为T2代转基因植株。对T2代转基因植株进行表型观察和表达分析。
1.2.6 拟南芥花粉活力染色检测
在上午10:00左右选取拟南芥植株主茎上完全开放成“十字形”的花10朵,置于1.5 mL离心管中,冰上保存。在载玻片上滴一滴亚力山大染液;解剖镜下小心剖去花瓣、萼片与雌蕊,将花药浸没在染液中并用镊子轻轻挤压使花粉散出;立即盖上盖玻片在显微镜下观察。有活力的花粉被染成红色,没有活力的花粉不被染色呈无色。选取显微镜下5个视野,统计没有活力花粉占视野中总花粉比例。
1.2.7 实时荧光定量PCR
提取黄瓜幼茎、幼嫩叶片、顶点、雌花芽、雄花芽、开放当天雄花、开放当天雌花、3 mm果实、1 cm果实、2 cm果实和3 cm果实的RNA,并反转录为cDNA;提取拟南芥col及相关转基因株系花序的RNA,反转录为cDNA。以黄瓜Ubiquitin(NM_001282241)和拟南芥Actin(AT5G09810)分别为内参基因。依照TB Green Premix Ex Taq II试剂盒(TaKaRa)说明进行加样与PCR。选用10 μL反应体系,PCR反应程序为:95℃预变性30 s;95℃变性5 s,58℃复性 34 s,40个循环。用2-△△CT分析方法计算基因相对表达量。试验采用3次技术重复,3次生物学重复。
1.2.8 mRNA原位杂交
取20 d黄瓜苗的生长点和盛果期1 cm的果,立即置于FAA固定液中4℃固定,并用石蜡包埋。选取CsRPL1(330 bp)和CsRPL2(309 bp)的特异片断设计上、下游引物,并在上、下游引物的5′端分别添加SP6和T7接头。利用DIG RNA Labeling Kit(SP6/T7)试剂盒合成探针,其中SP6探针为负对照,并利用点杂交的方法检测探针质量。将样品切成10 μm厚的切片,置于载玻片上,42℃烘片4—12 h,将烘干样品进行脱蜡、去蛋白、乙酰化、脱水处理后,敷探针50℃过夜,使探针与目的片段结合(此过程完成前所有步骤均要求RNase-free环境)。洗去探针,封闭处理后与抗体共孵育,之后洗去抗体用BICP(5-Bromo-3-Indolyl-4-Chloro Phosphate )和NBT(Nitroblue tetrazolium chloride)进行染色处理(避光),2—3 d后用显微镜观察样品组织染色情况,并拍片[24]。
2 结果
2.1 黄瓜CsRPL1/2的克隆
将拟南芥BELL亚家族基因蛋白序列比对到黄瓜库中,发现黄瓜中存在11个BELL亚家族基因。系统发育树分析发现黄瓜中Csa3G733340和Csa4G297540与拟南芥AtRPL位于同一分枝,蛋白序列相似度分别为41.43%和37.97%,依次命名为CsRPL1和CsRPL2(图1-A)。通过同源克隆得到黄瓜CsRPL1/2的CDS序列,与黄瓜基因组库(http:// www.cucurbitgenomics. org/)中序列一致。CsRPL1/2的CDS长度分别为1 443 bp和1 386 bp。基因结构与拟南芥AtRPL相似,都含有3个内含子和4个外显子,且第3个外显子长度高度保守(图1—B)。其中CsRPL1/2第3外显子长61 bp,AtRPL第3外显子长63 bp。图1
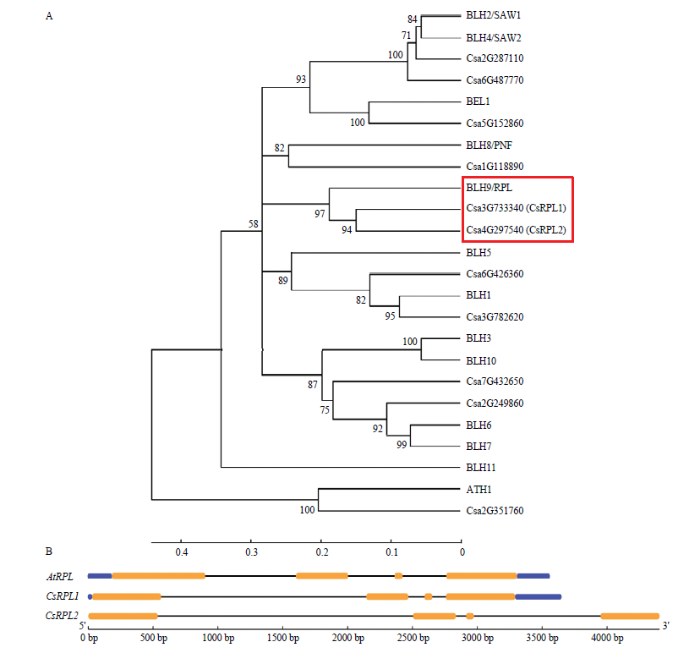 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1黄瓜CsRPL1/2与拟南芥AtRPL的进化树和基因结构分析
A:黄瓜与拟南芥BELL亚家族基因蛋白序列系统发育树分析,红色实线框内为AtRPL和与其同源的CsRPL1/2。BEL1(AT5G41410);ATH1(AT4G32980);BLH1(AT2G35940);BLH2/SAW1(AT4G36870);BLH3(AT1G75410);
BLH4/SAW2(AT2G23760);BLH5(AT2G27220);BLH6(AT4G34610);BLH7(AT2G16400);BLH8/PNF(AT2G27990);
BLH9/RPL(AT5G02030):BLH10(AT1G19700);BLH11(AT1G75430)。B:紫色方块代5′UTR、3′UTR;橙色方块代表外显子;直线代表内含子
Fig. 1The phylogenetic analysis and gene structure of cucumber CsRPL1/2 and Arabidopsis AtRPL
A: Phylogenetic analysis of BELL subfamily gene in cucumber and Arabidopsis thaliana, AtRPL and its homologous gene CsRPL1/2 in the red box. BEL1 (AT5G41410); ATH1 (AT4G32980); BLH1 (AT2G35940); BLH2/SAW1 (AT4G36870); BLH3 (AT1G75410); BLH4/SAW2 (AT2G23760); BLH5 (AT2G27220); BLH6 (AT4G34610); BLH7 (AT2G16400); BLH8/PNF (AT2G27990); BLH9/RPL (AT5G02030); BLH10 (AT1G19700); BLH11 (AT1G75430). B: Purple squares represent 5' UTR, 3' UTR; orange squares represent exons; straight lines represent introns
2.2 黄瓜CsRPL1/2蛋白序列分析
利用NCBI网站进行BLAST得到除黄瓜外13个物种RPL同源蛋白序列,并用MEGA 5.2中的Clustal W对蛋白序列进行比对。结果表明,RPL同源基因都含有BELL-Domain和Homeodomain两个保守域,且保守域内氨基酸在不同物种中高度保守(图2-A、B和C)。同时在RPL同源蛋白序列中存在两个EAR-Motif(图2-D和E),其中第二个EAR-Motif氨基酸序列完全保守其序列特征为LTLGL(图2-A和E);除了蔷薇科的葡萄、锦葵科的棉花以及单子叶的玉米、小麦和水稻外,第一个EAR-Motif序列也高度保守,序列特征符合EAR-Motif的序列特征LxLxL(x代表任意氨基酸)(图2-A和D)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2黄瓜CsRPL与其他物种RPL同源蛋白序列比对
A:橙色实线框内为BELL-Domain,蓝色实线框内为Homeodomain,红色虚线框内为EAR-Motif。AtRPL:拟南芥RPL(NP_195823.1);GmRPL:甜瓜RPL(XP_008448414.1);VvRPL:葡萄RPL(XP_010654234.1);PpRPL:桃RPL(XP_007208167.1);GhRPL:棉花RPL(XP_016738976.1);MdRPL:苹果RPL(XP_008363517.1);GmRPL:大豆RPL(XP_003516903.1);SlRPL:番茄RPL(XP_004246395.1);ZmRPL:玉米RPL:(NP_001168681.1);TaRPL:小麦RPL(BAJ04689.1);OsRPL:水稻RPL(XP_015641948.1)。B—E:BELL (B)、Homeodomain保守域(C)和EAR-Motif(D—E)的氨基酸保守性分析。D中的氨基酸序列不包括葡萄、棉花以及单子叶植物玉米、水稻和小麦;星号代表完全保守氨基酸
Fig. 2Sequence alignment of cucumber CsRPL with RPL homologs from other species
A: BELL-Domain in the orange box, Homeodomain in the blue box, and EAR-Motifs in the red dotted box. AtRPL: Arabidopsis RPL(NP_195823.1); GmRPL: Melon RPL (XP_008448414.1); VvRPL: Grape RPL (XP_010654234.1); PpRPL: Peach RPL (XP_007208167.1); GhRPL: Cotton RPL (XP_016738976.1); MdRPL: Apple RPL (XP_008363517.1); GmRPL: Soybean RPL (XP_003516903.1); SlRPL: Tomato RPL (XP_004246395.1); ZmRPL: Corn RPL: (NP_001168681.1); TaRPL: Wheat RPL (BAJ04689. 1); OsRPL: Rice RPL (XP_015641948.1); B-E: Amino acid conservation analysis of BELL-Domain (B), Homeodomain (C) and EAR-Motif (D and E); VvRPL, GhRPL and monocotyledon ZmRPL, TaRPL and OsRPL was not included; Asterisks represent the completely conserved amino acids
2.3 CsRPL1/2在黄瓜各部位的表达模式分析
CsRPL1在黄瓜地上部各个器官中均有表达,且表达量都远高于CsRPL2。CsRPL1在叶中表达量最少,在开放的雄花中表达量最高,同时随着果实的发育,CsRPL1的表达量逐渐下降。CsRPL2在茎中表达量最高,其次为开花当天的雄花,在果实中的表达量随着果实的发育而逐渐降低。原位杂交结果显示CsRPL1/2在黄瓜果实的胎座框中表达(图3-B和D);且杂交信号在植株顶点和侧生器官分生组织处的central zone(虚线框)区域富集(图3-C和E)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3CsRPL1/2表达模式分析
A:CsRPL1/2在黄瓜各器官中的表达分析;B—F:CsRPL1(B和C)和CsRPL2(D和E)在黄瓜果实与植株顶点处的原位杂交分析;B、D:CsRPL1/2在胎座框处表达;C、E:CsRPL1/2信号在植物顶端分生组织的CZ区域富集;白色虚线框代表信号富集区域;F:CsRPL-SP6探针作为负对照,未发现杂交信号。比例尺:100 μm
Fig. 3Gene expression pattern of CsRPL1/2
A: Relative expression of CsRPL1/2 in various parts of cucumber; B-F: In situ hybridization analysis of CsRPL1 (B, C) and CsRPL2 (D, E) in cucumber fruits and shoot tips; B, D: CsRPL1/2 expressed in placenta C, E: CsRPL1/2 signal was enriched in the central zone of the meristem in the shoot tips; White dotted box represents the signal enrichment region; F: CsRPL-SP6 probe was used as negative control, and no hybridization signal was found. Scale bar: 100 μm
2.4 CsRPL1/2过表达载体遗传转化拟南芥
为研究CsRPL的基因功能,用35S组成型启动子构建过表达载体,并遗传转化野生型拟南芥Columbia(Col)。得到T1代植株后提取DNA,用PCR鉴定的方法分别得到CsRPL1(#1-1、#1-2、#1-3)和CsRPL2(#2-1、#2-2、#2-3)各3株过表达转基因阳性植株(图4),取转基因拟南芥的花序提取RNA,对CsRPL1/2的表达量进行荧光定量分析,35S::CsRPL1和35S::CsRPL2转基因株系中的CsRPL1和CsRPL2的表达量相较于野生型表达量急剧升高(图5-H)。图4
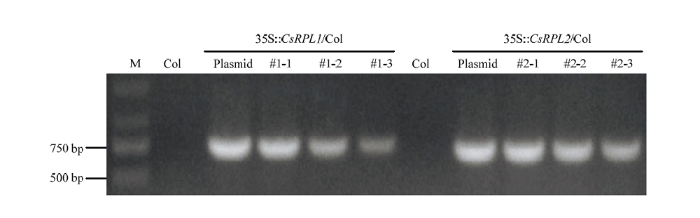 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4转基因拟南芥株系的PCR鉴定
M:2000 bp DNA标准分子量;Plasmid:阳性对照质粒;Col:野生型拟南芥
Fig. 4PCR identification of transgenic Arabidopsis
M: 2000 bp marker; Plasmid: positive control plasmid; Col: wild type Arabidopsis
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5CsRPL1/2异源表达拟南芥表型观察与统计分析
A:野生型与转基因植株表型(Col:野生型;#1-1:CsRPL1过表达株系;#2-1:CsRPL2过表达株系,比例尺:10 cm);B:果荚内种子发育情况,白色箭头指向败育的种子(比例尺:2 mm);C:果荚长度表型(比例尺:1 cm);D:果荚长度统计数据;E:花粉活力检测(比例尺1 mm);F:单个果荚种子数统计;G:花粉活力的统计数据;H:野生型与转基因植株中CsRPL1/2的表达量分析
Fig. 5Phenotypic observation and statistical analysis of ectopic expression of CsRPL1/2 in Arabidopsis
A: The phenotype of wild type and transgenic plants (Col: wild type; #1-1: CsRPL1 overexpressing line; #2-1: CsRPL2 overexpressing line, scale bar: 10 cm); B: Seeds development in the fruits, white arrows pointed to abortive seeds (scale bar: 2 mm); C: The character of silique length (scale bar: 1 cm) D: Statistical data of silique length; E: Pollen viability test (scale bar 1 mm); F: Statistical data of seed number per silique; G: Statistical data of pollen viability test; H: Expression analysis of CsRPL1/2 in wild type and transgenic plants
2.5 转基因拟南芥植株表型分析
将鉴定得到的CsRPL1和CsRPL2的各3个T1代转基因株系单株收种,用潮霉素抗性筛选阳性植株,同时播种野生型Col拟南芥植株作为对照。每个株系移栽6株(即为T2代株系),并对其进行表型观察。与野生型相比,除了果荚长度显著变短外,35S::CsRPL1和35S::CsRPL2转基因株系的整个植株的生长发育未发生明显变化(图5-A)。进一步表型观察,统计每株拟南芥主茎从下到上20个果荚长度,CsRPL1/2转基因株系果荚长度均明显短于野生型(图5-D)。将转基因拟南芥果荚沿着腹缝线剥开,发现部分种子败育(图5-B);且转基因拟南芥株系的成熟果荚种子数较野生型也显著减少(图5-F)。为探究种子败育原因,用亚历山大染料染色法观察花粉活力,发现转基因拟南芥未染色花粉比例明显高于野生型植株(图5-E和G)。3 讨论
TALE基因家族广泛存在于动植物中[25],RPL作为其中的一员,其功能涉及植物生长发育的多个方面,包括SAM(Shoot apical meristem)的起始与保持、侧生器官分化、茎伸长、成花转变、四轮花器官发育,以及果实发育等[1,18,20-22,26-35]。本研究中利用同源比对,发现黄瓜中存在两个RPL,且与拟南芥AtRPL相比,内含子与外显子数量相同;内含子长度相近,表明RPL基因长度和结构相对保守。CsRPL1和CsRPL2的蛋白序列均包含BELL-Domain、Homeodomain保守域,以及分别位于BELL-Domain上游和Homeodomain下游的两个EAR-Motif。EAR-Motif最早发现于TFⅢA型锌指蛋白中[36],与转录因子相连时转录因子表现出转录抑制作用[37],并且这种抑制作用在许多基因的研究中得到证明,如AGL15[38]、AUX/IAA[39]等。因此,推测黄瓜CsRPL在行使功能时可能表现为转录抑制作用。拟南芥中,AtRPL在叶中少量表达,在茎、花序和花中表达量较高[1]。在黄瓜中,CsRPL1在叶片中表达低,其次为顶点和茎,在生殖器官花芽和开放的花中表达量较高,特别是在开放的雄花中,其表达量是叶片中的25倍左右。值得注意的是,随着黄瓜果实的生长,CsRPL1表达量呈逐渐下降趋势,推测CsRPL1可能主要在黄瓜果实生长发育前期发挥作用。与CsRPL1相比,在所有检测部位中,CsRPL2的表达量极低,且在生殖器官中的表达模式与CsRPL1相似,因此推测CsRPL1和CsRPL2可能存在功能冗余,且在正常条件下,CsRPL1优先发挥功能。拟南芥中,RPL在胎座框处表达,通过限制SHP1/2异位表达从而促进拟南芥胎座框的形成[1]。黄瓜果实横切面的原位杂交结果显示CsRPL1/2同样在果实胎座处表达,但是其具体功能有待进一步探究。之前有报道AtRPL在拟南芥SAM的PZ(peripheral zone)区域表达[19,26],而本研究中黄瓜CsRPL1/2信号则主要聚集在分生组织的CZ(central zone)区域,推测黄瓜RPL在生长点处的功能与拟南芥RPL存在不同,有待进一步研究。
拟南芥CsRPL1/2过表达转基因植株均表现为果荚变短的表型,且果荚内部有很多胚珠败育,不能发育形成正常的种子,野生型植株种子则发育正常,之后对单个成熟果荚种子数的统计结果也证明了这一点。种子的发育需要雌雄配子的结合,花粉萌发后花粉管伸长运送精细胞至胚珠与卵细胞结合,完成授精进而发育成种子。转基因植株花粉活力检测结果显示(其雄蕊与野生型相比结构上无明显差异),转基因植株花粉约有30%左右不能被染色,而野生型植株花粉则几乎全部被染色,说明转基因株系花粉活力下降或许是种子败育的原因之一。拟南芥花粉发育是一个极为精细的过程,大约有3 500个基因参与表达[40]。在花药发育早期,AG在调控雄蕊原基形成的同时[41],也被报道通过正调控SPL来诱导花粉形成[42]。而SPL下游的一系列基因包括BAM1/2、TPD1、EMS、SERK1/2和RPK2都控制着花粉囊细胞的分化[43,44,45,46]。在成熟花粉形成阶段,DYT1作为EMS1下游基因,两者共同调控绒毡层发育[47,48],在ms1突变体中绒毡层细胞没有发生细胞凋亡,同时花粉壁合成异常导致花粉最终败育[49]。AMS在调控绒毡层形成的同时,在花粉壁发育网络中也起着至关重要的作用[50,51]。结合CsRPL1/2在雄花芽和开放雄花中的高表达模式,推测CsRPL1/2可能参与花粉的发育,但其在生殖器官发育中的功能还需要进一步探究。
4 结论
在黄瓜中存在两个RPL同源基因并命名为CsRPL1、CsRPL2,同属于BELL(BEL1-like homeodomain)家族。CsRPL1在黄瓜各器官中的表达量远高于CsRPL2;在拟南芥中异源过表达CsRPL1/2均会导致花粉育性降低,果荚变短,说明CsRPL1/2可能参与生殖器官的发育,且存在功能冗余,在正常生长条件下,CsRPL1优先发挥功能。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 5]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
