 ,, 陈明明, 张俊星, 张林林, 李新, 郭宏, 丁向彬
,, 陈明明, 张俊星, 张林林, 李新, 郭宏, 丁向彬 ,, 刘新峰
,, 刘新峰 ,天津农学院动物科学与动物医学学院,天津 300384
,天津农学院动物科学与动物医学学院,天津 300384Effects of Bovine LncRNA-133a on the Proliferation and Differentiation of Skeletal Muscle Satellite Cells
LI Yan ,, CHEN MingMing, ZHANG JunXing, ZHANG LinLin, LI Xin, GUO Hong, DING XiangBin
,, CHEN MingMing, ZHANG JunXing, ZHANG LinLin, LI Xin, GUO Hong, DING XiangBin ,, LIU XinFeng
,, LIU XinFeng ,College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384
,College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384通讯作者:
第一联系人:
收稿日期:2018-05-18接受日期:2018-09-28网络出版日期:2019-01-01
| 基金资助: |
Received:2018-05-18Accepted:2018-09-28Online:2019-01-01

摘要
关键词:
Abstract
Keywords:
PDF (3029KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李燕, 陈明明, 张俊星, 张林林, 李新, 郭宏, 丁向彬, 刘新峰. 牛LncRNA-133a对骨骼肌卫星细胞增殖分化的影响[J]. 中国农业科学, 2019, 52(1): 143-153 doi:10.3864/j.issn.0578-1752.2019.01.013
LI Yan, CHEN MingMing, ZHANG JunXing, ZHANG LinLin, LI Xin, GUO Hong, DING XiangBin, LIU XinFeng.
0 引言
【研究意义】骨骼肌作为动物躯体最重要的组织之一,占重比可高达40%[1],因此骨骼肌的发育对经济动物肉牛来说意义重大。肌细胞作为骨骼肌的基本形成单位,它的分化发育直接影响到骨骼肌的发育形成[2]。随着人们对LncRNAs研究的不断深入,研究者已经发现许多LncRNAs可以参与肌细胞的增殖和分化过程[3,4,5]。【前人研究进展】linc-MD1及lnc-mg作为内源性竞争性RNA(ceRNA)促进肌细胞的分化发育[6,7];lncRNA MAR1发挥其“sponge”吸附作用促进肌分化和再生[8];Linc-YY1 则通过与靶基因的互作作用促进肌分化和再生[9];此外,还有Lnc-SEMT,H19等,也都在肌形成过程中发挥其重要的生物学功能[10-12]]。【本研究切入点】有关LncRNAs参与肌肉发育的研究已有很多,但多集中于模式动物小鼠的研究上。近年来,随着生物检测技术及生物信息学分析技术的发展,通过对牛骨骼肌肌肉组织进行高通量测序分析,已经获得了大量与肌肉发育潜在相关的LncRNAs[13,14,15]。研究者针对这些 LncRNAs开展了一系列的功能研究,并证实了其中一些LncRNA如lncMD[14]、LncRNA-AK143003[15]、LncRNA HZ-5[16]、LncRNA H19[17]等,确实参与调节牛骨骼肌细胞的分化发育。但相较小鼠C2C12成肌细胞生长发育相关LncRNA的报道,参与调节牛骨骼肌细胞生长发育的LncRNAs的相关报道仍然较少,测序获得的与牛肌肉发育相关的海量LncRNAs,还需要研究者不断挖掘和证实它们的功能。【拟解决的关键问题】本研究利用前期在牛肌肉组织中鉴定到的长链非编码RNA LncRNA-133a,通过表达谱分析,并进一步以体外分化牛骨骼肌卫星细胞为模型,通过过表达/抑制LncRNA-133a处理,探究其对牛骨骼肌卫星细胞增殖和分化过程的影响,旨在为牛骨骼肌生长发育相关LncRNAs的功能研究积累更多的有益资料。
1 材料与方法
试验于2017—2018年在天津市农业动物繁育及健康养殖重点实验室完成。1.1 材料
1.1.1 肌肉组织与细胞来源 肌肉组织为天津市农业动物繁育及健康养殖重点实验室冻存的3、6、9月龄胎牛及24月龄成年和牛骨骼肌肌肉;细胞为该实验室分离冻存的原代牛骨骼肌卫星细胞。1.1.2 主要仪器与试剂 CO2恒温培养箱(日本三洋);LightCycle 96实时荧光定量PCR仪(Roche);Nano-Drop ND 2000c Spectrophotometer(Thermo- scientific);荧光显微镜(Leica)。
pCDNA3.1-EGFP(武汉淼灵生物科技有限公司); siRNA oligos(上海吉玛基因);Lipofectamine? 3000(Invitrogen);Trizol reagent(Invitrogen);PrimeScript II 1st Strand cDNA Synthesis Kit(Takara);All-in- OneTM qPCR Mix(GeneCopoeia);Hoechst 33342染色液(碧云天);一抗,Anti-Myosin VⅡα antibody ab3481(abcom);二抗,CY3-羊抗兔IgG(BA1032,博士德生物);Cell-Light EdU Apollo 567 In Vitro Imaging Kit(RN:R11053.2,广州市锐博生物科技有限公司);qRT-PCR检测相关引物(苏州金唯智生物科技有限公司)。
1.2 方法
1.2.1 牛肌肉组织中LncRNA-133a时序表达谱鉴定 qRT-PCR检测:3、6、9月龄胎牛及成年牛肌肉组织全基因组检测LncRNA-133a的表达情况,其中3月龄胎牛肌肉组织作为对照。1.2.2 细胞培养 常规复苏实验室前期冻存的原代牛骨骼肌卫星细胞[18],利用原代牛骨骼肌卫星细胞体外培养模拟牛肌肉的发育进程[19],使用增殖培养基:含体积分数20%的胎牛血清、100 IU·mL-1青霉素和链霉素的DMEM,置于37℃、5%CO2、饱和湿度培养箱中培养。待增殖细胞融合度达80%—90%时将培养基更换为含体积分数2%马血清的DMEM的分化培养基。
1.2.3 牛骨骼肌卫星细胞转染及收集 24孔培养板内增殖期细胞融合度达50%时,按生产商家说明书利用Lipfectamine3000转染已构建的过表达载体pCDNA3.1-EGFP-LncRNA-133a(简写为pcDE-LNC)及抑制物 si-lncRNA133a,以空质粒pCDNA3.1-EGFP(简写为pcDE-NC)及si-NC转染细胞作为对照,转染后24 h时培养基更换为分化培养基。
细胞总RNA及总蛋白收集。增殖期细胞(D0):更换分化培养基前收集;分化期细胞(D1—D3):于更换培养基后24 h(D1)、48 h(D2)、72 h(D3)时收集。按生产商家说明书利用Trizol及蛋白裂解液裂解并提取细胞总RNA及蛋白。
1.2.4 LncRNA-133a、分化标记基因表达水平的检测 qRT-PCR检测:收集24孔板中增殖期(D0)、分化期(D1—D3)未处理细胞、各转染处理细胞以及相应对照组细胞。取4 μg总RNA利用 All-in- OneTM First-Strand cDNA Synthesis Kit 试剂盒反转录为第一链cDNA,再采用qRT-PCR法检测各基因的表达。
qRT-PCR反应体系:10 μmol·L-1上游引物、10 μmol·L-1下游引物、 2.0 μL 5x稀释 cDNA、10 μL 2×All-in-OneTM qPCR Mix,Nuclease-free water 将体系补至20 μL。反应条件:95℃ 10 min;95℃ 10 s、60℃ 20 s、72℃ 15 s,重复35个循环。qRT-PCR反应引物信息如表1所示。
Table 1
表1
表1qRT-PCR引物信息
Table 1
| 基因 ID Gene ID | 引物序列(5′-3′) Primers sequence (5′-3′) | 产物长度 Product length (bp) | |
|---|---|---|---|
| LncRNA-133a | Forward | GCATAGCCGGTGTCTGAGAG | 108 |
| Reverse | CGGCCGCTTGTATATTGTCC | ||
| MHC | Forward | GTGGAATCCGGAGGCAGAA | 105 |
| Reverse | TTTTCGAAGGTAGGGAGCGG | ||
| MyoG | Forward | GGCTGACAAATGCCAGACTATCC | 140 |
| Reverse | TGGTCCCTTGCTTTATCTCCCT | ||
| MyoD | Forward | GACGGCTCTCTCTGCAACTT | 101 |
| Reverse | CGGCGCGGATCCAGGT | ||
| GAPDH-1 | Forward | ACAGTCAAGGCAGAGAACGG | 98 |
| Reverse | CCAGCATCACCCCACTTGAT | ||
| GAPDH-2 | Forward | CCTGCCCGTTCGACAGATAG | 153 |
| Reverse | ATGGCGACGATGTCCACTTT | ||
新窗口打开|下载CSV
1.2.5 牛骨骼肌卫星细胞增殖、分化能力的检测 qRT-PCR检测:24孔板中细胞在转染后DM2期时,检测肌细胞分化标记因子MYOD(muscle regulatory factors, MRFs成员)、MYOG(myogenin)及MHC(Myosin heavy chain)的转录组表达情况。
EdU细胞增殖检测:96孔板中细胞在转染后D0期时,按Cell-Light EdU Apollo 567 In Vitro Imaging Kit试剂盒说明书检测各处理细胞的增殖期细胞数。
MHC细胞免疫荧光检测:48孔板中各处理细胞在DM2期时,进行肌卫星细胞分化标记因子MHC的免疫荧光蛋白染色检测。
1.2.6 牛骨骼肌卫星细胞分化标记基因蛋白表达水平的检测 Western blotting检测:6孔板中细胞在DM2期时被蛋白裂解液裂解,收集蛋白,通过Western blotting检测分化标志基因MHC的蛋白表达。
1.2.7 统计分析 每组试验均设置3个生物学重复,所有数据均以“平均数±标准误”表示。每个生物学重复至少采集3个技术重复或视野。对于EdU细胞增殖效率(EdU阳性细胞数/ Hoechst 33342标记细胞数),分别计数5个200 x视野的细胞总数及EdU阳性细胞数,结果采用X2检验进行分析;荧光显微镜观察5个100 x视野的MHC蛋白的免疫荧光染色;qRT-PCR结果按2-ΔΔCt 法计算,采用t检验进行差异显著性分析,以GAPDH基因作为内参基因对检测的目的基因进行归一化。其中,“**”表示差异极显著(P<0.01),“*”表示差异显著(P<0.05),“N.S.”表示差异不显著(P>0.05)。
2 结果
2.1 LncRNA-133a的组织表达谱
LncRNA-133a是天津农学院动物分子育种与转基因创新中心实验室前期从3、6、9月龄胎牛肌肉组织的高通量测序数据中筛选鉴定出的组织时序表达呈下降趋势的一条LncRNA。本研究利用qRT-PCR法,验证不同月龄肌肉中LncRNA-133a的时序表达情况。如图1:除在6月龄肋间肌及背腰肌中有相对上调外,LncRNA-133a在3、6、9月龄胎牛及成年牛各肌肉组织中均呈下降的表达趋势。表明测序结果可靠,该LncRNA可进行下一步的功能研究。图1
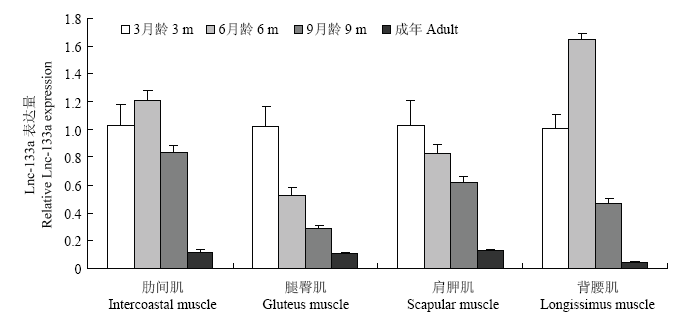 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图13、6、9月龄胎牛及成年牛不同肌肉组织中LncRNA-133a的表达
Fig. 1Expression of LncRNA-133a in different muscle tissue of 3, 6 and 9 months old fetus and adult cattle
2.2 牛骨骼肌卫星细胞LncRNA-133a及肌分化标记因子的表达水平
光镜下观察D0-D3时期牛骨骼肌卫星细胞的分化状态并于对应时期收集细胞总RNA,qRT-PCR法检测LncRNA-133a及肌分化标记因子的正常表达水平。如图2、图3:随着骨骼肌卫星细胞进入诱导分化阶段,牛骨骼肌卫星细胞分化形成的肌管清晰可见(图2),且肌分化标记因子MHC及MyoG均呈上升表达趋势,表明牛骨骼肌卫星细胞的体外诱导分化模型构建成功;LncRNA-133a在牛骨骼肌卫星细胞分化期间高表达,且分化48 h表达量最高,这提示其可能参与牛骨骼肌卫星细胞分化发育的调节过程。图2
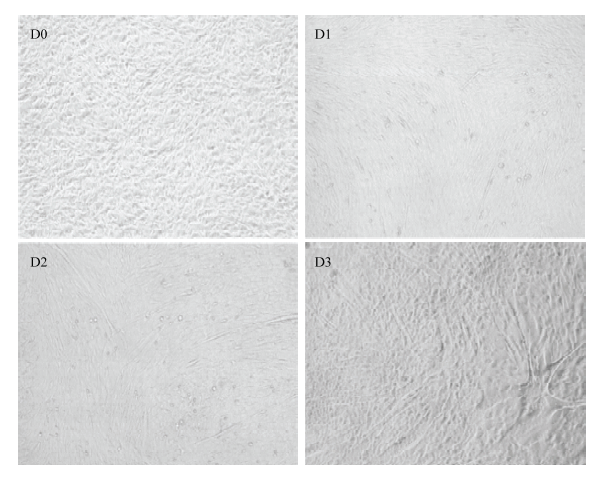 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2牛骨骼肌卫星细胞分化进程(100×)
Fig. 2Differentiation process of bovine skeletal muscle satellite cells (100×)
图3
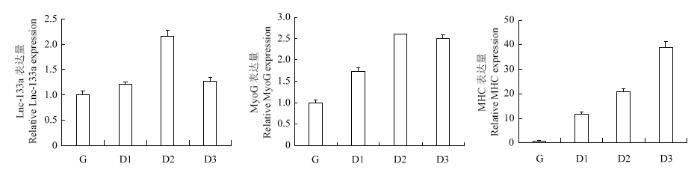 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3牛骨骼肌卫星细胞分化阶段 LncRNA-133a及分化标记因子的表达
Fig. 3Expression of LncRNA-133a and marker genes of myogenic differentiation during the differentiation of bovine skeletal muscle satellite cells
2.3 LncRNA-133a的过表达/抑制效率
牛骨骼肌卫星细胞转染过表达LncRNA-133a载体pcDE-LncRNA-133A或LncRNA-133a的抑制物 si-lncRNA133a 及相应对照载体pcDE-NC或对照抑制物si-NC。利用qRT- PCR法检测转染处理后D0及D2期过表达/抑制效率。相比pcDE-NC组,pcDE- LNC组LncRNA-133a在D0和D2期均有显著过表达效果(图4-A);si-lncRNA133a 组相比si-NC组,si-lncRNA133a组LncRNA-133a在D0和D2期均有显著抑制效果(图4-B)。结果表明构建过表达/抑制LncRNA-133a的牛骨骼肌卫星细胞模型成功,可进行下一步试验验证。2.4 LncRNA-133a对牛骨骼肌卫星细胞增殖能力的影响
过表达/抑制LncRNA-133a 后D0期对牛骨骼肌卫星细胞进行EdU染色检测牛骨骼肌卫星细胞增殖数。结果显示:过表达LncRNA-133a组EdU阳性细胞数显著增多;而抑制LncRNA-133a后,EdU阳性细胞数则显著减少。这表明LncRNA-133a可以促进牛骨骼肌卫星细胞的增殖,对牛骨骼肌卫星细胞的增殖有着正向调控作用(图5)。图4
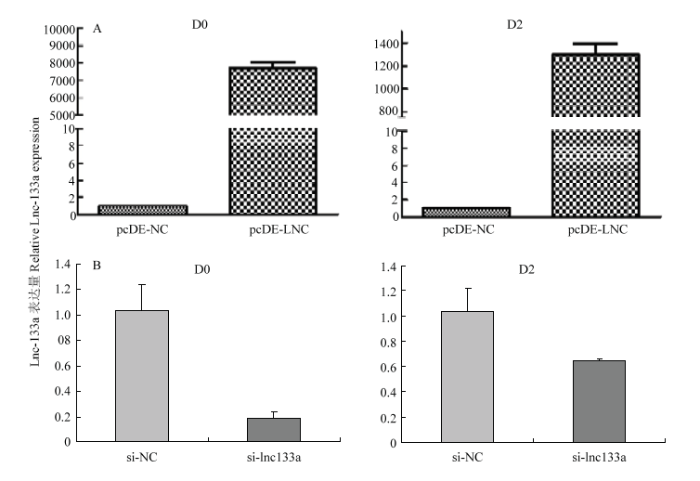 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4qRT-PCR检测LncRNA-133a的过表达(A)和抑制(B)效率
Fig. 4Overexpression(A) & knockdown(B) efficiency of LncRNA-133a by qRT-PCR analysis
图5
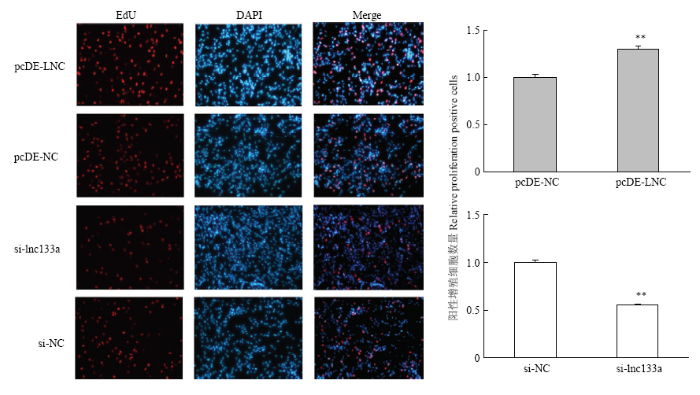 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5EdU检测过表达/抑制LncRNA-133a后牛骨骼肌卫星细胞的增殖细胞
Fig. 5Proliferating bovine skeletal muscle satellite cells were labeled with EdU after overexpression & knockdown of LncRNA- 133a (200×)
2.5 LncRNA-133a对牛骨骼肌卫星细胞分化能力的影响
qRT-PCR及Western blotting检测过表达/抑制LncRNA-133a后D2期牛骨骼肌卫星细胞分化标记因子MHC、MyoD及MyoG 的mRNA水平及蛋白水平表达。过表达LncRNA-133a后,MHC、MyoD及MyoG的mRNA水平表达均呈显著上调趋势(图6-A);同时MHC在蛋白水平均也显著上调(图6-B)。而抑制LncRNA-133a后,MHC、MyoD及MyoG的mRNA水平表达均呈下调趋势(图7-A);MHC在mRNA水平虽然下调不显著,但蛋白水平下调显著(图7-B)。此外,通过免疫荧光染色试验检测MHC融合细胞也得到一致结果,如图8:过表达LncRNA-133a后,MHC融合肌管数增多(图8-A);而抑制LncRNA-133a表达后,MHC融合肌管数减少(图8-B)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6过表达LncRNA-133a促进牛骨骼肌卫星细胞分化
Fig. 6Overexpression of LncRNA-133a promotes the differentiation of bovine skeletal muscle satellite cells
图7
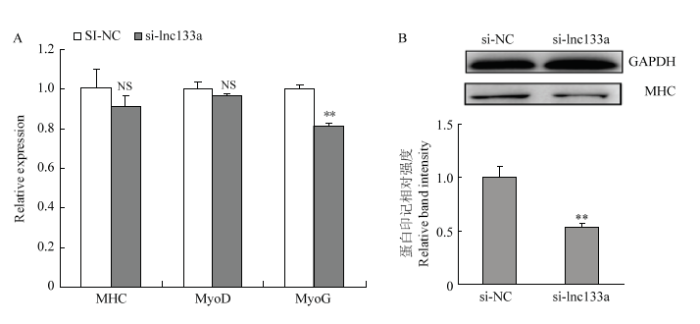 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7抑制LncRNA-133a表达阻滞牛骨骼肌卫星细胞分化
Fig. 7Knockdown of LncRNA-133a suppresses the differentiation of bovine skeletal muscle satellite cells
3 讨论
肌肉发育分化是发育生物学研究的重要主题。肌细胞作为骨骼肌的基本形成单位,它的分化发育直接影响到骨骼肌的发育形成。肌细胞的分化和生长发育过程是内在遗传、表观遗传和外在各种信号互作的结果,受到机体肌肉发育调节因子和调控通路的多层次精细调节。lncRNA作为一类调控因子参与肌肉的发育已是不争的事实,相关报道也不断涌现。有研究表明通过EdU、CCK8或流式细胞仪检测技术,甚至是细胞划痕试验得到如Sirt1[20]、lncRNA AK017368[21]、长非编码RNA-GTL2[22]等LncRNAs具有促进肌细胞增殖的作用。本研究中,在过表达/抑制LncRNA-133a处理后24h(D0),通过EdU细胞增殖检测初步验证LncRNA-133a具有促进牛骨骼肌卫星细胞增殖的作用。
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8免疫荧光检测过表达(A)/抑制(B)LncRNA-133a牛骨骼肌卫星细胞的分化标记因子MHC
Fig. 8Marker gene MHC of myogenic during the differentiation of bovine skeletal muscle satellite cells labeled with Immunofluorescence after overexpression(A) & knockdown(B) of LncRNA-133a (100×)
在肌细胞分化过程中,骨骼肌特异性标记基因如MyoD 、MyoG 及MHC 开始表达[23]。其中,MyoD诱导细胞周期的退出同时开启细胞分化[24,25],骨骼肌分化决定因子MyoG受MyoD的启动开始表达,并调控成肌细胞融合和肌纤维形成[26,27],而作为骨骼肌纤维内粗肌丝主要成分的MHC[28],在肌细胞分化后期表达量逐渐升高。在各肌分化相关的研究中,通常把MyoD、MyoG、MHC作为肌分化标记因子[29,30],利用qRT-PCR、Western blotting及免疫荧光蛋白染色分析等方法,检测它们mRNA水平、蛋白水平及基因蛋白融合表达的变化来验证肌细胞的分化进程。
通过这样的验证手法,现已证实如Linc-YY1、LncRNA Dum、LncMyoD、linc-MD1[9, 31-33]等LncRNAs参与调节肌细胞分化,且这类LncRNAs在成肌细胞分化阶段呈时序性上升表达,所以后续的功能研究基本都集中在这些LncRNAs的高表达时期。此外,ALBRECHT等[34]研究发现,在3月龄牛胎儿的初级纤维中便可检测到作为肌纤维成熟标记物的肌球蛋白,说明肌纤维的发育主要在妊娠早期。本研究中,通过对LncRNA-133a的时序表达谱分析发现:组织上,LncRNA-133a主要在3月龄胎牛肌肉组织中高表达;细胞上,LncRNA-133a在牛骨骼肌卫星细胞分化早期(D2)表达量最高。据此我们推测LncRNA-133可能参与调节牛骨骼肌卫星细胞的早期分化发育。在牛骨骼肌卫星细胞分化早期(D2)进行LncRNA-133a的功能验证发现,有效过表达LncRNA-133a后,肌分化标记因子如MHC、MyoG、MyoD的mRNA水平均显著上升,同时MHC在蛋白水平也显著上调。同样,在有效抑制LncRNA-133a后,对MyoG mRNA表达水平和MHC蛋白表达水平的下调影响是显著的,对MHC、MyoD mRNA表达水平有不显著的下调影响,这有可能是该检测时期这些分化标记因子自身表达特性的影响。此外,MHC融合细胞的免疫荧光试验分析也得到了一致结果。这些结果均表明LncRNA-133a可以促进牛骨骼肌卫星细胞分化。
现阶段研究对LncRNAs在肌细胞增殖分化过程中扮演的经典调节角色已很明确:在肌细胞的增殖分化过程中,肌生长发育相关LncRNAs对成肌细胞的增殖与分化两个生物学过程的促进或抑制调节作用是相反的,或只参与两者其中一个,但这并不排除LncRNAs 对肌细胞增殖及分化有一致的促进或抑制作用,进而促进或抑制成肌纤维的形成,影响肌肉的生长发育。YUE[35]等研究发现,过表达lncYYW后通过mRNA microarray分析得到,GH1及其下游基因AKT1和PIK3CD上调,上调的GH1激活JAK促进成肌细胞增殖,同时也发现过表达lncYYW促进了成肌细胞的分化。相对于LncRNAs在其他生物学过程中(如肿瘤)调节作用机制的研究,LncRNAs对肌细胞增殖分化的研究仍有短缺,如2017年研究发现与乳腺癌转移相关的LncRNA H19,在肿瘤转移的不同阶段(EMT和MET),通过吸附不同的miRNA均发挥其促肿瘤转移作用[36]。综上,本研究中LncRNA-133a促进肌卫星细胞的增殖分化,可能是LncRNA-133a通过某种互作机制对肌细胞增殖分化相关靶标的调控所致。
4 结论
利用前期鉴定的牛肌肉发育相关的长链非编码RNA LncRNA-133a为研究靶标,经组织表达谱分析发现,LncRNA-133a在3、6、9月龄胎牛及成年牛骨骼肌中时序表达呈下降趋势;进一步利用牛骨骼肌卫星细胞体外分化模型,经过表达和抑制LncRNA-133a后发现,LncRNA-133a对牛骨骼肌卫星细胞的增殖及分化均有促进作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.3864/j.issn.0578-1752.2014.06.016URLMagsci [本文引用: 1]

骨骼肌是动物躯体最重要的组成部分,占到产肉动物躯体的40%,肌纤维作为骨骼肌组织的主要成分,其类型的差异是影响产肉动物肌肉品质的重要因素之一。因此,骨骼肌的生长发育与产肉动物肉的产量有着密切的联系,而其生理生化特性的差异也将会直接影响到产肉动物屠宰之后肉品的质量。一般而言,动物骨骼肌肌纤维数目在胚胎发育期间基本上就已固定,出生之后,由于肌纤维的肥大,动物躯体肌肉块才表现出增大增粗。另外,动物肌肉块在生长发育过程中,其肌纤维组成类型并不是完全固定的,它们会随着骨骼肌对代谢与功能需求的改变而发生转变。骨骼肌的生长发育,以及骨骼肌肌纤维类型的发生与发展是一个非常复杂的生物学过程,受到许多信号通路与因子的调控。随着分子生物学技术的飞速发展,各种先进技术相继被应用于生物学现象的研究中,利用这些分子生物学技术很好的阐明了许多复杂生物学现象形成的分子机制。目前,骨骼肌生长发育的分子遗传调控机制取得了长足的进展,许多与骨骼肌形成发育相关的关键因子已被鉴定出来。然而,在早期研究中,人们对于骨骼肌肌纤维的研究主要集中在类型的鉴定,以及不同肌纤维类型生理生化特性的分析,对于骨骼肌肌纤维形成的具体分子遗传调控机制的研究相对较少。近年来,随着研究的不断深入,骨骼肌肌纤维形成的分子遗传调控机制也取得了突破性的进展。因此,有必要进一步对骨骼肌肌纤维类型的特性,骨骼肌肌纤维类型形成的分子机制,以及肌纤维类型与肌肉品质的关系进行全面的综述。本文首先对肌纤维的类型、特性进行了综述;进一步分别对慢型肌纤维与快型肌纤维形成的分子调控机制的研究进展进行了回顾;最后对肌纤维类型与肉品质的关系进行了讨论。总之,本综述的撰写将有助于对骨骼肌肌纤维类型形成的遗传机制的进一步了解,为将来进一步的深入研究肌纤维形成的分子机制提供参考;同时也将有助于揭示肌肉品质形成的分子遗传调控机制,为利用分子生物学技术培育高品质新品种或新品系产肉动物提供分子理论依据。
DOI:10.3864/j.issn.0578-1752.2014.06.016URLMagsci [本文引用: 1]

骨骼肌是动物躯体最重要的组成部分,占到产肉动物躯体的40%,肌纤维作为骨骼肌组织的主要成分,其类型的差异是影响产肉动物肌肉品质的重要因素之一。因此,骨骼肌的生长发育与产肉动物肉的产量有着密切的联系,而其生理生化特性的差异也将会直接影响到产肉动物屠宰之后肉品的质量。一般而言,动物骨骼肌肌纤维数目在胚胎发育期间基本上就已固定,出生之后,由于肌纤维的肥大,动物躯体肌肉块才表现出增大增粗。另外,动物肌肉块在生长发育过程中,其肌纤维组成类型并不是完全固定的,它们会随着骨骼肌对代谢与功能需求的改变而发生转变。骨骼肌的生长发育,以及骨骼肌肌纤维类型的发生与发展是一个非常复杂的生物学过程,受到许多信号通路与因子的调控。随着分子生物学技术的飞速发展,各种先进技术相继被应用于生物学现象的研究中,利用这些分子生物学技术很好的阐明了许多复杂生物学现象形成的分子机制。目前,骨骼肌生长发育的分子遗传调控机制取得了长足的进展,许多与骨骼肌形成发育相关的关键因子已被鉴定出来。然而,在早期研究中,人们对于骨骼肌肌纤维的研究主要集中在类型的鉴定,以及不同肌纤维类型生理生化特性的分析,对于骨骼肌肌纤维形成的具体分子遗传调控机制的研究相对较少。近年来,随着研究的不断深入,骨骼肌肌纤维形成的分子遗传调控机制也取得了突破性的进展。因此,有必要进一步对骨骼肌肌纤维类型的特性,骨骼肌肌纤维类型形成的分子机制,以及肌纤维类型与肌肉品质的关系进行全面的综述。本文首先对肌纤维的类型、特性进行了综述;进一步分别对慢型肌纤维与快型肌纤维形成的分子调控机制的研究进展进行了回顾;最后对肌纤维类型与肉品质的关系进行了讨论。总之,本综述的撰写将有助于对骨骼肌肌纤维类型形成的遗传机制的进一步了解,为将来进一步的深入研究肌纤维形成的分子机制提供参考;同时也将有助于揭示肌肉品质形成的分子遗传调控机制,为利用分子生物学技术培育高品质新品种或新品系产肉动物提供分子理论依据。
DOI:10.1016/j.gde.2009.08.001URLPMID:19762225 [本文引用: 1]

Myogenic cells in the body of vertebrates derive from the dorsal somite, the dermomyotome, where multipotent cells are present. Regulation of cell fate choice is discussed, as is that of progenitor cell self-renewal once cells have entered the myogenic programme. Ongoing research on the formation of the first skeletal muscle, the myotome, is presented with emphasis on mechanisms controlling the early segregation of slow and fast muscle lineages that characterizes this process in the zebrafish embryo. Further insights into myogenic populations that contribute to trunk and limb development at different stages are summarized and the distinct regulatory networks that underlie the formation of head muscles are discussed.
DOI:10.3864/j.issn.0578-1752.2014.20.016URL [本文引用: 1]

生长发育性状是受遗传和环境因素共同作用和/或相互作用的复杂性状,尽管利用全基因组关联研究可分析基因组上全部基因,筛选出与某类性状关联的SNP,但很难综合评价某个基因对其确切的作用。寻找与生长发育相关的精准基因是育种研究的目标之一。长链非编码RNA(long noncoding RNA,LncRNA)在细胞增殖分化、个体发育、信号转导、干细胞维持、代谢等几乎所有重要生命活动中发挥关键的调控作用,在表观遗传水平、转录水平及转录后水平等方面具有控制基因表达的作用,与多种重大疾病的发生密切相关。LncRNA是一类长度大于200个nt,且不表现出蛋白质编码潜能的RNAs,通过多种机制发挥生物学功能,参与染色质修饰、X染色体沉默以及基因组印记、转录干扰、转录激活、核内运输等多种重要调控过程,涉及表观遗传调控、转录调控及转录后调控等多个层面。深入探讨LncRNA调控生肌因子进而调节肌肉发育分化的新路径,阐释哺乳动物生肌分子时间,寻找肌肉组织中与生长发育相关的新LncRNA分子,深入研究与生长发育密切相关的LncRNA分子及其靶基因的生物学功能,阐明LncRNA在肌肉生长发育的调控机制,是肌肉发育遗传育种的主要研究内容。本文就LncRNA在哺乳动物肌肉生长发育、细胞生长、分化、增殖中的作用进行综述。
DOI:10.3864/j.issn.0578-1752.2014.20.016URL [本文引用: 1]

生长发育性状是受遗传和环境因素共同作用和/或相互作用的复杂性状,尽管利用全基因组关联研究可分析基因组上全部基因,筛选出与某类性状关联的SNP,但很难综合评价某个基因对其确切的作用。寻找与生长发育相关的精准基因是育种研究的目标之一。长链非编码RNA(long noncoding RNA,LncRNA)在细胞增殖分化、个体发育、信号转导、干细胞维持、代谢等几乎所有重要生命活动中发挥关键的调控作用,在表观遗传水平、转录水平及转录后水平等方面具有控制基因表达的作用,与多种重大疾病的发生密切相关。LncRNA是一类长度大于200个nt,且不表现出蛋白质编码潜能的RNAs,通过多种机制发挥生物学功能,参与染色质修饰、X染色体沉默以及基因组印记、转录干扰、转录激活、核内运输等多种重要调控过程,涉及表观遗传调控、转录调控及转录后调控等多个层面。深入探讨LncRNA调控生肌因子进而调节肌肉发育分化的新路径,阐释哺乳动物生肌分子时间,寻找肌肉组织中与生长发育相关的新LncRNA分子,深入研究与生长发育密切相关的LncRNA分子及其靶基因的生物学功能,阐明LncRNA在肌肉生长发育的调控机制,是肌肉发育遗传育种的主要研究内容。本文就LncRNA在哺乳动物肌肉生长发育、细胞生长、分化、增殖中的作用进行综述。
DOI:10.5505/tjtes.2015.04831URLPMID:27598585 [本文引用: 1]

Severe thermal trauma covering more than 30% of the total body surface area (TBSA) triggers a sustained pathophysiological response, which includes, but is not limited to, hypermetabolism, chronic inflammation, and severe skeletal muscle wasting. Long non-coding RNAs (lncRNAs) are an important class of pervasive genes involved in a variety of biological functions. However, the functions of lncRNAs in the regulation of responses of skeletal muscle wasting after severe burn have remained untested. Presently examined were the expression profiles of lncRNAs and messenger RNAs (mRNAs) in skeletal muscle tissues of 3 pairs of burned rats at the early flow phase, compared with sham rats, using microarray. Each potential lncRNA-mRNA pair identified is a strong candidate in the definitive confirmation of the presence of specific lncRNA-mRNA interactions, thus providing a detailed picture of the pathogenesis of skeletal muscle wasting in burned rats. LncRNA expression levels were compared among 3 injured tissues and matched normal tissues from microarray data. An average of 117 significantly differentially expressed lncRNAs (1.5-fold) were identified. Only 202 mRNAs were significantly upregulated or downregulated, an average of 92 mRNAs were upregulated in injured, compared to matched normal, tissues, while an average of 110 mRNAs) were downregulated. Presently identified were lncRNAs differentially expressed in skeletal muscles of burned rats, compared to normal tissues. Regulatory pathways may be involved in the pathogenesis of skeletal muscle wasting. Each lncRNA-mRNA pair identified is a strong candidate for a future study to definitively confirm the presence of specific lncRNA-mRNA interactions, thus providing a more detailed picture of the pathogenesis of skeletal muscle wasting in burned rats.
URL [本文引用: 1]
DOI:10.1016/j.cell.2011.09.028URLPMID:22000014 [本文引用: 1]

A long noncoding RNA, linc-MD1, governs muscle cell differentiation by competitively binding to microRNAs that target muscle-specific transcription factors. linc-MD1 functions not only as a decoy RNA but also as a precursor pri-microRNA.
DOI:10.1038/ncomms14718URLPMID:28281528 [本文引用: 1]

Recent studies indicate important roles for long noncoding RNAs (lncRNAs) as essential regulators of myogenesis and adult skeletal muscle regeneration. However, the specific roles of lncRNAs in myogenic differentiation of adult skeletal muscle stem cells and myogenesis are still largely unknown. Here we identify a lncRNA that is specifically enriched in skeletal muscle (myogenesis-associated lncRNA, in short, lnc-mg). In mice, conditional knockout of lnc-mg in skeletal muscle results in muscle atrophy and the loss of muscular endurance during exercise. Alternatively, skeletal muscle-specific overexpression of lnc-mg promotes muscle hypertrophy. In vitro analysis of primary skeletal muscle cells shows that lnc-mg increases gradually during myogenic differentiation and its overexpression improves cell differentiation. Mechanistically, lnc-mg promotes myogenesis, by functioning as a competing endogenous RNA (ceRNA) for microRNA-125b to control protein abundance of insulin-like growth factor 2. These findings identify lnc-mg as a novel noncoding regulator for muscle cell differentiation and skeletal muscle development.
URL [本文引用: 1]
DOI:10.1038/ncomms10026URLPMID:26658965 [本文引用: 2]

Abstract Little is known how lincRNAs are involved in skeletal myogenesis. Here we describe the discovery of Linc-YY1 from the promoter of the transcription factor (TF) Yin Yang 1 (YY1) gene. We demonstrate that Linc-YY1 is dynamically regulated during myogenesis in vitro and in vivo. Gain or loss of function of Linc-YY1 in C2C12 myoblasts or muscle satellite cells alters myogenic differentiation and in injured muscles has an impact on the course of regeneration. Linc-YY1 interacts with YY1 through its middle domain, to evict YY1/Polycomb repressive complex (PRC2) from target promoters, thus activating the gene expression in trans. In addition, Linc-YY1 also regulates PRC2-independent function of YY1. Finally, we identify a human Linc-YY1 orthologue with conserved function and show that many human and mouse TF genes are associated with lincRNAs that may modulate their activity. Altogether, we show that Linc-YY1 regulates skeletal myogenesis and uncover a previously unappreciated mechanism of gene regulation by lincRNA.
[D].
[D].
DOI:10.1016/j.molcel.2013.08.027URLPMID:24055342

Abundantly expressed in fetal tissues and adult muscle, the developmentally regulated H19 long noncoding RNA (IncRNA) has been implicated in human genetic disorders and cancer. However, how H19 acts to regulate gene function has remained enigmatic, despite the recent implication of its encoded miR-675 in limiting placental growth. We noted that vertebrate H19 harbors both canonical and noncanonical binding sites for the let-7 family of microRNAs, which plays important roles in development, cancer, and metabolism. Using H19 knockdown and overexpression, combined with in vivo crosslinking and genome-wide transcriptome analysis, we demonstrate that H19 modulates let-7 availability by acting as a molecular sponge. The physiological significance of this interaction is highlighted in cultures in which H19 depletion causes precocious muscle differentiation, a phenotype recapitulated by let-7 overexpression. Our results reveal an unexpected mode of action of H19 and identify this IncRNA as an important regulator of the major let-7 family of microRNAs.
DOI:10.1101/gad.234419.113URL
DOI:10.1111/age.12539URLPMID:28262958 [本文引用: 1]

Long noncoding RNAs (lncRNAs) have various biological functions and have been extensively studied in recent years. However, the identification and characterization of bovine lncRNAs in skeletal muscle has been very limited compared with that of lncRNAs in other model organisms. In this study, 7188 bovine skeletal muscle lncRNAs were identified by RNA-Seq and a stringent screening procedure in four different muscle tissues. These lncRNAs shared many characteristics with other mammalian lncRNAs, such as a shorter open reading frame and lower expression level than for mRNAs. Furthermore, the chromosomal locations and global expression patterns for these lncRNAs are also described in detail. More importantly, we detected the important interaction relationships of lncRNAs-miRNAs-mRNAs related to muscle development among 36 lncRNAs, 62 miRNAs and 12 mRNAs. Our results provide a global expression pattern of lncRNAs specific to bovine skeletal muscle and provide important targets for revealing the function of bovine muscle development by thoroughly studying the interaction relationships of lncRNAs-miRNAs-mRNAs.
DOI:10.1016/j.bbamcr.2016.08.014URLPMID:27589905 [本文引用: 2]

61We report here the first analysis of lncRNA landscape during muscle development.61lncMDis a muscle-specific lncRNA which is tightly regulated during myogenesis.61lncMDpromotes muscle differentiation by acting as a ceRNA for miR-125b.61IGF2is an important target of miR-125b in bovine myogenesis.61lncMDincreasesIGF2expression in a miR-125b-dependent manner.
DOI:10.1038/s41598-017-03071-7URLPMID:28588232 [本文引用: 2]

Abstract Myogenic differentiation factor (MyoD) is a master transcription factor in muscle development and differentiation. Although several long non-coding RNAs (lncRNAs) linked to MyoD have been found to influence muscle development, the functions of many lncRNAs have not been explored. Here we utilized lncRNA and mRNA microarray analysis to identify potential lncRNAs regulated by MyoD in muscle cells. A total of 997 differentially expressed lncRNAs (335 up-regulated and 662 down-regulated) and 1,817 differentially expressed mRNAs (148 up-regulated and 1,669 down-regulated) were identified after MyoD knockdown in C2C12 cells. Functional predictions suggested that most lncRNAs are involved in the biological pathways related to muscle differentiation and cell cycle with co-expressed genes. To gain further insight into the MyoD-mediated lncRNA expression in muscle differentiation, tissue expression profiles and MyoD overexpression were performed, and we found one of the candidate lncRNAs-AK143003 was significantly regulated by MyoD. Further analyses showed its noncoding ability and cytoplasmic localisation. Silencing of AK143003 stimulated the accumulation of myogenic marker genes, whereas AK143003 overexpression led to their decreased synthesis. This study identified a multitude of MyoD-mediated lncRNAs for further investigation and identified a novel lncRNA, lnc-AK143003, which plays a role in controlling muscle differentiation.
DOI:10.3969/j.issn.1008-5394.2017.03.015URL [本文引用: 1]

为探究lncRNA对牛骨骼肌卫星细胞成肌分化过程的影响,选取前期测序结果中在牛肌卫星细胞成肌分化前后差异表达且表达丰度较高的lncRNA-HZ5进行成肌分化调控研究,利用qRT-PCR技术对其在牛骨骼肌卫星细胞成肌分化前后的表达变化进行检测,然后设计合成lncRNA-HZ5的siRNA,转染牛骨骼肌卫星细胞,抑制lncRNA-HZ5表达,之后将肌卫星细胞进行成肌分化诱导,通过肌管形成状态和MHC表达水平检测分析lncRNA-HZ5表达水平改变对肌卫星细胞成肌分化过程的影响.结果表明:lncRNA-HZ5在成肌分化前后的表达存在显著差异,干扰lncRNA-HZ5表达后的肌卫星细胞经成肌分化诱导,肌管数量及MHC的表达量均显著高于对照组,说明下调lncRNA-HZ5表达可以显著促进肌卫星细胞的成肌分化过程.本研究结果表明,lncRNA-HZ5可以负调控牛骨骼肌卫星细胞的成肌分化过程.
.
DOI:10.3969/j.issn.1008-5394.2017.03.015URL [本文引用: 1]

为探究lncRNA对牛骨骼肌卫星细胞成肌分化过程的影响,选取前期测序结果中在牛肌卫星细胞成肌分化前后差异表达且表达丰度较高的lncRNA-HZ5进行成肌分化调控研究,利用qRT-PCR技术对其在牛骨骼肌卫星细胞成肌分化前后的表达变化进行检测,然后设计合成lncRNA-HZ5的siRNA,转染牛骨骼肌卫星细胞,抑制lncRNA-HZ5表达,之后将肌卫星细胞进行成肌分化诱导,通过肌管形成状态和MHC表达水平检测分析lncRNA-HZ5表达水平改变对肌卫星细胞成肌分化过程的影响.结果表明:lncRNA-HZ5在成肌分化前后的表达存在显著差异,干扰lncRNA-HZ5表达后的肌卫星细胞经成肌分化诱导,肌管数量及MHC的表达量均显著高于对照组,说明下调lncRNA-HZ5表达可以显著促进肌卫星细胞的成肌分化过程.本研究结果表明,lncRNA-HZ5可以负调控牛骨骼肌卫星细胞的成肌分化过程.
DOI:10.1186/s11658-017-0040-6URLPMID:5481879 [本文引用: 1]

H19 is a well-characterized Long noncoding RNA (lncRNA) that has been proven to promote myoblast differentiation in humans and mice. However, its mechanism of action is still not fully interpreted. Using RT-qPCR, we examined H19 RNA levels in various tissues from 1-week, 1-month, 6-month and 36-month old male cattle (i.e., newborn, infant, young and adult). The protein and mRNA levels of MyoG, MyHC, Sirt1 and FoxO1 in the satellite and C2C12cells with an H19 silencing or overexpression vector were respectively detected using western blot and real-time qPCR. H19 was highly expressed in skeletal muscle at all the studied ages. High expression of H19 was required for the differentiation of bovine satellite cells. Knockdown of H19 caused a remarkable increase in the myoblast-inhibitory genes Sirt1/FoxO1, suggesting that H19 suppresses Sirt1/FoxO1 expression during myogenesis. Western blotting analysis of co-transfection of Sirt1 or FoxO1 expression vectors with pcDNA-H19 indicated that Sirt1/FoxO1 overexpression neutralized the promotion of myoblast differentiation through transfection of pcDNA-H19. H19 promoted the differentiation of bovine skeletal muscle satellite cells by suppressing Sirt1/FoxO1. The online version of this article (doi:10.1186/s11658-017-0040-6) contains supplementary material, which is available to authorized users.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/j.scr.2009.10.003URLPMID:19962952 [本文引用: 1]

The satellite cell of skeletal muscle provides a paradigm for quiescent and activated tissue stem cell states. We have carried out transcriptome analyses on satellite cells purified by flow cytometry from Pax3 GFP/+ mice. We compared samples from adult skeletal muscles where satellite cells are mainly quiescent, with samples from growing muscles or regenerating ( mdx) muscles, where they are activated. Analysis of regulation that is shared by both activated states avoids other effects due to immature or pathological conditions. This in vivo profile differs from that of previously analyzed satellite cells activated after cell culture. It reveals how the satellite cell protects itself from damage and maintains quiescence, while being primed for activation on receipt of the appropriate signal. This is illustrated by manipulation of the corepressor Dach1, and by the demonstration that quiescent satellite cells are better protected from oxidative stress than those from mdx or 1-week-old muscles. The quiescent versus in vivo activated comparison also gives new insights into how the satellite cell controls its niche on the muscle fiber through cell adhesion and matrix remodeling. The latter also potentiates growth factor activity through proteoglycan modification. Dismantling the extracellular matrix is important for satellite cell activation when the expression of proteinases is up-regulated, whereas transcripts for their inhibitors are high in quiescent cells. In keeping with this, we demonstrate that metalloproteinase function is required for efficient regeneration in vivo.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
.
DOI:10.1096/fj.201700560RRURLPMID:28904016 [本文引用: 1]

Long noncoding RNAs (lncRNAs) have been reported to play diverse roles in biologic and pathologic processes, including myogenesis. We found that lncRNA AK017368 is highly expressed in skeletal muscle cells. Functional analyses showed that overexpression of AK017368 promoted proliferation and restrained differentiation of myoblasts; whereas inhibition of AK017368 had completely opposite effects in vitro In mice, knockdown of AK017368 promoted muscle hypertrophy in vivo RNA molecules of AK017368 acted mechanistically as competing endogenous RNAs to target micro-RNA (miR)-30c, which was supported by the results of bioinformatics analyses and dual-luciferase reporter assays. It has been shown that lncRNA AK017368 competes with trinucleotide repeat containing-6A (Tnrc6a) for miR-30c. Tnrc6a was previously reported to promote proliferation and inhibit differentiation of myoblast cells, whereas miR-30c targets the 3'-UTR of Tnrc6a mRNA to weaken its function. Taken together, lncRNA AK017368 promotes proliferation and inhibits differentiation of myoblast cells by attenuating function of miR-30c.-Liang, T., Zhou, B., Shi, L., Wang, H., Chu, Q., Xu, F., Li, Y., Chen, R., Shen, C., Schinckel, A. P. lncRNA AK017368 promotes proliferation and suppresses differentiation of myoblasts in skeletal muscle development by attenuating the function of miR-30c.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
URL [本文引用: 1]

旨在探讨MSTN基因对绵羊成肌细胞增殖和分化的作用及相关机制,进一步揭示MSTN在绵羊成肌细胞中的生物学功能。本研究利用重组腺病毒介导shRNA干扰绵羊成肌细胞内源性MSTN基因表达,通过CCK-8法检测成肌细胞增殖能力,流式细胞仪检测细胞周期变化,qRT-PCR检测p21基因表达。成肌细胞诱导分化后利用免疫细胞化学技术检测肌管的形成情况,统计细胞融合率,qRT-PCR检测诱导48、72、96h后MyoD、MyoG、Myf5、Myf6和HACD1基因的表达量变化。结果发现,干扰MSTN后成肌细胞的增殖受到抑制,细胞周期停滞在G0/G1期,并且p21的表达呈上升趋势。在对成肌细胞分化作用的研究中,发现干扰组与阴性对照组相比,诱导48h4个生肌调节因子的表达量均呈下降趋势,MyoG基因表达显著下调(P0.05);诱导72h干扰组成肌细胞融合率极显著高于阴性对照组(P0.01),Myf5和Myf6基因表达量极显著降低(P0.01),MyoD基因表达量升高不显著(P0.05),MyoG基因表达量极显著增加(P0.01),诱导96h,Myf5基因表达量显著降低(P0.05),MyoD基因表达量降低不显著(P0.05),MyoG基因表达量极显著降低(P0.01),Myf6基因表达量升高不显著(P0.05)。另外诱导72h,HACD1基因的表达量在干扰组中显著高于阴性对照组(P0.05),诱导48和96h无显著变化。研究表明,干扰MSTN表达对成肌细胞的增殖有抑制作用,对分化有促进作用,HACD1基因与成肌细胞融合相关。
URL [本文引用: 1]

旨在探讨MSTN基因对绵羊成肌细胞增殖和分化的作用及相关机制,进一步揭示MSTN在绵羊成肌细胞中的生物学功能。本研究利用重组腺病毒介导shRNA干扰绵羊成肌细胞内源性MSTN基因表达,通过CCK-8法检测成肌细胞增殖能力,流式细胞仪检测细胞周期变化,qRT-PCR检测p21基因表达。成肌细胞诱导分化后利用免疫细胞化学技术检测肌管的形成情况,统计细胞融合率,qRT-PCR检测诱导48、72、96h后MyoD、MyoG、Myf5、Myf6和HACD1基因的表达量变化。结果发现,干扰MSTN后成肌细胞的增殖受到抑制,细胞周期停滞在G0/G1期,并且p21的表达呈上升趋势。在对成肌细胞分化作用的研究中,发现干扰组与阴性对照组相比,诱导48h4个生肌调节因子的表达量均呈下降趋势,MyoG基因表达显著下调(P0.05);诱导72h干扰组成肌细胞融合率极显著高于阴性对照组(P0.01),Myf5和Myf6基因表达量极显著降低(P0.01),MyoD基因表达量升高不显著(P0.05),MyoG基因表达量极显著增加(P0.01),诱导96h,Myf5基因表达量显著降低(P0.05),MyoD基因表达量降低不显著(P0.05),MyoG基因表达量极显著降低(P0.01),Myf6基因表达量升高不显著(P0.05)。另外诱导72h,HACD1基因的表达量在干扰组中显著高于阴性对照组(P0.05),诱导48和96h无显著变化。研究表明,干扰MSTN表达对成肌细胞的增殖有抑制作用,对分化有促进作用,HACD1基因与成肌细胞融合相关。
DOI:10.1186/1471-2091-6-27URLPMID:1322219 [本文引用: 1]

Background The two myogenic regulatory factors Myf5 and MyoD are basic helix-loop-helix muscle transcription factors undergoing differential cell cycle dependent proteolysis in proliferating myoblasts. This regulated degradation results in the striking expression of these two factors at distinct phases of the cell cycle, and suggests that their precise and alternated disappearance is an important feature of myoblasts, maybe connected to the maintenance of the proliferative status and/or commitment to the myogenic lineage of these cells. One way to understand the biological function(s) of the cyclic expression of these proteins is to specifically alter their degradation, and to analyze the effects of their stabilization on cells. To this aim, we undertook the biochemical analysis of the mechanisms governing Myf5 mitotic degradation, using heterologous systems. Results We show here that mitotic degradation of Myf5 is conserved in non-myogenic cells, and is thus strictly under the control of the cell cycle apparatus. Using Xenopus egg extracts as an in vitro system to dissect the main steps of Myf5 mitotic proteolysis, we show that (1) Myf5 stability is regulated by a complex interplay of phosphorylation/dephosphorylation, probably involving various kinases and phosphatases, (2) Myf5 is ubiquitylated in mitotic extracts, and this is a prerequisite to its degradation by the proteasome and (3) at least in the Xenopus system, the E3 responsible for its mitotic degradation is not the APC/C (the major E3 during mitosis). Conclusion Altogether, our data strongly suggest that the mitotic degradation of Myf5 by the ubiquitin-proteasome system is precisely controlled by multiple phosphorylation of the protein, and that the APC/C is not involved in this process.
DOI:10.1006/excr.2000.4973URLPMID:10942602 [本文引用: 1]

Proliferating myoblasts already express MyoD before the induction of differentiation. Overexpression of MyoD in normal and transformed cell lines was shown to block cells from entering S phase, suggesting that the MyoD growth suppressive effect must be tightly controlled in growing myoblasts. Here we show that during G1 phase, but not in G2, MyoD abundance is down-regulated by the ubiquitin–proteasome pathway through phosphorylation of serine 200. Roscovitine, a specific inhibitor of cyclin–Cdk2 complexes, prevents both phosphorylation and degradation of MyoD in G1. Inhibition of the ubiquitin-dependent proteasome pathway by MG132 results in stabilization of MyoD-wt, with little effect on a MyoD mutant where serine 200 is replaced by an alanine. Our results show that MyoD Ser200 is the substrate for phosphorylation by cyclin E–Cdk2 stimulating its degradation by the ubiquitin–proteasome system which controls MyoD levels in G1. Phosphorylation/degradation of MyoD at the end of G1 thus represents the regulatory checkpoint in growing myoblasts allowing progression into S phase in a manner similar to the recently examplified cdk2-phosphorylation/degradation of p27Kip1.
DOI:10.1016/j.devcel.2013.12.020URLPMID:24525185 [本文引用: 1]

We discuss the upstream regulators of myogenesis that lead to the activation of myogenic determination genes and subsequent differentiation, focusing on the mouse model. Key upstream genes, such as Pax3 and Pax7, Six1 and Six4, or Pitx2, participate in gene regulatory networks at different sites of skeletal muscle formation. MicroRNAs also intervene, with emerging evidence for the role of other noncoding RNAs. Myogenic determination and subsequent differentiation depend on members of the MyoD family. We discuss new insights into mechanisms underlying the transcriptional activity of these factors.
.
DOI:10.1016/S0074-7696(02)16006-2URLPMID:9551857 [本文引用: 1]

Abstract Adult muscle is composed of different fiber types distinguished by their speed of contraction and metabolism. The generation of these differences is related both to the sequence in which muscle fibers form and to differences between the myogenic cells involved. Fibers form in two successive waves (primary and secondary) whose time of appearance can be correlated with the existence of successive populations of myogenic cells (embryonic and fetal). The differences between fibers arise through an interplay between heritable cellular commitment, where cells are preprogrammed to produce particular types of fiber and influences from the limb environment. The techniques of genetically marking cells and clonal analysis in vivo and in vitro are starting to reveal the relationship between these different influences. Although the process of myogenesis is similar in birds and mammals it is likely that cell autonomous behaviour plays a more important role during avian development as compared to mammals. The identification of muscle specific transcription factors has provided some clues to the mechanisms by which development is controlled but the expression of relatively few of these has been correlated with the sequence of events seen in myogenesis.
DOI:10.1016/B978-0-12-394307-1.00004-7URLPMID:22559940 [本文引用: 1]

Abstract Muscle is a contractile tissue of animals, dedicated to produce force and cause motion. In higher animals, there are two types of muscle tissue: (a) striated muscle, including all voluntary skeletal muscles and involuntary cardiac muscle, and (b) smooth muscle consisting of involuntary muscles, including those of the viscera, blood vessels, and uterus. Although muscle growth and regeneration take place throughout vertebrate life, the heart is the first organ to start functioning, with continued development until delivery. Skeletal muscles, on the other hand, develop in four successive, temporally distinct phases of embryonic, fetal, neonatal, and adult muscle with the postnatal phase being basically hypertrophy. Unlike terminally differentiated skeletal and cardiac muscles in adults, smooth muscle cells retain their plasticity and the phenotype can change reversibly in response to environmental changes. For the past 20 years, the availability of gene recombination technology directed the focus of studies on transcription factors and signaling molecules, and we would like to review what has been explored by recent studies on myogenesis. Copyright 2012 Elsevier Inc. All rights reserved.
DOI:10.1111/j.1365-2303.1994.tb00538.xURLPMID:7600968 [本文引用: 1]

Embryonic and fetal skeletal myoblasts were grown in culture in the presence of TGF beta. Under the conditions employed, TGF beta inhibited differentiation of fetal but not of embryonic myoblasts. To investigate the possible relevance of these data to skeletal muscle histogenesis in vivo, we studied the proliferation/differentiation state of mesodermal cells in the proximal region of the limb bud at the time of primary fiber formation. BrdU labeling and immunostaining for myosin heavy chains revealed that very few mesodermal cells enter the S phase of the cycle when differentiated primary fibers first appear. However, a few hours later, many cells in S phase surround newly formed muscle fibers, suggesting that the latter may be a source of mitogens for undifferentiated myoblasts. Co-culture experiments supported this hypothesis, showing that medium conditioned by fiber-containing explants can stimulate myoblast proliferation. Taken together these data suggested a possible mechanism for the regulation of muscle fiber formation. The model assumes that fibers form in the proximal region of the limb bud, where TGF beta is known to be present, and BrdU labeling experiments did not reveal cells in S phase. It is conceivable that non-dividing embryonic myoblasts (which do not respond to TGF beta) can undergo differentiation, while fetal myoblasts are inhibited by TGF beta. Once formed, primary fibers may stimulate a new wave of proliferation in fetal myoblasts, in order to expand the pool of cells needed to form secondary fibers.(ABSTRACT TRUNCATED AT 250 WORDS)
DOI:10.1034/j.1399-0004.2000.570103.xURLPMID:10733231 [本文引用: 1]

Over the past years, several studies have unraveled important mechanisms by which the four myogenic regulatory factors (MRFs: MyoD, Myf-5, myogenin, and MRF4) control the specification and the differentiation of the muscle lineage. Early experiments led to the hypothesis that these factors were redundant and could functionally replace one another. However, recent experiments using in vivo and in vitro models have demonstrated that in fact different aspects of the myogenic program are controlled by different factors in vivo , suggesting that these factors play distinct roles during myogenesis. The activity of the MRFs during proliferation and differentiation of muscle precursor cells has clearly been demonstrated to be dependent on specific cell-cycle control mechanisms as well as distinct interactions with other regulatory molecules, such as the ubiquitously expressed E proteins and several other transcription factors. Furthermore, the observation that the MRFs can recruit chromatin remodeling proteins has shed some light on the mechanisms by which the MRFs activate gene expression. Recently, a functional role for MyoD during satellite cell activation and muscle repair has been identified in vivo , which cannot be substituted for by the other MRFs. This has put forward the hypothesis that these factors also play specific biological roles following muscle injury and repair.
DOI:10.1038/cr.2015.21URL [本文引用: 1]
DOI:10.1016/j.devcel.2015.05.009URLPMID:26143994

Long non-coding RNAs are regulators of various biological functions. Gong and Li et al. show that LncMyoD is a LncRNA target of MyoD during myogenesis and is required for myoblast differentiation by affecting IMP2-mediated mRNA translation. LncMyoD is functionally conserved between mouse and human, despite limited sequence homology.
DOI:10.1016/j.molcel.2013.12.012URLPMID:3919156 [本文引用: 1]

The muscle-specific long noncoding RNA linc-MD1 was shown to be expressed during early phases of muscle differentiation and to trigger the switch to later stages by acting as a sponge for miR-133 and miR-135. Notably, linc-MD1 is also the host transcript of miR-133b, and their biogenesis is mutually exclusive. Here, we describe that this alternative synthesis is controlled by the HuR protein, which favors linc-MD1 accumulation through its ability to02bind linc-MD1 and repress Drosha cleavage. We show that HuR is under the repressive control of miR-133 and that the sponging activity of linc-MD1 consolidates HuR expression in a feedforward positive loop. Finally, we show that HuR also acts in the cytoplasm, reinforcing linc-MD1 sponge activity by cooperating for miRNA recruitment. An increase in miR-133 synthesis, mainly from the two unrelated miR-133a coding genomic loci, is likely to trigger the02exit from this circuitry and progression to later differentiation stages. Graphical Abstract 61 A feedforward positive loop exists between linc-MD1 and HuR during myogenesis 61 HuR controls the relative biogenesis of miR-133b and its host linc-MD1 RNA 61 Linc-MD1, by sponging miR-133, alleviates its repression on HuR expression 61 Cytoplasmic HuR reinforces linc-MD1 activity by cooperating for miRNA recruitment A feedforward positive loop exists between linc-MD1 and HuR during myogenesis HuR controls the relative biogenesis of miR-133b and its host linc-MD1 RNA Linc-MD1, by sponging miR-133, alleviates its repression on HuR expression Cytoplasmic HuR reinforces linc-MD1 activity by cooperating for miRNA recruitment linc-MD1 and miR-133 are alternatively processed from the same precursor RNA. These RNAs play opposing roles in early phases of myogenesis. Legnini et02al. now show that the balance between the RNAs is regulated by HuR, which inhibits generation of miR-133 by inhibiting microprocessor activity on the precursor RNA.
DOI:10.2527/jas.2013-6258URLPMID:23658343 [本文引用: 1]

Muscle fiber development during gestation determines the muscle structure at birth and establishes the conditions for muscle development in growing cattle. Differences in muscle structure among beef cattle breeds and between beef and dairy cattle are obvious already shortly after birth. The objective of the study was to investigate the development of muscle fibers and muscle fiber bundle structure in semitendinosus muscle of divergent cattle breeds from 3 mo of gestation until birth. Fetuses of German Angus (GA), Galloway (GW), Belgian Blue (BB), and Holstein Friesian (HF) were harvested at 3, 4.5, 6, or 9 mo of gestation. Muscle sections were analyzed for fiber size and types as well as for bundle structure. The results confirmed that primary muscle fiber development occurs mainly during the first trimester of gestation. All fibers were initially positive for fetal fast myosin. Slow myosin as a marker for fiber maturation was detected in primary fibers at 3 mo of gestation showing a weak immunostaining. During the second trimester, the intensity of immunostaining strongly increased indicating increased slow myosin protein expression. Concurrently, the shape of primary fibers changed from myotubes to myofibers whereas the size stayed nearly constant. The main increase in muscle mass during the second trimester was caused by secondary fiber development. As an example, the ratio between secondary and primary fibers increased in Holstein Friesian fetuses from 5.9 at 4.5 mo of gestation to 21.6 at 6 mo of gestation. Primary and secondary fibers continued to growth during the third trimester. Regional differences in the density of slow muscle fibers were detected leading to greater variation within the muscle than among breeds. Structural organization of muscle fibers in muscle fiber bundles developed early in fetal life. At first, large main bundles were visible. Smaller structural units defined as primary bundles were measurable at 6 mo of gestation when most fibers were developed. The size of primary bundles nearly doubled from 6 mo of gestation to birth in all breeds. In summary, differences among breeds in the early fetal muscle fiber development were detected in contractile differentiation and partly in muscle fiber bundle structure. A prolonged secondary fiber generation and altered contractile differentiation may be involved in breed differences of postnatal muscle development.
DOI:10.1007/s11626-017-0180-zURLPMID:28726188 [本文引用: 1]

Abstract Long noncoding RNAs (lncRNAs) are key regulatory factors for gene expression in a variety of biological processes; however, the role of lncRNAs in muscle formation and development is poorly understood, particularly in cattle. Here, we identified a highly expressed lncRNA in muscle, lncYYW, by high-throughput sequencing in bovine longissimus, scapular, intercostal, and gluteus muscles. The expression of lncYYW increased gradually during myoblast differentiation. Overexpression of lncYYW increased the number of cells in the DNA synthesis (S) stage of the cell cycle and upregulated the expression of two well-established myogenic markers, myogenin and myosin heavy chain. A microarray analysis showed that lncYYW positively regulates the expression of growth hormone 1 and its downstream genes, AKT1 and PIK3CD, in bovine myoblasts. This discovery provides a good foundation for further study of the mechanism of action of lncYYW during bovine myoblast development. Taken together, our results reveal a novel lncRNA associated with bovine myoblast proliferation and differentiation. This lncRNA will play a crucial and critical role in future studies of bovine muscle development.
URLPMID:28611183 [本文引用: 1]

Abstract Metastasis is a multistep process by which tumor cells disseminate from their primary site and form secondary tumors at a distant site. The pathophysiological course of metastasis is mediated by the dynamic plasticity of cancer cells, which enables them to shift between epithelial and mesenchymal phenotypes through a transcriptionally regulated program termed epithelial-to-mesenchymal transition (EMT) and its reverse process, mesenchymal-to-epithelial transition (MET). Using a mouse model of spontaneous metastatic breast cancer, we investigated the molecular mediators of metastatic competence within a heterogeneous primary tumor and how these cells then manipulated their epithelial-mesenchymal plasticity during the metastatic process. We isolated cells from the primary mammary tumor, the circulation, and metastatic lesions in the lung in TA2 mice and found that the long noncoding RNA (lncRNA) H19 mediated EMT and MET by differentially acting as a sponge for the microRNAs miR-200b/c and let-7b. We found that this ability enabled H19 to modulate the expression of the microRNA targets Git2 and Cyth3 , respectively, which encode regulators of the RAS superfamily member adenosine 5'-diphosphate (ADP) ribosylation factor (ARF), a guanosine triphosphatase (GTPase) that promotes cell migration associated with EMT and disseminating tumor cells. Decreasing the abundance of H19 or manipulating that of members in its axis prevented metastasis from grafts in syngeneic mice. Abundance of H19, GIT2, and CYTH3 in patient samples further suggests that H19 might be exploited as a biomarker for metastatic cells within breast tumors and perhaps as a therapeutic target to prevent metastasis. Copyright 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
