 ,, 王玲, 丛胜波, 王金涛, 李文静, 万鹏湖北省农业科学院植保土肥研究所/农业部华中作物有害生物综合治理重点实验室/农作物重大病虫草害防控湖北省重点实验室,武汉 430064
,, 王玲, 丛胜波, 王金涛, 李文静, 万鹏湖北省农业科学院植保土肥研究所/农业部华中作物有害生物综合治理重点实验室/农作物重大病虫草害防控湖北省重点实验室,武汉 430064Cloning, Sequence Analysis and Expression of Pheromone Biosynthesis Activating Neuropeptide (PBAN) Gene in Different Development Stages of Pectinophora gossypiella
XU Dong ,, WANG Ling, CONG ShengBo, WANG JinTao, LI WenJing, WAN PengInstitute of Plant Protection and Soil Science, Hubei Academy of Agricultural Sciences/Key Laboratory of Integrated Pest Management on Crops in Central China, Ministry of Agriculture/Hubei Key Laboratory of Crop Disease, Insect Pests and Weeds Control, Wuhan 430064
,, WANG Ling, CONG ShengBo, WANG JinTao, LI WenJing, WAN PengInstitute of Plant Protection and Soil Science, Hubei Academy of Agricultural Sciences/Key Laboratory of Integrated Pest Management on Crops in Central China, Ministry of Agriculture/Hubei Key Laboratory of Crop Disease, Insect Pests and Weeds Control, Wuhan 430064第一联系人:
责任编辑: 岳梅
收稿日期:2018-08-6接受日期:2018-09-19网络出版日期:2018-12-01
| 基金资助: |
Received:2018-08-6Accepted:2018-09-19Online:2018-12-01

摘要
关键词:
Abstract
Keywords:
PDF (512KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
许冬, 王玲, 丛胜波, 王金涛, 李文静, 万鹏. 红铃虫性信息素合成激活肽基因克隆、序列特征及在不同发育阶段的表达分析[J]. 中国农业科学, 2018, 51(23): 4449-4458 doi:10.3864/j.issn.0578-1752.2018.23.005
XU Dong, WANG Ling, CONG ShengBo, WANG JinTao, LI WenJing, WAN Peng.
0 引言
【研究意义】红铃虫(Pectinophora gossypiella)是一种世界性的重要棉花害虫,以幼虫钻入棉花的蕾、花、铃等繁殖器官进行危害。因其在组织外滞留时间短,导致各种传统防治方法效果有限,给棉花的生长造成严重损失。20世纪70年代末,美国成功研发出红铃虫性信息素,并在生产应用中取得良好效果[1]。我国也是较早利用红铃虫性信息素开展虫情监测和害虫防治的国家[2]。目前,各国在红铃虫性信息素化学结构、组分比例、田间应用等方面作了大量研究[3],但有关红铃虫性信息素在虫体内的合成、释放及作用的分子机制尚缺乏深入了解。蛾类昆虫性信息素合成与释放受其体内性信息素合成激活肽(pheromone biosynthesis activating neuropeptide,PBAN)控制。该活性肽是一种C端具有FXPRL五肽序列的昆虫神经肽,除了与昆虫性信息素合成释放相关外,还在表皮色素控制、胚胎滞育以及刺激内脏肌肉收缩等生理过程中发挥着重要作用[4,5]。因此,研究PBAN在红铃虫生长发育、求偶交配及与寄主化学通讯过程中的表达规律,以揭示红铃虫性信息素合成释放的调控机制,可为进一步探讨棉花害虫遗传防治的新途径,如设计具有激活或抑制作用的类似物,研制高效、专一性强、无公害的性信息素提供思路[6]。【前人研究进展】大多数蛾类昆虫的性信息素释放具有明显的昼夜节律性,而昆虫性信息素合成激活神经肽PBAN是控制其合成和释放的最主要调控因子[7]。PBAN最初从谷实夜蛾(Helicoverpa zea)中分离得到,后陆续又在棉铃虫(Helicoverpa armigera)[8]、烟草天蛾(Manduca sexta)[9]、家蚕(Bombyx mandarina)[10]、水稻二化螟(Chilo supperssalis)[11]、小菜蛾(Plutella xylostella)[12]、分月扇舟蛾(Clostera anastomosis)[13]等多种鳞翅目昆虫中以及双翅目昆虫埃及伊蚊(Aedes aegypti)[14]中克隆鉴定。PBAN主要是通过影响合成途径中不同的关键酶,最终导致这些反应的不同组合产生各种昆虫所特有的信息素混合物。TSFADIA等[15]研究了印度谷螟(Plodia interpunctella)和棉铃虫的酶抑制剂对性信息素的生物合成途径及限制速率,证实PBAN可对性腺分泌的乙酰辅酶A产生影响,并通过刺激脂肪酸合成酶系中的乙酰辅酶A羧化酶来增加其活性,进而促使性信息素合成[7,15]。但目前的研究显示,不同物种中PBAN的调控机制有所不同,如玉米螟PBAN可通过增加脂肪酸生物合成时的底物来调控信息素生物合成,而家蚕体内PBAN的作用却是减少脂肪酸生物合成时的底物供应[16,17]。值得注意的是,PBAN对蛾类性信息素的调节不仅仅局限在雌虫中,随后的研究表明PBAN在雄性个体中也有表达,并且在雄性信息素产生中起着关键作用[18]。研究进一步发现,PBAN并不是调控性信息素合成的唯一物质,在一些昆虫中,保幼激素(juvenile hormone,JH)和章鱼胺(octopamine,OA)对调控性信息素也起着一些作用[19,20]。RAFAELI等****认为保幼激素可通过控制PBAN的释放进而控制信息素的生物合成,并且能够控制PBAN等进入血淋巴,进而调控其体色多态现象[21]。RAFAELI等在细胞水平揭示章鱼胺在刺激产生性信息素的过程中起到中间传递信息的作用,它能与PBAN相互作用抑制性信息素生物合成[22]。研究还发现,大多鳞翅目害虫PBAN的转录前体除了编码PBAN外,还可编码α-神经肽、β-神经肽、滞育激素(diapause hormone,DH)以及γ-神经肽等[23]。如PBAN编码的滞育激素在有的昆虫蛹滞育期可作用于卵巢诱导其胚胎滞育,而在另外一些昆虫体内却能缩短滞育时期甚至打破滞育[24],甚至有些昆虫PBAN的多肽序列还包含了黑化作用和红化作用激素,可在幼虫阶段对表皮起黑化作用,成虫发育过程调节信息素生物合成[25]。【本研究切入点】红铃虫性信息素已被应用于棉花害虫绿色防控,但红铃虫本身性信息素合成释放的机制尚不清楚。【拟解决的关键问题】明确红铃虫PBAN基因(PgosPBAN)序列特征及其在不同发育阶段的表达规律,探索昆虫交配行为及棉花挥发物对PgosPBAN表达水平的影响,为阐明红铃虫性信息素合成、释放机制打下基础。1 材料与方法
试验于2016年5月至2018年6月在湖北省农业科学院植保土肥研究所完成。1.1 试验材料
红铃虫为敏感品系,于2003年采自湖北省潜江地区(N 30°20′55.63″、E 112°55′58.41″)棉田,经笔者实验室人工饲料连续饲养,期间不接触任何农药。供试红铃虫幼虫在(27±1)℃、相对湿度50%左右的养虫间饲养,自然光照。待其化蛹后,将其置于光照培养箱中,温度(27±1)℃,相对湿度70%左右,光周期L﹕D=14 h﹕10 h。鄂棉24为常规棉,由湖北省农业科学院植保土肥研究所棉花病虫害课题组提供。于2016年5月上旬种植于湖北省农业科学院试验田中,N 30°28′53.33″、E114°18′40.24″。
1.2 PgosPBAN全长cDNA克隆
1.2.1 PgosPBAN cDNA片段序列扩增 红铃虫总RNA提取采用TRIzol法,提取试剂盒购自Invitrogen。反转录参考Promega公司的Reverse Transcription System试剂盒说明书进行。利用DNAMAN Version 6软件,分析已经报道的鳞翅目害虫PBAN的氨基酸序列,根据保守区结合密码子偏好特性,利用Primer 5.0设计兼并引物PgosPBAN-F、PgosPBAN-R(表1),以合成的cDNA第一链为模板进行PCR。反应体系:10×LA Taq buffer 2.5 μL,dNTPMix(2.5 mmol·L-1 each)2 μL,LA Taq 0.25 μL,正反引物各1.25 μL,cDNA 2 μL,ddH2O 15.75 μL。反应条件:95℃预变性5 min,95℃变性30 s,60℃退火30 s,72℃延伸1 min,32个循环,最后72℃延伸10 min,4℃保存。扩增产物用1.5%琼脂糖凝胶电泳检测,目的条带用Gel Extraction Kit凝胶回收试剂盒回收纯化。后连接到T-easy克隆载体,转化到Dh5α感受态细胞,涂布到含有氨苄青霉素(Amp)的LB固体培养基。37℃培育过夜,经蓝白斑筛选,挑取白色单菌落放入含有Amp的LB液体培养基中,37℃,200 r/min培养12 h。经菌液PCR验证,将目的条带大小合适的菌液采用质粒提取试剂盒(AXYGEN)提取质粒,并送北京奥科鼎盛生物科技有限公司测序验证。Table 1
表1
表1PgosPBAN扩增所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 用途 Primer use |
|---|---|---|
| PgosPBAN-F | CTBTGGTTCGGYCCYMGACTMGG | cDNA克隆 cDNA cloning |
| PgosPBAN-R | CATSGTBGKSCCBAGCCTKGGBGAGAAGT | |
| 3′ PgosPBAN-Outer | CAAACCTACCTCCGTCTTCTT | 3′端cDNA扩增 3′-cDNA end amplification |
| 3′ PgosPBAN-Inner | CGCCCTAAAATACTACTACGAC | |
| 5′ PgosPBAN-Outer | GTCAGCTAACCTCCTTCCGAG | 5′端cDNA扩增 5′-cDNA end amplification |
| 5′ PgosPBAN-Inner | CACTCTCGTATCGCTTCCTCT | |
| PgosPBAN-O-F | GCAACAGTAAATCGTAGAAACA | 开放阅读框扩增 ORF amplification |
| PgosPBAN-O-R | GATGAGCATAAACCAGCCAAT | |
| PgosPBAN-Q-F | GGCAAGCGTTCTTTCCATCC | 实时荧光定量PCR qRT-PCR |
| PgosPBAN-Q-R | GCCTCGTCATCAGCCTGTG | |
| ACS1 | CACCGTGCCCATCTATGAAGG | |
| ACR1 | GACGATTTCCCTCTCAGCGGT |
新窗口打开|下载CSV
1.2.2 PgosPBAN 3′和5′-末端cDNA序列扩增 设计目的基因序列片段外侧和内侧特异性引物,采用TaKaRa RACE试剂盒扩增该基因3′和5′-末端cDNA序列。扩增产物处理方法同上,测序得到目的基因3′和5′-末端cDNA序列片段,用DNAMAN软件拼接得到目的基因全长cDNA序列,最后再根据所拼接序列设计引物进行扩增全长的PCR验证。
1.3 PgosPBAN序列分析、进化树构建和蛋白二级结构预测
采用生物信息学在线工具Protparam(https://web.expasy.org/protparam/)预测蛋白理论分子量、等电点、亲脂性;利用SignaIP 4.1 Serve(http://www.cbs.dtu.dk/ services/SignalP/)预测信号肽;蛋白二级结构利用Chou & Fasman二级结构服务器进行预测;跨膜预测分析(http:// www.cbs.dtu.dk/services/TMHMM-2.0/,TMHMM);利用美国国家生物技术信息中心(NCBI)的BLASTx程序搜索序列同源相似性;采用DANMAN V6软件比对蛋白序列的同源相似性;采用MEGA7.0软件构建蛋白的系统发育进化树,用邻接法(neighbor-joining,NJ)并经bootstrap重复1 000次抽样分析。1.4 PgosPBAN在红铃虫不同发育阶段的表达
分别选取5日龄幼虫12头、10日龄(4龄幼虫)幼虫5头、1日龄和5日龄蛹各5头,以及羽化0 d(羽化当天)、1、3、5、8 d的雌、雄成虫各5头用于PgosPBAN表达量测定,采用双标准曲线法[26]。荧光定量PCR(qRT-PCR)反应体系:SYBR? Premix Ex TaqTM(Tli RNaseH Plus)(2X)10 μL,上、下游引物各1 μL,cDNA 1 μL,无RNA酶水7 L。采用两步法PCR标准法,扩增程序:95℃ 2 min;95℃ 5 s,60℃ 30 s,共40个循环。以红铃虫的actin为内参基因,采用Primer 5.0软件分别设计目的基因和内参基因引物(表1)。将含有目的基因和内参基因的质粒标准品与不同发育阶段的红铃虫样品,在同一96孔板上进行qRT-PCR反应并制作标准曲线。每处理设5个生物学重复及3个技术重复,以无菌水为对照。根据目的和内参基因质粒标准品构建的标准曲线获取基因的拷贝数含量,通过内参基因的均一化处理计算红铃虫各处理中目的基因相对于内参基因的表达量。同时,以羽化当天(0 d)雌虫表达量为标准定量,计算红铃虫不同发育阶段基因相对表达量。1.5 交配行为对PgosPBAN表达的影响
选取刚羽化的红铃虫处女蛾,按性比1﹕1的比例,将红铃虫雌、雄成虫放置于塑料产卵罐中(直径10 cm,高15 cm),产卵罐顶端用50目的纱网覆盖,底端密封,内置10%的蜂蜜水和供红铃虫成虫休息、隐藏的折叠纸片。在首个暗期处理8 h时,用微红光源每隔0.5 h观察一次交配情况。选取有交配行为的成虫作为试验用虫。在首次交配后第1个和第3个暗期3 h左右,取红铃虫雌、雄成虫各5头,用于测定红铃虫雌、雄成虫体内PgosPBAN表达量,重复4次。基因表达量测定方法同1.4。1.6 棉花挥发物对PgosPBAN表达的影响
选取刚羽化的红铃虫处女雌、雄蛾,分别放置于不同的产卵罐后,将其置于四周放置有棉苗(5—7叶期)的封闭塑料盒中(40 cm×50 cm)饲养,以不放棉苗处理作为对照。设置处理时间为1、3、5、8 d。每重复取5头成虫,重复4次。待各处理时间到达后,将处理的雌、雄成虫取出,用于测定PgosPBAN表达量。测定方法同1.4。1.7 数据统计与分析
利用SPSS 16.0统计软件对红铃虫不同发育阶段内PgosPBAN表达量进行方差分析,采用One-way ANOVA用于显著性检验,多重比较采用Duncan’s新复极差法分析(P<0.05)。2 结果
2.1 PgosPBAN全长cDNA克隆及序列分析
通过RACE技术扩增获得PgosPBAN cDNA全长序列(GenBank登录号:KY987647)。该序列全长1 461 bp,其中开放阅读框(ORF)618 bp,编码205个氨基酸。5′端的非翻译区(5′-UTR:untranslated regions)长121 bp,3′-UTR长722 bp。根据PgosPBAN基因拼接结果的序列设计特异性引物,以红铃虫幼虫和成虫混合的cDNA为模板进行扩增,可以克隆到ORF区的cDNA全长序列(图1),且电泳条带与预测片段大小一致。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1PgosPBAN开放阅读框PCR扩增产物核酸凝胶电泳
M:DL2000 DNA marker;1:PgosPBAN ORF区PCR产物PCR products of the coding sequence of PgosPBAN
Fig. 1Agarose gel electrophoresis of PCR product of the coding sequence of PgosPBAN
以SignalP 4.0推导出蛋白序列后,发现其N端有一条潜在的信号肽:M1-A23,紧随后面的是DH类似肽(S24-W54)。同时,PgosPBAN全长序列中含有6个典型的识别切割位点,分别为G55-K56-R57、K102-K103、G111-R112、G135-R136-R137、G171-P172和G181- R182。根据基因的剪切位点,找到了该基因编码的5个神经肽,分别为滞育激素、α-食下神经节(α-SGNP,V104-L110)、β-食下神经节(β-SGNP,S113-L134)、PBAN(L138-L170)以及γ-食下神经节(γ-SGNP,N173-L180)。每个神经肽的C末端都有FXPR/KL序列。
利用生物信息学在线工具Protparam分析氨基酸序列,该蛋白的预测分子量为2.41 kD;等电点为9.25;含酸性氨基酸(Asp+Glu)31个,占15.2%,碱性氨基酸(Arg+Lys)36个,占17.6%;分子式为C1073H1670N308O314S5;不稳定系数为46.84;脂肪指数为69.46,总平均疏水指数为-0.773。TMHMM显示,该蛋白为非跨膜蛋白,蛋白二级结构组成为13.2%的H(α-helix)、10.2%的E(β-sheet)、76.6%的C(loop或coil)。
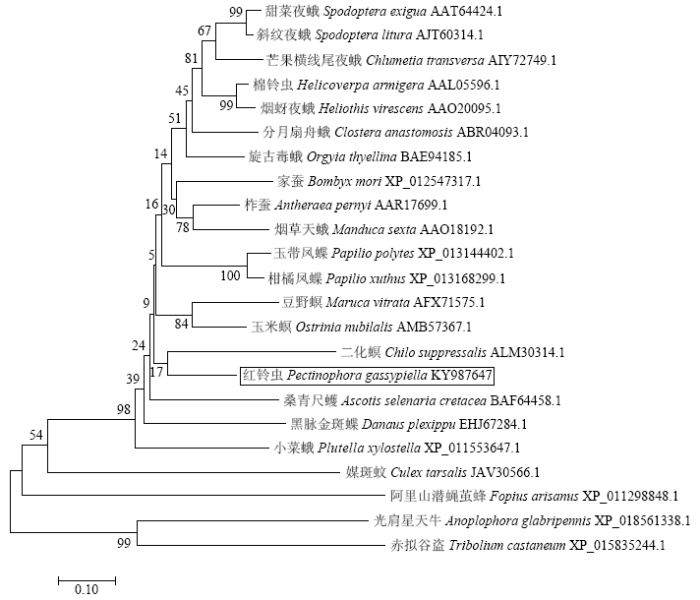
2.2 PgosPBAN氨基酸序列系统进化树构建
采用MEGA 7.0的邻接法对4个目16科23种昆虫PBAN构建了进化树(图2)。结果表明,PgosPBAN与15种鳞翅目昆虫的PBAN位于同一分支。其中与PgosPBAN进化关系最近的是二化螟的PBAN(GenBank登录号:ALM30314.1),表明这两个基因可能有共同的祖先基因;其次是甜菜夜蛾(Spodoptera exigua,GenBank登录号:AAT64424.1)、斜纹夜蛾(S. litura,GenBank登录号:AJT60314.1)等其他科14种鳞翅目害虫,表明PgosPBAN与大多鳞翅目昆虫PBAN的亲缘关系较近。PgosPBAN与鞘翅目的光肩星天牛(Anoplophora glabripennis,GenBank登录号:XP_018561338.1)、赤拟谷盗(Tribolium castaneum,GenBank登录号:XP_015835244.1)以及双翅目媒斑蚊(Culex tarsalis,GenBank登录号:JAV30566.1)、膜翅目阿里山潜蝇茧蜂(Fopius arisanus,GenBank登录号:XP_011298848.1)的PBAN位于不同分支上,其亲缘关系较远。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2PBAN蛋白系统进化分析
Fig. 2The phylogenetic analysis of PBAN protein from different species
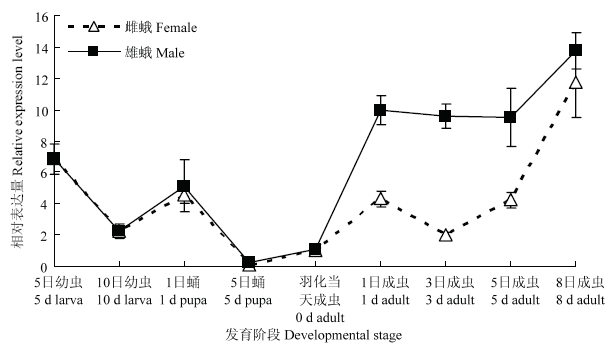
2.3 PgosPBAN在不同发育阶段的表达水平
采用qRT-PCR方法,以羽化当天的处女雌蛾的PgosPBAN表达量为基准,分析了红铃虫不同发育阶段PgosPBAN表达水平。结果表明,PgosPBAN在红铃虫不同发育阶段存在表达特异性,其中在成虫期的表达量较高。羽化1 d的处女雌、雄成虫,相对表达量分别为标准参量的4.3和10.0倍,羽化8 d则分别为11.7和13.8倍。可见,PgosPBAN在红铃虫雌、雄成虫体内均有表达,且雄性成虫1—5 d的相对表达量显著高于雌成虫。在幼虫期表达量相对较低,其中,5 d的幼虫比10 d的相对表达量高,分别为6.9和2.3倍。在蛹期相对表达量最低,5 d雌、雄蛹的相对表达量仅为0.06和0.20倍(图3)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3PgosPBAN在红铃虫不同发育阶段的相对表达量
Fig. 3The relative expression level of PgosPBAN in different developmental stages of P. gossypiella
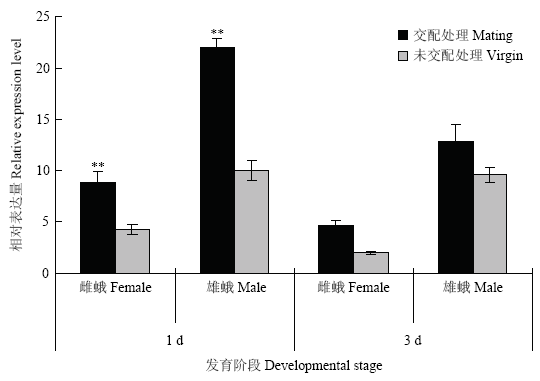
2.4 交配行为对不同发育阶段PgosPBAN表达的影响
通过比较红铃虫成虫在交配前后PgosPBAN表达水平,发现有交配行为的红铃虫雌、雄成虫在交配后1 d(首次交配后13 h)和交配后3 d时的表达量都高于未交配处理。其中,在首次交配后13 h,雌、雄成虫体内的PgosPBAN相对表达量分别是标准参量的8.9和22.0倍,而未交配的处女雌、雄成虫体则仅为4.3和10.0倍,显著低于交配处理(图4)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4交配行为对红铃虫成虫PgosPBAN表达量的影响
Fig. 4The effect of mating on the expression level of PgosPBAN at adult stage of P. gossypiella
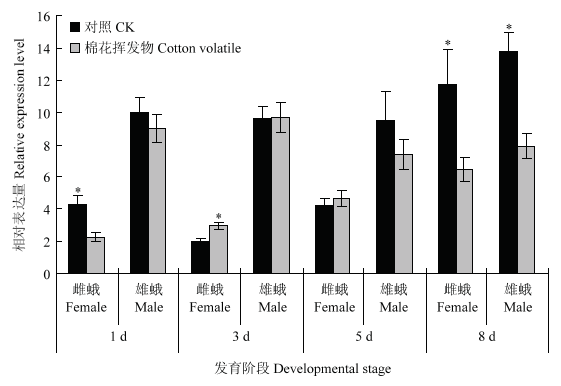
2.5 棉花挥发物对红铃虫成虫PgosPBAN的影响
通过分析棉花挥发物对红铃虫PgosPBAN表达水平的影响发现, 1—5 d雄成虫PgosPBAN表达水平未受到棉花寄主挥发物显著影响,而棉花挥发物处理后1、8 d雌成虫及8 d雄成虫PgosPBAN表达水平均显著低于对照处理(图5)。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5棉花挥发物对红铃虫成虫PgosPBAN表达量的影响
Fig. 5The effect of cotton volatile on the expression level of PgosPBAN at adult stage of P. gossypiella
3 讨论
大多数昆虫PBAN编码的5个神经肽都非常保守,而大部分氨基酸残基存在较大的变异多样性[27],这为遗传改造PBAN、干扰成虫交配以及开发高选择性或广谱性的PBAN类似物用于绿色防控开拓了思路[28]。本研究鉴定了PgosPBAN,其cDNA全长序列包含6个识别切割位点,编码5个神经肽,且C末端都含有FXPR/KL序列,具有典型的PBAN结构。同源性及系统进化树结果显示,PgosPBAN与大多鳞翅目昆虫的PBAN位于同一个分支,其中与PgosPBAN进化关系最近的是二化螟PBAN,表明这两个基因可能有共同的祖先基因。PgosPBAN在红铃虫不同发育阶段中存在明显的表达特异性。其中,成虫期的相对表达量最高,幼虫期次之,蛹期最低。这与甜菜夜蛾、斜纹夜蛾等昆虫的发育表达模式相似[29,30],与烟草天蛾等昆虫的发育表达模式则完全不同[9]。PBAN在红铃虫幼虫期和蛹期可能仅行使了部分功能。有研究显示,PBAN编码的滞育激素在部分昆虫的幼虫期可行使推迟若虫进入成熟期的时间、促使若虫群体发育整齐及调节若虫体色变化等功能[30],在蛹期则可能存在刺激蛹发育和解除滞育的功能[31]。成虫期,PgosPBAN表达量表现出随着日龄增大逐渐升高的趋势,暗示其在此时期可能已开启性信息素的合成功能,为分泌大量性信息素合成其相关前体物质[32]。此结果与红铃虫羽化前期具有旺盛的交配能力相吻合。虽然本试验只能在mRNA水平而不是在蛋白水平直接证明PgosPBAN在红铃虫成虫1—8 d内呈上升趋势,但结合红铃虫求偶行为的研究结果(未发表),处女蛾在羽化前期(1—13 d)的暗期求偶持续时间也随日龄的增加而上升,表明其求偶行为与PgosPBAN表达量有密切的联系,这在一些昆虫中已有详细的研究[33,34]。另外,本研究还发现,与美洲棉铃虫[18]等昆虫相似,PgosPBAN表达不局限于红铃虫雌虫,其雄虫体内也有较高水平表达,且表达量高于雌虫,说明PgosPBAN在红铃虫雄性性信息素合成释放以及生长发育等生理过程可能也发挥着重要作用。有研究显示,大多数昆虫雄蛾性信息素主要是近距离激发雌虫性欲,抑制同种其他雄虫前来交配[35,36]。本试验将多头雌、雄成虫置于同一容器中,雄蛾个体间为了趋避同种、避免交配竞争势必会刺激合成PBAN,从而产生更多的性信息素来吸引雌性。PBAN与雄性信息素间的关系有待于进一步研究。
交配行为是影响性信息素以及PBAN合成释放的一个重要因素。有研究显示,大多数昆虫交配后,其体内PBAN短期内会先受到显著抑制后又逐渐恢复[30,37-38]。但有的昆虫交配后,性信息素合成释放短时间内虽会受到抑制,但体内性腺的活性以及PBAN在食下神经节内的合成并不受影响[39]。说明交配行为对不同昆虫PBAN合成释放的调控影响差异较大。本研究结果显示,红铃虫雌、雄成虫在交配处理后1 d和3 d,其体内PgosPBAN相对表达量均显著高于处女蛾,证实交配行为对1—3 d红铃虫成虫的PgosPBAN调控作用显著。鉴于PBAN相对表达量与性信息素含量变化往往呈反比[18],笔者推测红铃虫处女蛾为了交配成功,可能会加大合成释放性信息素,从而导致其体内前体物质PBAN的大量损耗,而有交配行为的红铃虫,对再次交配的投入会相应减少,其体内合成释放PBAN的速率也会受到抑制。
另外,本研究还发现棉花挥发物对PgosPBAN存在一定的调控作用。暴露于棉花挥发物1—5 d雄成虫PgosPBAN表达水平未受到显著影响,而1、8 d雌成虫及8 d雄成虫PgosPBAN表达水平均显著低于对照。这与植物挥发物对小菜蛾PBAN的调控机制相似。有研究显示,暴露于植物挥发物异硫氰酸丙烯酯的小菜蛾在羽化后18 h和60 h时PBAN表达量均显著低于对照处理[40]。说明寄主植物挥发物对昆虫PBAN合成及性信息素释放存在一定的调控及刺激作用。对于红铃虫羽化后期成虫,棉花挥发物可能会激发其对性信息素的积极行为,通过这种增效及补偿机制以达到成功交配,但势必会导致红铃虫体内大量前体物质的损耗。
4 结论
利用RACE技术分离得到PgosPBAN全长序列1 461 bp,ORF为618 bp,编码205个氨基酸,且N端有一条潜在的信号肽。该基因含有6个保守的识别切割位点,编码5个神经肽,每个神经肽的C末端都有FXPR/KL序列,其编码的蛋白与大多鳞翅目昆虫具有高度同源性。PgosPBAN在红铃虫不同发育阶段存在明显的表达特异性。PgosPBAN表达不局限于红铃虫雌蛾,雄性个体也有大量表达,且表达量高于雌蛾。根据PgosPBAN在红铃虫不同发育阶段的表达规律以及与交配行为、棉花挥发物的调控关系,推测该基因不仅参与了红铃虫雌性信息素的合成释放,在调控雄性信息素以及调节生长发育等方面可能也扮演着重要角色。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]

1986-1993年在棉红铃 虫(pectinophoragossypiellaSaunders)发生中密度、高密度区应用红铃虫性信息素2%微胶囊、2%改进微胶囊、塑料纤维夹 片、PVC胶带、聚乙烯塑料管,采用干扰交配方式进行防治技术的研究。经过22个年次试验结果表明:在红铃虫发生中密度地区以自然沟、渠为界,5-8公顷 连片棉田为使用单位,所用剂型均能有效控制红铃虫的为害。青铃活虫、籽棉含虫量、僵瓣花比常规农药防治区减少70-80%以上。在高密度区,试验区四周需 有100公尺以上隔离带,使用PVC胶带剂型,在越冬代始蛾时施挂一次,干扰交配时间可达三个月以上,性信息素释放稳定,控制青铃活虫、籽棉含虫量与使用 4-5次菊酯农药的效果相当。
URL [本文引用: 1]

1986-1993年在棉红铃 虫(pectinophoragossypiellaSaunders)发生中密度、高密度区应用红铃虫性信息素2%微胶囊、2%改进微胶囊、塑料纤维夹 片、PVC胶带、聚乙烯塑料管,采用干扰交配方式进行防治技术的研究。经过22个年次试验结果表明:在红铃虫发生中密度地区以自然沟、渠为界,5-8公顷 连片棉田为使用单位,所用剂型均能有效控制红铃虫的为害。青铃活虫、籽棉含虫量、僵瓣花比常规农药防治区减少70-80%以上。在高密度区,试验区四周需 有100公尺以上隔离带,使用PVC胶带剂型,在越冬代始蛾时施挂一次,干扰交配时间可达三个月以上,性信息素释放稳定,控制青铃活虫、籽棉含虫量与使用 4-5次菊酯农药的效果相当。
URL [本文引用: 1]

本文综述了性信息素在棉红铃虫防治中的应用,以及性信息素在害虫综合治理中的发展前景。
URL [本文引用: 1]

本文综述了性信息素在棉红铃虫防治中的应用,以及性信息素在害虫综合治理中的发展前景。
DOI:10.1007/BF01014723URLPMID:24271678 [本文引用: 1]

Abstract Heritability of variation in male pheromone response by pink bollworm moths,Pectinophora gossypiella (Saunders), was examined using a still-air, wing-fanning bioassay. Heritability (00±SE) of overall responsiveness, as measured by the mean duration of wing fanning to the blend of pheromone components produced by females [4409080956 ratio of (Z, E)- to (Z, Z)-7,11-hexadecadienyl acetate], was 0.385 00± 0.095. Heritabilities of wingfanning duration to blends with 25 and 65%Z, E isomer were 0.377 00± 0.113 and -0.145 00± 0.103, respectively. These findings indicate an asymmetry in the genetic component of variation in response to pheromone blends with high and low proportions of theZ, E isomer. An index of response specificity for individual males was developed based on the response to an off-blend (either 25 or 65%Z, E isomer) relative to the response to the 44%Z, E blend. Heritabilities of response specificity were 0.117 00± 0.059 and -0.043 00± 0.067 for the 25 and 65%Z, E blends, respectively.
DOI:10.1016/S0301-0082(02)00057-6URLPMID:12427481 [本文引用: 1]

Neuropeptides in insects act as neuromodulators in the central and peripheral nervous system and as regulatory hormones released into the circulation. The functional roles of insect neuropeptides encompass regulation of homeostasis, organization of behaviors, initiation and coordination of developmental processes and modulation of neuronal and muscular activity. With the completion of the sequencing of the Drosophila genome we have obtained a fairly good estimate of the total number of genes encoding neuropeptide precursors and thus the total number of neuropeptides in an insect. At present there are 23 identified genes that encode predicted neuropeptides and an additional seven encoding insulin-like peptides in Drosophila. Since the number of G-protein-coupled neuropeptide receptors in Drosophila is estimated to be around 40, the total number of neuropeptide genes in this insect will probably not exceed three dozen. The neuropeptides can be grouped into families, and it is suggested here that related peptides encoded on a Drosophila gene constitute a family and that peptides from related genes (orthologs) in other species belong to the same family. Some peptides are encoded as multiple related isoforms on a precursor and it is possible that many of these isoforms are functionally redundant. The distribution and possible functions of members of the 23 neuropeptide families and the insulin-like peptides are discussed. It is clear that each of the distinct neuropeptides are present in specific small sets of neurons and/or neurosecretory cells and in some cases in cells of the intestine or certain peripheral sites. The distribution patterns vary extensively between types of neuropeptides. Another feature emerging for many insect neuropeptides is that they appear to be multifunctional. One and the same peptide may act both in the CNS and as a circulating hormone and play different functional roles at different central and peripheral targets. A neuropeptide can, for instance, act as a coreleased signal that modulates the action of a classical transmitter and the peptide action depends on the cotransmitter and the specific circuit where it is released. Some peptides, however, may work as molecular switches and trigger specific global responses at a given time. Drosophila, in spite of its small size, is now emerging as a very favorable organism for the studies of neuropeptide function due to the arsenal of molecular genetics methods available.
URL [本文引用: 1]

昆虫在其生长发育过程中,如胚胎发育、蜕皮变态、滞育、迁飞、代谢、生殖等都离不开神经肽的调控。信息素合成激活肽(pheromone biosynthesis activating neuropeptide,PBAN)和Pyrokinin神经肽是C端具有五肽FXPRL(X=S,V,T,G等)(苯丙-X-脯-精-亮氨酸)序列的一类神经肽,在昆虫的生长发育中起重要的生理功能,如性信息素的合成、控制表皮色素、促进胚胎滞育和刺激内脏肌肉收缩等重要的生理功能。因此近几年对PBAN/pyrokinin神经肽的鉴定、加工、作用和降解方式的研究成为研究的热点,为研制高效、低毒、专一性强、无公害的杀虫剂提供了思路。介绍了PBAN/pyrokinin神经肽类及其基因的研究进展,并对PBAN/pyrokinin神经肽在害虫防治中的应用进行了展望。
URL [本文引用: 1]

昆虫在其生长发育过程中,如胚胎发育、蜕皮变态、滞育、迁飞、代谢、生殖等都离不开神经肽的调控。信息素合成激活肽(pheromone biosynthesis activating neuropeptide,PBAN)和Pyrokinin神经肽是C端具有五肽FXPRL(X=S,V,T,G等)(苯丙-X-脯-精-亮氨酸)序列的一类神经肽,在昆虫的生长发育中起重要的生理功能,如性信息素的合成、控制表皮色素、促进胚胎滞育和刺激内脏肌肉收缩等重要的生理功能。因此近几年对PBAN/pyrokinin神经肽的鉴定、加工、作用和降解方式的研究成为研究的热点,为研制高效、低毒、专一性强、无公害的杀虫剂提供了思路。介绍了PBAN/pyrokinin神经肽类及其基因的研究进展,并对PBAN/pyrokinin神经肽在害虫防治中的应用进行了展望。
DOI:10.3969/j.issn.1005-0507.2004.01.011URL [本文引用: 1]

德国小蠊的聚集行为是由个体分泌的聚集信息素引起的 ,德国小蠊若虫的体表和粪便中均含有聚集信息素。用气相色谱 (GC)和气 质谱 (GC MS)联用技术测定了德国小蠊体表的二氯甲烷提取物和粪便的正己烷提取物 ,结果表明体表提取物中的活性组分全部是外表皮分泌的一类碳氢化合物 ,而雌成虫和若虫的粪便提取物的GC MS谱图完全一致 ,与体表提取物相比 ,多了棕榈酸、亚油酸、油酸、十八碳酸、二十烷醇和二十二烷醇 6种组分 ,其余 2 5种组分则完全相同。这些结果表明德国小蠊聚集信息素的主要组分是表皮碳氢化合物。在体表提取物中含量最高的是 3 ,7 /3 ,9 /3 ,1 1 二甲基二十九碳烷 (占总量的 2 2 2 % )。其次是 9 1 1 /1 3 /1 5 甲基二十九碳烷、3 甲基二十九碳烷、 5 甲基二十九碳烷、正二十九碳烷、1 1 ,1 5 1 3 ,1 7 二甲基二十九碳烷和 5 ,9 /5 ,1 1 二甲基二十九碳烷 ,含量依次是 1 3 5 5 %、 1 0 46%、 7 93 % 6 48%、6 1 4%和 5 5 2 %。粪便提取物中也是 3 ,7 /3 ,9 /3 ,1 1二甲基二十九碳烷的含量最高 (占总量的 1 2 86% )其次是 9 1 1 /1 3 /1 5 甲基二十九碳烷、油酸、 3 甲基二十九碳烷、正二十九碳烷、 1 1 /1 3 /1 5 甲基三十一碳烷和 1 1 ,1 5 1 3 ,1 7 二甲基二十九碳
DOI:10.3969/j.issn.1005-0507.2004.01.011URL [本文引用: 1]

德国小蠊的聚集行为是由个体分泌的聚集信息素引起的 ,德国小蠊若虫的体表和粪便中均含有聚集信息素。用气相色谱 (GC)和气 质谱 (GC MS)联用技术测定了德国小蠊体表的二氯甲烷提取物和粪便的正己烷提取物 ,结果表明体表提取物中的活性组分全部是外表皮分泌的一类碳氢化合物 ,而雌成虫和若虫的粪便提取物的GC MS谱图完全一致 ,与体表提取物相比 ,多了棕榈酸、亚油酸、油酸、十八碳酸、二十烷醇和二十二烷醇 6种组分 ,其余 2 5种组分则完全相同。这些结果表明德国小蠊聚集信息素的主要组分是表皮碳氢化合物。在体表提取物中含量最高的是 3 ,7 /3 ,9 /3 ,1 1 二甲基二十九碳烷 (占总量的 2 2 2 % )。其次是 9 1 1 /1 3 /1 5 甲基二十九碳烷、3 甲基二十九碳烷、 5 甲基二十九碳烷、正二十九碳烷、1 1 ,1 5 1 3 ,1 7 二甲基二十九碳烷和 5 ,9 /5 ,1 1 二甲基二十九碳烷 ,含量依次是 1 3 5 5 %、 1 0 46%、 7 93 % 6 48%、6 1 4%和 5 5 2 %。粪便提取物中也是 3 ,7 /3 ,9 /3 ,1 1二甲基二十九碳烷的含量最高 (占总量的 1 2 86% )其次是 9 1 1 /1 3 /1 5 甲基二十九碳烷、油酸、 3 甲基二十九碳烷、正二十九碳烷、 1 1 /1 3 /1 5 甲基三十一碳烷和 1 1 ,1 5 1 3 ,1 7 二甲基二十九碳
DOI:10.1016/j.ygcen.2008.04.004URLPMID:18495120 [本文引用: 2]

This review focuses on the endocrine regulation of reproductive behavior in moth species with particular emphasis on Helicoverpa spp. Reproductive behavior in most adult moths is dependent on the release of a unique blend of sex pheromones by the females to attract conspecific males. Mating, on the other hand, results in a loss of sexual receptivity due to the transfer of secretions from the male accessory glands, which renders females unattractive to ensuing mates. Synchronization of sexual behavior is attained by the timely release of Pheromone- Biosynthesis- Activating Neuropeptide (PBAN), a member of the PBAN/Pyrokinin neuropeptide family, characterized by a common amino acid sequence FXPRLamide motif in the C-terminus. PBAN is released into the hemolymph of females during the scotophase and is drastically reduced after mating, contributing to the loss in female receptivity. Pheromone production is age-dependent and Juvenile Hormone is involved in its regulation. PBAN activates pheromone production through its binding to a PBAN-Receptor (PBAN-R) and subsequent up-regulation of key enzymes in the biosynthetic pathway. The PBAN-R gene was identified as a member of the G-protein coupled receptor family (GPCRs), classified with the vertebrate subfamily of neuromedin U receptors. Using both biochemical and in silico mutagenesis studies, putative binding sites are predicted. Differential expression studies reveal its localization in pheromone glands, neural tissues and the male aedeagus. In the latter tissue, no activity and/or receptor-binding can be detected in response to PBAN. These results raise many questions concerning the evolutionary role of the PBAN/Pyrokinin receptors belonging to the GPCR family.
DOI:10.1016/j.jinsphys.2003.09.006URLPMID:15037090 [本文引用: 1]

Diapause hormone (DH) and pheromone biosynthesis activating neuropeptide (PBAN) are encoded by a single mRNA in the suboesophegeal ganglion (SG) and are responsible for induction of embryonic diapause in of DH to terminate pupal diapause is 20 pmol/pupae. The other four FXPRLamide neuropeptides from the DH-PBAN polyprotein precursor have cross activity for diapause termination. These observations therefore suggest a potential role for these FXPRL family peptides in promoting continuous development in several noctuid species. The high expression of this gene in pharate adults and adults indicates that the FXPRL family peptides may have multiple physiological functions.
[本文引用: 2]
URL [本文引用: 1]

根据家蚕 (Bombyxmori)性信息素合成激活肽 (pheromonebiosynthesisactivatingneuropeptide,PBAN)基因DNA序列设计引物 ,扩增获得中国野桑蚕 (BombyxmandarinaChina)PBAN基因。分析表明 ,PBAN由 33个氨基酸组成 ,在第 1 4个氨基酸异亮氨酸和第 1 5个氨基酸酪氨酸之间插入了 6 98bp的内含子。根据PBAN及其基因cDNA、DNA序列分别构建分子进化树 ,结果显示 3个水平比对结果构建的分子进化树有较好的一致性 ,推测PBAN基因可能适合于科、属之间的进化分析 ;并且PBAN基因内含子没有表现出特有的进化信息 ,推测PBAN基因内含子的进化与PBAN全长基因的进化在进化速率上并没有显著差别。相对于PBAN及α SGNP、γ SGNP ,β SGNP的进化速率相对较快 ,推测 β SGNP序列可能适合用于种间的进化分析。
URL [本文引用: 1]

根据家蚕 (Bombyxmori)性信息素合成激活肽 (pheromonebiosynthesisactivatingneuropeptide,PBAN)基因DNA序列设计引物 ,扩增获得中国野桑蚕 (BombyxmandarinaChina)PBAN基因。分析表明 ,PBAN由 33个氨基酸组成 ,在第 1 4个氨基酸异亮氨酸和第 1 5个氨基酸酪氨酸之间插入了 6 98bp的内含子。根据PBAN及其基因cDNA、DNA序列分别构建分子进化树 ,结果显示 3个水平比对结果构建的分子进化树有较好的一致性 ,推测PBAN基因可能适合于科、属之间的进化分析 ;并且PBAN基因内含子没有表现出特有的进化信息 ,推测PBAN基因内含子的进化与PBAN全长基因的进化在进化速率上并没有显著差别。相对于PBAN及α SGNP、γ SGNP ,β SGNP的进化速率相对较快 ,推测 β SGNP序列可能适合用于种间的进化分析。
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.peptides.2005.04.016URLPMID:16005110 [本文引用: 1]

Pheromone biosynthesis activating neuropeptide (PBAN) produced in the subesophageal ganglion stimulates pheromone production in the pheromone gland. A cDNA isolated from female adult heads of the diamondback moth ( Plutella xylostella (L.)) encodes 193 amino acids including PBAN, designated as Plx-PBAN, and four other neuropeptides (NPs): diapause hormone (DH) homologue, α-NP, β-NP and γ-NP. All of the peptides are amidated in their C-termini and shared a conserved motif, FXPR(or K)L structure, as reported from other PBAN cDNAs. Plx-PBAN consists of 30 amino acids, the shortest PBAN so far reported. Plx-PBAN exhibited below 50% homology, compared with other known PBANs. The Plx-DH homologue is structurally different from DH of Bombyx mori. The length of Plx-β-NP (16 amino acids) was the shortest and showed relatively low similarity, whereas γ-NP (10 amino acids in length) was the longest among examined γ-NPs. When female adults were injected with synthetic Plx-PBAN, pheromone production showed a maximal increase 1 h post-injection. RT-PCR screening revealed that Plx-PBAN cDNA was expressed in all examined body parts, with the highest expression level in the head of female adults. Analysis of RT-PCR products indicated the Plx-PBAN sequence was identical in all examined body parts of both sexes. Phylogenetic analysis revealed that the Plx-PBAN gene is distantly related to other PBANs, demonstrated by the relatively low similarity.
DOI:10.1016/j.ibmb.2007.07.012URLPMID:17967345 [本文引用: 1]

Using a strategy of rapid amplification of cDNA ends, the cDNA of diapause hormone (DH) and pheromone biosynthesis activating neuropeptide (PBAN) was cloned from the head of Clostera anastomosis (L.). The Cloan-DH-PBAN cDNA contains an open reading frame encoding a 196-amino acid preprohormone, from which five putative FXPRL peptides, DH, PBAN, -SGNP(SGNP, suboesophageal ganglion neuropeptide), -SGNP and -SGNP, are released. Comparing the deduced amino acid sequences from cDNAs of these five FXPRL peptides to those known from other insects, Cloan-DH shows highest similarity of 93.1% to that from Agrotis ipsilon, Cloan-PBAN 93.9% to those from Helicoverpa armigera, Helicoverpa zea and Helicoverpa assulta, which show the highest similarity to species of Noctuidae. Phylogenetic analysis revealed that the Cloan-DH-PBAN gene is relatively closely related to those from Noctuoidea, but distant from those from Tortricoidea, Yponomeutoidea and Bombycoidea species. The DNA sequence encoding Cloan-DH-PBAN was cloned by PCR, which is 3698 bp in size and comprises six exons interspersed by five introns. Developmental expression of the DH-PBAN transcripts in the head was also showed by a semi-quantitative RT-PCR method, which was relatively low in larvae and remained low in pupae of both sexes, increased sharply in adults of both sexes.
[本文引用: 1]
DOI:10.1016/j.ibmb.2008.01.005URLPMID:18405833 [本文引用: 2]

We combine the use of labeled precursors with enzyme inhibitors to decipher the biosynthetic pathway of pheromone biosynthesis and the rate-limiting step/s that are regulated by pheromone biosynthesis activating neuropeptide (PBAN). We demonstrate that Plodia interpunctella is able to utilize hexadecanoic acid, and to a lesser extent tetradecanoic acid, for the biosynthesis of the main pheromone component (Z,E)-9,12-tetradecadienyl acetate. This indicated that the main pathway involves a 11 desaturase, chain shortening, followed by a 12 desaturase, but that a functional 9 desaturase could also be utilized. Using reverse transcription-quantitative real-time polymerase chain reaction (RT-QPCR) we distinguish two out of nine possible desaturase gene transcripts in P. interpunctella that are expressed at the highest levels. The rate-limiting step for PBAN-stimulation was studied in two moth species so as to compare the biosynthesis of a diene (P. interpunctella) and a monoene (Helicoverpa armigera) main pheromone component. In both species, incorporation of label from the 13C sodium acetate precursor was activated by PBAN whereas no stimulatory action was observed in the incorporation of the precursors: 13C malonyl coenzyme A; hexadecanoic 16,16,16-2H3 or tetradecanoic 14,14,14-2H3 acids. The acetyl coenzyme A carboxylase (ACCase) inhibitor, Tralkoxydim, inhibited the PBAN-stimulation of incorporation of stable isotope whereas the fatty-acyl reductase inhibitor, Mevastatin, failed to influence the stimulatory action of PBAN. These results provide irrefutable support to the hypothesis that PBAN affects the production of malonyl coenzyme A from acetate by the action of ACCase in the pheromone glands of these moths.
.
DOI:10.1073/pnas.86.6.1806URLPMID:16594018 [本文引用: 1]

Experiments were performed to characterize the action of a brain hormone on pheromone biosynthesis in female redbanded leafroller and cabbage looper moths. Results showed that the two species differed in their respective control mechanisms. In the cabbage looper, pheromone titer from decapitated females that received either saline or brain extract injections was not significantly different from control females, suggesting that pheromone biosynthesis was not dependent on the presence of the brain hormone. In contrast, with redbanded leafroller females, studies using radiolabeled acetate incorporation as well as incorporation of deuterium-labeled hexadecanoic acid showed that (i) the brain hormone was required for pheromone biosynthesis, (ii) the brain hormone regulated pheromone biosynthesis by activating synthesis of octadecanoyl and hexadecanoyl intermediates, and (iii) the brain hormone did not control other enzymes in the pathway. Regulation of fatty acid synthetase was unlikely since assays of the enzyme from decapitated and normal females showed no differences in the amount or distribution of the 18- and 16-carbon acyl end products. These results in conjunction with those from organ cultures of the pheromone gland suggest that the brain hormone acts by increasing the substrate supply for fatty acid synthesis.
DOI:10.1271/bbb.57.2144URL [本文引用: 1]

The sex pheromone of Bombyx mori, bombykol [(10E,12Z)-10,12-hexadecadien-1-ol], can be biosynthesized in four steps: construction of a hexadecanoic moiety from acetyl CoA, 62-11-desaturation, .62-10,12-desaturation, and reduction of the acyl group. This biosynthesis is regulated by a hormone named the pheromone biosynthesis activating neuropeptide (PBAN). To examine the steps that are accelerated by this neurohormone, pheromone glands excised from decapitated females were incubated in vitro with either 14C-Iabeled sodium acetate or one of three fatty acids [hexadecanoic acid, (Z)-11-hexadecenoic acid, or (10E,12Z)-10,12-hexadecadienoic acid]. After analyzing the radioactivity that was incorporated from each precursor into bombykol and the biosynthetic precursors, it was observed that the first three steps proceeded in glands both treated and untreated with synthetic PBAN of B. mori; however, the last step proceeded only in the treated glands. From this in vitro experiment, it can be concluded that the main regulatory role of PBAN is in the reduction of the acyl group in B. mori, as was shown by our previous in vivo experiment.
DOI:10.3389/fendo.2011.00046URLPMID:3356091 [本文引用: 3]

Both males and females of heliothine moths utilize sex-pheromones during the mating process. Females produce and release a sex pheromone for the long ange attraction of males for mating. Production of sex pheromone in females is controlled by the peptide hormone (pheromone biosynthesis activating neuropeptide, PBAN). This review will highlight what is known about the role PBAN plays in controlling pheromone production in female moths. Male moths produce compounds associated with a hairpencil structure associated with the aedaegus that are used as short-range aphrodisiacs during the mating process. We will discuss the role that PBAN plays in regulating male production of hairpencil pheromones.
DOI:10.1016/j.jinsphys.2004.04.001URLPMID:15183285 [本文引用: 1]

Isolated pheromone glands of Helicoverpa zea were utilized to investigate the physiological action of pheromone biosynthesis activating neuropeptide (PBAN) with regard to the role of calcium ions in stimulating pheromone biosynthesis under various incubation conditions. Incubation of glands with 1 渭M or 1 nM PBAN produced a significant amount of pheromone after a 5 min incubation period and reached maximum pheromone production after 30 min. Glands incubated with PBAN for 1 min, and then without PBAN for 30 min, produced pheromone whether or not extracellular calcium was present during the first 1 min. The presence of lanthanum as a calcium channel blocker did not affect pheromone production if present during the first 1 min of incubation with PBAN. However, if calcium was absent or lanthanum ion was present during the 30 min of incubation, no pheromone was produced. A maximum amount of pheromone was reached when glands were incubated for 1 min with PBAN and for 10 min without PBAN, and repeated three times. The present results indicate that a time interval exists between PBAN binding to a receptor and opening of extracellular calcium channels. Calcium influx into the cytosol from extracellular stores is required for PBAN to stimulate pheromone production. This could be achieved by PBAN either binding periodically to the receptor or the plasma membrane calcium channel could remain activated for a period of time after the initial activation.
DOI:10.3969/j.issn.0452-8255.2005.02.004URL [本文引用: 1]

综述了铃夜蛾属Helicoverpa昆虫性信息素生物合成途径及内分泌因子的调控作用 ,包括信息素生物合成激活神经肽 (PBAN)和信息素生物合成抑制肽 (PSP)等的来源、结构和作用机制及一些种中保幼激素 (JH)和章鱼胺 (OA)对性信息素生物合成的作用 ,并展望了未来的研究方向。
DOI:10.3969/j.issn.0452-8255.2005.02.004URL [本文引用: 1]

综述了铃夜蛾属Helicoverpa昆虫性信息素生物合成途径及内分泌因子的调控作用 ,包括信息素生物合成激活神经肽 (PBAN)和信息素生物合成抑制肽 (PSP)等的来源、结构和作用机制及一些种中保幼激素 (JH)和章鱼胺 (OA)对性信息素生物合成的作用 ,并展望了未来的研究方向。
DOI:10.1016/S0965-1748(02)00264-3URLPMID:12609521 [本文引用: 1]

The present study was designed to determine the age and female specificity of a membrane protein that binds to a pheromone biosynthesis activating neuropeptide (PBAN) ligand and to elucidate the effect of Juvenile Hormone (JH) on binding as well as pheromone activation. The precise age at which developing adult females of Helicoverpa armigera begin to respond to PBAN was determined. PBAN activates in vitro pheromone biosynthesis as well as its intracellular second messenger, cAMP, only in intersegments of newly emerged adult female pheromone glands (i.e. 1-day-old females). An increase in response was observed in 2-day-old females. Intersegments of female pupae and the homologous tissues of adult males do not respond to PBAN. However, in the presence of Juvenile Hormone II (JH II) PBAN induced a response in females, 1 day before emergence (pharate females), but not in younger female pupae. This phenomenon was also observed after topical applications of the JH analog fenoxycarb (FX). In addition the response to PBAN by intersegments of FX-treated emerged adults increased significantly to the level of 2-day-old females. JH II also stimulated the level of incorporation of 35S-labelled amino acids in female pupae into membrane proteins that are typical in adult intersegments. Using a photoaffinity-biotin labelled PBAN analog we demonstrate specific binding of a membrane protein (estimated MW: 50kD) in adult females. This binding was not detected in female pupae 3 days before emergence. However, in such female pupae specific binding of the 50kD protein by the photoaffinity-biotin labelled PBAN analog was induced after JH II or FX treatments thereby providing evidence that JH may up-regulate this putative receptor protein.
DOI:10.1016/S0965-1748(96)00029-XURL [本文引用: 1]

ABSTRACT The biochemical second messenger system during pheromonotropic and pheromonostatic activities was assessed and compared. The involvement of G-proteins was implicated by the stimulatory action of sodium fluoride (NaF), at a range of 1 2 mM, on both pheromone biosynthesis and intracellular cAMP levels in isolated intersegmental membranes of Helicoverpa armigera. However, cholera toxin did not mimic the pheromonotropic response of PBAN. The stimulatory action of NaF was significantly inhibited by adrenergic agonists (tyramine and clonidine) as was observed at low levels of PBAN. At high levels of PBAN, although cAMP production was inhibited, pheromone biosynthesis was unaffected by clonidine. A similar phenomenon was observed with the ionophore, thapsigargin, in which adrenergic agonists did not inhibit pheromone biosynthesis but reduced intracellular cAMP to basal levels. Thus pheromonotropic activity exhibited both cAMP-independent and cAMP-dependent stimulatory responses. The calcium calmodulin inhibitor, W7, inhibited pheromone biosynthesis and intracellular cAMP production which was induced either by Hez-PBAN, NaF or thapsigargin. The pheromonostatic activity by clonidine was prevented in the presence of pertussis toxin, thereby indicating the involvement of inhibitory G-protein (Gi) in the inhibitory action of adrenergic agonists on the activity of Hez-PBAN. From the results we hypothesized that negative regulation of pheromonotropic activity occurs at the membrane receptor level by the interaction of an adrenergic receptor.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/0022-1910(90)90060-SURL [本文引用: 1]

Melanization and reddish colouration hormone (MRCH) which regulates colour polymorphism associated with phase variation in the armyworm species has been purified from a head extract of the silkworm, Bombyx mori. Amino acid sequence analysis of MRCH-I established its primary structure and demonstrated that MRCH-I was the same molecule as pheromone biosynthesis activating neuropeptide (PBAN-I) purified from the same extract. Natural and synthetic MRCH-I (PBAN-I) revealed interspecific activity both in cuticular melanization and in sex pheromone production. In the common cutworm, Spodoptera litura, synthetic MRCH-I(PBAN-I) induced cuticular melanization in the larval stage and sex pheromone production in the adult stage. These findings suggest that some lepidopteran insect utilize the same neurohormone, MRCH and PBAN, in different physiological events according to developmental stages and species.
URL [本文引用: 1]

实时荧光定量PCR(FQ-PCR)是一种准确有效的核酸定量分析技术,具有易操作、高通量、高敏感性、高特异性、高度自动化和低污染等优点,并随新定量PCR仪及新操作方法的发展而得到广泛应用,但是,定量PCR的高敏感性特点使得实验操作严格而繁琐。阐述了一种改进的相对定量方法——双标准曲线法的试验原理和特点,描述了定量PCR体系的优化方式,探讨了试验误差分析方法及试验操作技巧,并就试验数据的处理方法进行讨论。试验证明,双标准曲线法是一种经济、简单而准确的定量方法。
.
URL [本文引用: 1]

实时荧光定量PCR(FQ-PCR)是一种准确有效的核酸定量分析技术,具有易操作、高通量、高敏感性、高特异性、高度自动化和低污染等优点,并随新定量PCR仪及新操作方法的发展而得到广泛应用,但是,定量PCR的高敏感性特点使得实验操作严格而繁琐。阐述了一种改进的相对定量方法——双标准曲线法的试验原理和特点,描述了定量PCR体系的优化方式,探讨了试验误差分析方法及试验操作技巧,并就试验数据的处理方法进行讨论。试验证明,双标准曲线法是一种经济、简单而准确的定量方法。
DOI:10.1126/science.244.4906.796URLPMID:17802237 [本文引用: 1]

A pheromone biosynthesis activating neuropeptide (PBAN) hormone that controls sex pheromone production in female moths was identified from the brain-subesophageal ganglion complexes of the adult corn earworm, Heliothis zea. PBAN has 33 amino acid residues and a molecular weight of 3900. Its amino acid sequence has no significant homology with any of the fully characterized peptide hormones. The synthetic peptide, at a dose of between 2 and 4 picomoles, induced production of a normal quantity of sex pheromone in ligated H. zea females. The peptide also induced pheromone production in six other species of moths, thus indicating that this or similar peptides may be responsible for the regulation of pheromone production in moths.
DOI:10.1016/j.euprot.2014.02.008URL [本文引用: 1]

Applied peptidomics: A PK active core analog incorporating a novel transPro conformational-mimetic motif, the dihydroimidazole moiety, was found to demonstrate pure, selective agonism in pyrokinin (PK) family bioassays. A second PK core analog incorporating the dihydroimidazole moiety proved to be an antagonist of the diapause-termination activity of another PK assay. The dihydroimidazole analogs feature a modification adjacent to the primary tissue-bound peptidase hydrolysis site that is expected to enhance biostability over natural PK peptides identified by peptidomics. The research identifies a novel scaffold to design mimetic PK analogs as potential environmentally favorable pest management agents capable of disrupting PK-regulated systems.
DOI:10.1080/10425170601060806URLPMID:17364826 [本文引用: 1]

Abstract Diapause hormone (DH) and pheromone biosynthesis activating neuropeptide (PBAN), two important insect neuropeptides, regulate insect development and sex pheromone biosynthesis, respectively. DH-like immunoreactivity has been detected in the suboesophageal ganglion (SG) of pharate adult of Spodoptera exigua (Spe) by using an antiserum against Helicoverpa armigera DH. A full-length of Spe-DH-PBAN cDNA was obtained based on reverse transcription-PCR and rapid amplification of cDNA ends strategies. The open reading frame of this cDNA encodes a 197-amino acid precursor protein that contains DH, PBAN, and three other SG neuropeptides, all of which share a conservative C-terminal pentapeptide motif FXPR/KL (X = G, T or S). Northern blot analysis demonstrates the presence of an 800 bp transcript in the SG. The Spe-DH-PBAN mRNA is detectable at high levels at larval and adult stages, suggesting that Spe-DH-PBAN gene might be correlated with larval development and sex pheromone biosynthesis in moths.
[本文引用: 3]
[本文引用: 3]
DOI:10.1016/j.jinsphys.2004.03.011URLPMID:15183284 [本文引用: 1]

FXPRLamide peptides encoded by the DH-PBAN (diapause hormone-pheromone biosynthesis activating neuropeptide) gene induce embryonic diapause in Bombyx mori, but terminate pupal diapause in Helicoverpa armigera (Har). Here, we explore the mechanisms of terminating pupal diapause by the FXPRLamide peptides. Using quantitative RT-PCR, we observed that expression of Har-DH-PBAN mRNA in the SG of nondiapause-type pupae was significantly higher than in diapause-type pupae. Immunocytochemical results indicated that the level of FXPRLamide peptides and axonal release are related to the diapause decision. Ecdysteroidogenesis in prothoracic glands (PGs) was stimulated by synthetic Har-DH in vivo and in vitro, and labeled Har-DH bound to the membrane of the PG, thus suggesting that DH breaks diapause by activating the PG to synthesize ecdysone. Furthermore, the response of DH in terminating diapause was temperature dependent. Decerebration experiments showed that the brain can control pupal development through the regulation of DH, and DH can terminate diapause and promote development without the brain. This result suggests a possible mechanism of response for the signals of DH and other FXPRLamide peptides in H. armigera.
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/aesa/79.1.128URL [本文引用: 1]

ABSTRACT Ovipositors of the female corn earworm, Heliothis zea (Boddie), contained large amounts of extractable sex pheromone only during scotophase. Quantitative gas chromatographic analysis of ovipositor extracts for (Z)-11-hexadecenal, the species' major pheromonal component, indicated that female sex pheromone titer was maximal on the third night after adult emergence. Females in their third night began calling within 1 h after onset of scotophase, and peak calling activity coincided with maximal pheromone titer. Compared to that of virgin females of similar age, mating on the third night caused a 93% reduction in pheromone titer. Titer in mated females increased during subsequent nights, and 3 days later it was similar to that of virgin females of the same age.
DOI:10.1016/j.jinsphys.2005.01.004URLPMID:15890183 [本文引用: 1]

In a previous study we showed that juvenile hormone (JH) or its analog, fenoxycarb (FX), is involved in the up-regulation of pheromone biosynthesis-activating neuropeptide (PBAN) competence. JH causes induction of binding to a putative PBAN-receptor (PBAN-R) and the subsequent pheromone production by pheromone glands of pharate females. The present study demonstrates that pheromone production by the adult female is age-dependent. The pheromonotropic response increased to reach a maximum at 4 days, after which a decreased response was observed. Binding of the PBAN-R was also age-dependent. Treatment with FX inhibited both binding of PBAN to the PBAN-R and the pheromonotropic response as reflected by the production of the main pheromone component, Z-11-hexadecenal. Thus, in contrast to its up-regulatory role in pharate females, FX treatment of adult females causes down-regulation of both pheromone production and specific binding to the PBAN-R. In addition, behavioural observations showed that calling behaviour, mating success and subsequent egg-fertility are affected by treating females with FX.
DOI:10.3969/j.issn.1673-9868.2001.02.018URL [本文引用: 1]

介绍了烟夜蛾性信息素的生物学、行为学、化学组成、影响因子及其应用方面的研究进展。
DOI:10.3969/j.issn.1673-9868.2001.02.018URL [本文引用: 1]

介绍了烟夜蛾性信息素的生物学、行为学、化学组成、影响因子及其应用方面的研究进展。
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1002/arch.20167URLPMID:17294422 [本文引用: 1]

Insect males produce accessory gland (MAG) factors that are transferred in the seminal fluid to females during copulation, and elicit changes in the mated female's behavior and physiology. Our previous studies showed that the injection of synthetic Drosophila melanogaster sex-peptide ( Drm SP) into virgin females of the moth Helicoverpa armigera causes a significant inhibition of pheromone production. In this and other moth species, pheromone production, correlated with female receptivity, is under neuroendocrine control due to the circadian release of the neuropeptide PBAN. In this study, we show that PBAN, present in the hemolymph during the scotophase in females, is drastically reduced after mating. We also identify 4 Drm SP-like HPLC peaks (Peaks A, S1, S2, and B) in MAGs, with increasing levels of Drm SP immunoreactivity during the scotophase, when compared to their levels observed during the photophase. In H. armigera MAGs, a significant reduction in the pheromonostatic peak (Peak B) was already evident after 15 min of copulation, and depletion of an additional peak (Peak S2) was evident after complete mating. Peak A is also detected in female brains, increasing significantly 1 h after mating, at which time inhibition of pheromone biosynthesis also occurs. However, changes corresponding to the other MAG peaks were not detected in mated female tissues. Arch. Insect Biochem. Physiol. 64:142-155, 2007. 2007 Wiley-Liss, Inc.
DOI:10.1016/S1226-8615(08)60131-4URL [本文引用: 1]

This study was undertaken to clarify the suppression phenomenon of sex pheromone production after mating and its relationship to the physiological mechanism in adult females of Helicoverpa assulta, and determine the mating factor from males causing depletion of sex pheromonc production. Sex pheromone production of H. assulta females was mostly terminated in 3 hours after mating. Mated females maintained with a low titer of sex pheromone until 3 days when it started to increase again, which showed a characteristic of species mating more than once. The mated female again produced pheromone upon injection of pheromone biosynthesis activating neuropeptide (PBAN) or extracts of brain-suboesophageal ganglion complexes (Br-Sg) of mated female, which were shown similar pheromonotropic activities as compared with virgin females. These results indicated that the mating did not inhibit the receptivity of pheromone gland itself and PBAN biosynthesis in suboesophageal ganglion of the mated females. And it seems to support that the depletion of sex pheromone production is responsible for blocking of PBAN release from head. To investigate the mating factor from adult males, when extracts of reproductive organs of male were injected into hemocoel of virgin females evoking depletion of sex pheromone production as shown in mated female. The results suggest that a chemical substance(s) from the male reproductive organs could be responsible for the loss of sex pheromone biosynthesis in H. assulta.
[本文引用: 1]
[本文引用: 1]
