 ,1, 林席跃2, 雷正平2, 丁在松
,1, 林席跃2, 雷正平2, 丁在松 ,1,*, 赵明1
,1,*, 赵明1Physiological characters of carbon, nitrogen, and hormones in ratooning rice cultivars with strong regeneration ability
HUANG Su-Hua ,1, LIN Xi-Yue2, LEI Zheng-Ping2, DING Zai-Song
,1, LIN Xi-Yue2, LEI Zheng-Ping2, DING Zai-Song ,1,*, ZHAO Ming1
,1,*, ZHAO Ming1通讯作者: * 丁在松, E-mail:dingzaisong@caas.cn
收稿日期:2020-10-23接受日期:2021-04-27网络出版日期:2021-05-20
| 基金资助: |
Corresponding authors: * E-mail:dingzaisong@caas.cn
Received:2020-10-23Accepted:2021-04-27Published online:2021-05-20
| Fund supported: |
作者简介 About authors
E-mail:hsuhua@163.com

摘要
明确强再生力品种腋芽萌发的生理基础与激素调控特点对于再生稻品种筛选和栽培技术调控具有重要意义。本研究利用在江西崇义县建立的再生稻品种筛选平台, 分析了2019年筛选的13个品种头季收获时不同部位的非结构性碳水化合物(non-structural carbohydrate, NSC)及全氮含量, 并对促进和抑制腋芽萌发的主要激素油菜素内酯和独脚金内酯的合成和信号转导关键基因的表达进行了研究。结果表明, 13个品种的再生力存在较大的差异, 变化范围为1.26~2.38; 不同品种之间, 不同节位之间的叶片、叶鞘和茎秆的可溶性糖、淀粉和非结构性碳水化合物含量均存在极显著的差异(P值均小于0.001); 而全氮含量除了上下节位茎秆的差异不显著外, 其余的也均存在极显著的差异; 与再生力的相关性分析表明仅有下部节位茎秆的可溶性糖、淀粉和NSC含量与再生力相关性达到显著或极显著水平(R2分别为0.4442*、0.9000**和0.8303**), 而其他均无显著相关性。强再生力品种谷优676中BR合成和信号途径中促进分蘖的基因CYP90A、CYP852A、D2、BRI、BSK和CYCD3表达水平增高, 而抑制分蘖的基因CYP734A1、BZR和BKI表达水平较低。可见, 可以利用下部节位茎秆的淀粉含量作为强再生力品种的筛选指标, 同时以BR途径相关基因表达水平作为辅助指标。
关键词:
Abstract
It is of great significance to clarify the physiological basis and hormone regulation characteristics of axillary bud germination for the selection of ratoon rice cultivars with strong ratooning ability and the regulation of cultivation techniques. In this study, the content of non-structural carbohydrate (NSC) and total nitrogen in different parts of 13 cultivars selected in 2019 were analyzed at the first harvest stage using the screening platform established in Chongyi County, Jiangxi Province. The biosynthesis and signal transduction genes of brassinolides and strigolactones, which promoted and inhibited axillary bud germination, were also studied. The results showed that there were significant differences in the regeneration rate of 13 cultivars, ranging from 1.26 to 2.38. The contents of soluble sugar, starch and non-structural carbohydrate in leaves, leaf sheaths, and stems were significantly different among different cultivars at different node positions (P-values were all less than 0.001). The total nitrogen content was also significant difference among them except for the upper and lower node stems. The correlation analysis showed that the contents of the soluble sugar, starch and NSC of the stem at the lower node had significant or extremely significant correlation with the regeneration rates (R2 = 0.4442*, 0.9000**, and 0.8303**, respectively), while there was no significant correlation in others. The relative expression levels of tillering promoting genes (CYP90A, CYP85A2, D2, BRI, BSK, and CYCD3) in BR synthesis and signal pathway were higher, while the inhibited tillering genes (CYP734A1, BZR, and BKI) were lower in Guyou 676 with higher regeneration rates. In conclusion, the starch content in the stem at the lower node could be used as the screening index of strong regeneration ability cultivar, and also the relative expression levels of BR pathway related genes could be used as supplementary indexes.
Keywords:
PDF (964KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
黄素华, 林席跃, 雷正平, 丁在松, 赵明. 强再生力水稻品种碳氮营养与激素生理特征研究. 作物学报, 2021, 47(11): 2278-2289 DOI:10.3724/SP.J.1006.2021.02070
HUANG Su-Hua, LIN Xi-Yue, LEI Zheng-Ping, DING Zai-Song, ZHAO Ming.
再生稻是利用头季收割后留桩的腋芽萌发成穗, 进行二季收获的水稻种植方式[1], 具有一种两收, 轻简化和资源高效利用的特点。近年来, 随着社会发展和城镇化, 农村劳动力向城市转移, 农业劳力短缺, 南方稻作区出现“双改单”甚或抛荒的现象; 再生稻成为了一种南方稻区缓解劳动力紧张、提高种粮效益的重要稻作制度[2]。然而, 由于缺乏专门的再生稻品种, 导致再生季产量低且不稳定[3]。其主要原因在于品种生态适应性不强导致结实率低下和较低的再生力所导致的亩穗数不足。因此, 培育和筛选强再生力的水稻品种是提高再生稻再生季产量、发挥再生稻作用的关键技术之一。
早期的研究表明, 强再生力品种头季稻的农艺性状主要表现为分蘖力强、有效穗多、穗粒数偏少、齐穗期叶粒比大的特征[4,5]。任天举等[6]对229个水稻杂交组合再生力与农艺性状的通径分析指出成熟期单茎的茎鞘干重对再生力的直接效应最大或接近最大, 可将茎鞘干重作为衡量品种再生力的重要指标[7,8]。进一步的研究发现头季稻收获后稻桩的干物质量与再生力和再生季产量具有显著的相关性[9]。通过去除部分叶片和/或小穗对头季稻进行源库调节的研究也表明其对再生季再生力的影响也与稻桩的干物质及非结构性碳水化合物(non-structural carbohydrates, NSC)含量的改变有关[10]。栽培技术调控的研究表明适时施用促芽肥是提高再生稻再生力的有效技术措施[11,12], 头季收获后使用促苗肥并进行土壤干湿交替的水分管理可以通过促进再生季根系生长和腋芽萌发提高再生力[13]。徐富贤等[1]认为其机制在于齐穗后施用氮肥延缓了叶片衰老, 提高了叶片光合物质生产, 从而增加了母茎茎鞘的干物质积累。可见, 茎鞘干物质很可能是其中储藏的养分物质对再生芽的发育起着重要作用。然而, 对再生稻头季收获时的碳、氮养分及其在不同器官(叶、茎和鞘), 不同节位的分布特征尚缺乏深入分析。另外, 近年来研究表明油菜素内酯(brassinosteroids, BRs)和独脚金内酯(strigolactones, SLs)两种激素信号分别在促进和抑制水稻分蘖发生中起着重要作用[14,15], 而在强再生力水稻品种再生芽的萌发过程中, 这2种激素的合成和信号转导途径是否也参与调控还未见报道。
本研究利用江西崇义县建立的适于全程机械化的再生稻品种筛选平台, 分析不同再生率水稻品种头季收获时不同节位(高、低)、不同器官(叶、茎和鞘)的NSC和氮素含量特点, 再生腋芽中BR和SL合成和信号通路关键基因的表达特点, 为揭示再生稻再生腋芽萌发的生理及分子机制奠定基础, 并为再生稻品种选育和高产栽培调控技术提供理论依据。
1 材料与方法
1.1 试验地概况
试验于2019年3月至11月在江西省崇义县全程机械化再生稻品种筛选平台(25°27′32.46″N, 114°20′12.92″E)进行, 试验基地属中亚热带季风气候, 地形为典型低山丘陵, 年均气温19.1℃, ≥10℃积温6480℃, 年均降雨量1645 mm, 无霜期约为289 d, 年日照数1551 h。试验地为沙壤土, 土壤pH 5.23, 有机质27.07 g kg-1, 全氮1.56 g kg-1, 全磷0.66 g kg-1, 全钾19.21 g kg-1, 碱解氮134.35 mg kg-1, 速效磷17.02 mg kg-1, 速效钾75.50 mg kg-1。1.2 供试品种与田间管理
试验材料为进入筛选平台的可用于江西南部水稻生产的品种13个(表1)。试验采用随机区组设计, 小区面积为15.0 m2, 3次重复。头季于4月1日播种, 4月18日移栽, 8月8日至23日收割, 留桩25 cm。再生季于10月25日至11月4日收割。头季稻每公顷施纯N 195 kg、P2O5 156 kg、K2O 195 kg, 氮肥作为基肥、分蘖肥(分蘖中期)和穂肥(孕穗初期)分3次平均分配, 磷肥一次性作为基肥施入, 钾肥分基肥和穂肥2次平均施用; 再生季每公顷施纯氮195 kg、P2O5 45 kg、K2O 120 kg, 1/2的氮和全部磷、钾肥在头季稻开花后15~20 d施用, 另外50%的氮在头季稻收获后3 d施用。田间水分管理遵循“两次晒田, 湿润管理”原则, 重点防控纹枯病、稻瘟病、稻飞虱、螟虫等病虫害。Table 1
表1
表1试验材料名称、类型及产量特性
Table 1
| 品种类型 Cultivar type | 品种名称 Cultivar name | 品种来源 Variety source |
|---|---|---|
| 籼型三系杂交稻 Three-line indica hybrid rice | 谷优676 Guyou 676 (GY676) | 谷丰A×福恢676 Gufeng A×Fuhui 676 |
| 赣优7076 Ganyou 7076 (GY7076) | 赣香A×福恢7076 Ganxiang A×Fuhui 7076 | |
| 泸优明占 Luyoumingzhan (LYMZ) | 泸香078A×华占 Luxiang 078A×Huazhan | |
| 籼型两系杂交稻 Two-line indica hybrid rice | 晶两优534 Jingliangyou 534 (JLY534) | 晶4155S×R534 Jing 4155S×R534 |
| 晶两优华占 Jingliangyouhuazhan (JLYHZ) | 晶4155S×华占 Jing 4155S×Huazhan | |
| 智两优5336 Zhiliangyou 5336 (ZLY5336) | 智农S×闽恢5336 Zhinong S×Minhui 5336 | |
| 晶两优粤农丝苗 Jingliangyouyuenongsimiao (JLYSM) | 4155S×R1212 | |
| 隆两优华占 Longliangyouhuazhan (LLYHZ) | 隆科638S×华占 Longke 638S×Huazhan | |
| 隆两优3463 Longliangyou 3463 (LLY3463) | 隆科638S×R3463 Longke 638S×R3463 | |
| 晶两优1377 Jingliangyou 1377 (JLY1377) | 晶4155S×R1377 Jing 4155S×R1377 | |
| 徽两优丝苗 Huiliangyousimiao (HLYSM) | 1892S×五山丝苗 1892S×Wushansimiao | |
| 深两优7011 Shenliangyou 7011 (SLY7011) | 深08S×福恢7011 Shen 08S×Fuhui 7011 | |
| 籼粳三系杂交稻 Three-line indica-japonica hybrid rice | 甬优4949 Yongyou 4949 (YY4949) | 甬粳49A×F9249 Yongjing 49A×F9249 |
新窗口打开|下载CSV
1.3 测定项目及方法
1.3.1 取样 于头季稻收割前2 d取样, 选取有效穗数与群体平均数一样或相近的6穴稻株, 从每穴稻株中各选取3个生长状态一致的单茎合并为1个样本。再生季萌发的腋芽一般位于倒2节以下节位, 因此将样品分为上部2节位和下部节位2个部分。将样品分解为上部2节位叶片, 上部2节位茎秆、上部2节位叶鞘、下部节位叶片、下部节位茎秆和下部节位叶鞘6个部分, 105℃杀青1 h后, 80℃继续烘干至恒重, 粉碎后过40目筛子, 用于可溶性糖、淀粉和全氮含量测定。另取剑叶, 下部节位腋芽液氮速冻, -80℃保存, 用于RNA提取和基因表达分析。1.3.2 产量和再生力 头季稻和再生季收获时, 每个小区取2 m2, 调查有效穗数, 粒重、含水量, 并换算成每公顷产量(按含水量13%计)。再生力为再生季和头季的有效穗数比值。
1.3.3 NSC含量测定 取1.3.1准备的烘干样品约200 mg, 加入8 mL 80%的乙醇, 60℃水浴20 min, 离心后上清倒入50 mL容量瓶, 重复3次, 定容后用于可溶性糖的测定。剩余的残渣加入10 mL 3mol L-1盐酸, 沸水浴40 min后, 冷却加入10 mL 3 mol L-1的NaOH中和, 冷却后定容至50 mL, 取部分离心, 上清用于淀粉含量的测定。可溶性糖和淀粉样品稀释合适的倍数后, 用蒽酮比色法在620 nm测试吸光度(Evolution 201紫外/可见分光光度计, Thermo), 以葡萄糖作标准曲线计算样品中可溶性糖含量, 淀粉的含量计算时乘以0.9的转换系数。
1.3.4 全氮含量测定 利用VELP-UDK169型凯氏定氮仪测定样品的氮素含量。
1.3.5 基因表达水平检测 选用BR和SL生物合成、代谢和信号转导途径上的关键基因19个, 其功能和所用的引物序列见表2, 内参使用GAPDH基因, 所有引物均由北京华大基因研究中心合成, 引物设计来自qPCR引物数据库[16]。采用TRIzol法提取头季稻收获前2 d下部节位腋芽和叶片样品的总RNA, 按照RevertAid RT Kit (Thermo Scientific)进行cDNA单链合成, 稀释10倍后作为qRT-PCR模板; 采用ABI Quant Studio 6荧光定量PCR仪(美国)进行qRT-PCR检测, 并按照KOD SYBR qPCR Mix (Toyobo)方法配置反应体系, 采用2-ΔΔCt法计算基因相对表达量。
Table 2
表2
表2基因表达分析的引物
Table 2
| 基因功能 Gene function | 基因 Gene | 基因座位 Locus | 上游引物 Forward primer (5′-3′) | 下游引物 Reverse primer (5′-3′) |
|---|---|---|---|---|
| 内参References | GAPDH | Os02g0601300 | CATGTTCAAGTATGACACCGTC | CACCAGTAGACTCAACAACGTA |
| BR合成 BR synthesis | CYP90B | Os03g0227700 | GAAGATCCTGCCGGTGTTAG | TACTCTTCCATCTCCAGGGATT |
| CYP90A | Os12g0139300 | AGCCTCATCAATCTCACTCATT | GTCTTGTACGCCGAGATGAG | |
| D2 | Os01g0197100 | CCAACTGGAAGAGGAGAACATA | ACATGTAGTCTGTCCATTGCAA | |
| CYP85A2 | Os03g0602300 | TGGAGAAGAACATGGAATCACA | GGAATGTTGCAATTTCTACGGT | |
| CYP734A1-1 | Os06g0600400 | GCTAGCTAGGAAAAGACAGGAA | GTACCACTAGTCTGTTAGCGAG | |
| CYP 734A1-2 | Os01g0388000 | ACCACCACTGGAAGAAAATCTA | CGTCTTGTTCTTGGTTGTTGTT | |
| BR信号转导 BR signal transduction | BRI | Os01g0718300 | GATGGCAATGTTCAAGGAGATC | GAATGACTGTTGTTTCTACGGG |
| BSK | Os10g0571300 | CTTTTGAGTGGGAAGCACATAC | TTAGCATACTGCCCTTCTAAGG | |
| BKI | Os08g0474500 | GTCGTCCTTGTCCAATAATCG | CCGGAGATGATGAACGAGTG | |
| BZR | Os07g0580500 | ATTTGGGCGATTTCATTCTAGC | CGTGAATAAAATCAGCCGTGAT | |
| CYCD3 | Os09g0111100 | AGGGTTCAGTCCAAGAAAAAGA | GACAAAACAGCTTCTTCCTCAC | |
| SLs合成 SLs synthesis | D10 | Os04g0550600 | TGCAAAGAAGATAGGGACAGAG | GGAATCCCATTGGAAAAGTGAG |
| D17 | Os01g0746400 | CTGTACAAGTTCGAGTGGCA | GTTGATGAAGTGGAACGTCAC | |
| D27 | Os11g0587000 | AGAAGCTTCTGGGCTAAAGAAT | ACCTTGATCATTGTGAGGATGT | |
| SLs信号转导 SLs signal transduction | D3 | Os06g0154200 | ATCTTTCACTATGGGAGCGATT | CATGGATGAAAAGCTTCCTGAG |
| D14 | Os03g0203200 | AGAAAGAGAGAGAAGAAGCGAG | CGCGCTCCCCTTTTATATACTA | |
| D53 | Os11g0104300 | CTTCCTCTCCAAATTCCCCTT | AGAGAAGAGAAAAGGCTTGACC | |
| SPL14 | Os08g0509600 | GATGGATTGGTCTCTGTAGAGG | TTGAACACAAAATAAGGGCAGG | |
| TB1 | Os03g0706500 | CAATCTTGTGAGCACCGAATTG | GTGTGTGGATGGATGATCACAT |
新窗口打开|下载CSV
1.4 数据处理分析
用Microsoft Excel 2013软件进行数据整理、计算并进行方差分析和差异显著性分析, 用SigmaPlot 10.0作图。2 结果与分析
2.1 不同水稻品种的再生率
13个水稻品种的再生力存在着较大的差异(表3), 其变化范围为1.26~2.38。其中谷优676最高, 泸优明占再生力最低。13个品种头季产量变化范围为7.65~ 10.13 t hm-2, 再生季产量范围为4.05~6.57 t hm-2, 品种间头季、再生季和周年产量均存在显著差异(表3)。然而, 13个品种的再生力与再生季、头季及周年产量之间均无显著的相关性, 决定系数R2分别仅为0.0383、0.0131和0.0364 (图1)。Table 3
表3
表3不同品种的生育期、产量和再生力
Table 3
| 品种 Cultivar | 头季生育期 Main crop duration (d) | 再生季生育期 Ratoon crop duration (d) | 总生育期 Whole crop duration (d) | 头季产量 Main yield (t hm-2) | 再生季产量 Ratoon yield (t hm-2) | 年产量 Annual yield (t hm-2) | 再生力 Regeneration rate |
|---|---|---|---|---|---|---|---|
| 谷优676 GY676 | 136 | 71 | 207 | 9.42 b | 5.25 c | 14.37 cd | 2.38 a |
| 晶两优534 JLY534 | 145 | 72 | 217 | 9.48 b | 6.17 b | 15.65 a | 1.99 b |
| 晶两优华占 JLYHZ | 140 | 72 | 212 | 8.98 c | 5.03 c | 14.01 cd | 1.97 b |
| 智两优5336 ZLY5336 | 145 | 72 | 217 | 7.65 e | 4.45 d | 12.10 f | 1.94 b |
| 晶两优粤农丝苗 JLYSM | 145 | 72 | 217 | 8.80 c | 4.58 d | 13.38 e | 1.86 b |
| 隆两优华占 LLYHZ | 142 | 72 | 214 | 10.13 a | 4.53 d | 14.66 b | 1.85 b |
| 隆两优3463 LLY3463 | 145 | 72 | 217 | 9.90 a | 4.68 d | 14.60 b | 1.71 c |
| 晶两优1377 JLY1377 | 147 | 74 | 221 | 9.83 a | 5.10 c | 14.93 b | 1.68 c |
| 深两优7011 SLY7011 | 147 | 74 | 221 | 8.40 d | 4.58 d | 12.98 e | 1.60 c |
| 甬优4949 YY4949 | 134 | 77 | 210 | 9.08 c | 6.57 a | 15.65 a | 1.57 d |
| 徽两优丝苗 HLYSM | 145 | 72 | 217 | 8.18 d | 4.05 e | 12.23 f | 1.55 d |
| 赣优7076 GY7076 | 136 | 71 | 207 | 8.94 c | 4.73 d | 13.67 c | 1.43 d |
| 泸优明占 LYMZ | 135 | 71 | 206 | 9.20 bc | 4.65 d | 13.85 c | 1.26 e |
新窗口打开|下载CSV
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图113个杂交稻品种再生力与再生季、头季及周年产量的相关性
Fig. 1Relationships between regeneration rate and ratoon season, main season, and annual yields for 13 hybrid rice cultivars
2.2 头季稻收获前不同节位叶片、叶鞘和茎秆的NSC含量
图2显示了13个水稻品种头季收获前植株上、下不同节位叶片、叶鞘和茎秆的可溶性糖(soluble sugar, SS), 淀粉和NSC含量。叶片中SS的变化范围分别为13.9~32.3 mg g-1和12.0~32.9 mg g-1, 淀粉含量的变化范围分别为70.9~101.5 mg g-1和75.3~106.8 mg g-1, NSC含量变化范围为87.6~129.1 mg g-1和90.8~129.9 mg g-1; 叶鞘中SS含量变化范围分别为10.6~38.5 mg g-1和13.8~111.4 mg g-1, 淀粉含量的变化范围分别为50.7~154.7 mg g-1和87.1~203.8 mg g-1, NSC含量变化范围为95.8~193.2 mg g-1和105.9~315.2 mg g-1; 茎秆中SS含量变化范围分别为54.8~168.6 mg g-1和41.4~213.0 mg g-1, 淀粉含量的变化范围分别为75.9~173.5 mg g-1和79.3~394.2 mg g-1, NSC含量变化范围为109.5~304.7 mg g-1和125.3~565.9 mg g-1。方差分析表明品种间以及上下节位间的SS、淀粉和NSC含量均存在极显著的差异(表4)。各个组分在上、下部节位叶片之间的差异明显小于叶鞘和茎秆。图2
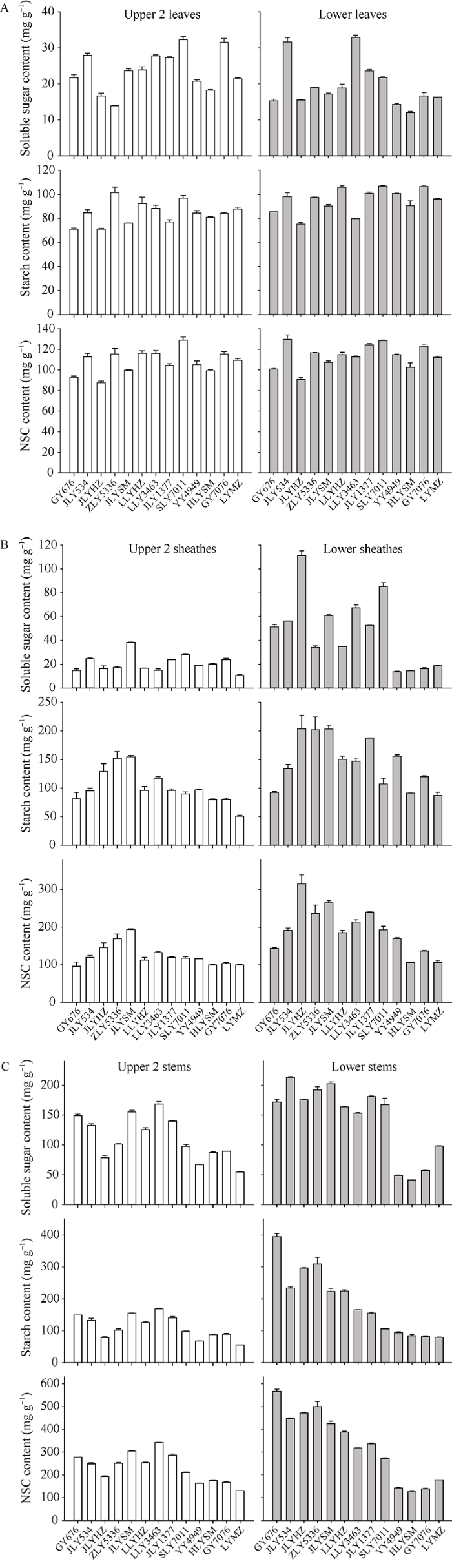 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2头季收获前水稻植株上部与下部节位叶片(A)、叶鞘(B)和茎秆(C)的可溶性糖、淀粉和非结构性碳水化合物含量
缩略词同
Fig. 2Soluble sugar, starch, and NSC content of the upper and lower leaves (A), sheaths (B), and stems (C) before main crop harvest in rice
Abbreviations are the same as those given in
Table 4
表4
表4不同水稻品种不同节位间可溶性糖、淀粉和NSC含量方差分析
Table 4
| 器官 Organ | 差异源 Source of variation | 自由度 df | 可溶性糖SS | 淀粉Starch | 非结构性碳水化合物NSC | |||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||
| 叶片Leaf | 节位 Node site | 1 | 2113.78 | 8.66E-44 | 1727.37 | 1.44E-41 | 594.95 | 3.92E-30 |
| 品种 Cultivar | 12 | 854.64 | 3.12E-55 | 387.53 | 2.20E-46 | 497.91 | 3.51E-49 | |
| 叶鞘Sheath | 节位 Node site | 1 | 12,932.43 | 5.13E-64 | 832.86 | 1.13E-33 | 2473.73 | 1.59E-45 |
| 品种 Cultivar | 12 | 1391.03 | 1.05E-60 | 173.85 | 1.63E-37 | 308.29 | 7.72E-44 | |
| 茎秆Stem | 节位 Node site | 1 | 3384.81 | 5.26E-49 | 1396.50 | 3.03E-39 | 3923.92 | 1.19E-50 |
| 品种 Cultivar | 12 | 2564.40 | 1.35E-67 | 1476.25 | 2.24E-61 | 3189.50 | 4.71E-70 | |
新窗口打开|下载CSV
图3-A、B和C分别显示了13个品种上部和下部节位的叶片, 叶鞘和茎秆中SS、淀粉和NSC含量的分布特征。可见上部和下部节位叶片可溶性糖、淀粉和NSC含量在13个品种间变异幅度比较一致, 没有出现较大的偏离值。而在叶鞘和茎秆中, 下部节位的箱体高度明显高于上部节位, 且出现较大的偏离值。表明一些品种在下部节位的叶鞘和茎秆中积累了更多的NSC。
图3
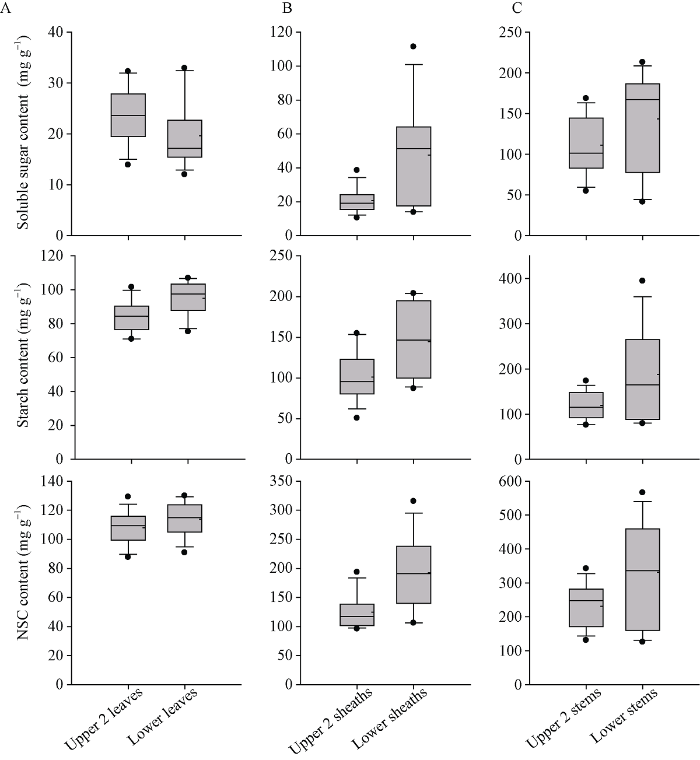 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3头季收获前水稻植株上部与下部节位叶片(A)、叶鞘(B)和茎秆(C)的可溶性糖、淀粉和非结构性碳水化合物含量的统计特征
箱图内的实线和虚线分别表示中位值和平均值; 箱图的上下边表示所有数据的上、下四分位数, 底部和顶部条形表示第5和第95个百分位, 底部和顶部圆点分别表示异常值。
Fig. 3Descriptive statistics of soluble sugar, starch, and NSC content of the upper and lower leaves (A), sheaths (B), and stems (C) before main crop harvest in rice
The solid and dashed lines within the boxes indicate medians and means, respectively; the upper and lower box edges of the boxes represent the 25th and 75th percentiles of all the data, the bottom and top bars represent the 5th and 95th percentiles, and the bottom and top dots represent the outliers, respectively.
进一步将不同节位不同器官的NSC含量分别与品种的再生率进行相关性分析表明, 下部茎秆的NSC含量与再生率高度相关(表5)。特别是下部节位茎秆的淀粉含量和NSC含量与品种的再生率决定系数分别达到0.9000和0.8303, 统计显著性P值远小于0.001。试验表明水稻下部节位茎秆的储藏物质淀粉对于腋芽的萌发可能具有极为重要的作用。
Table 5
表5
表5再生力与不同部位叶片、叶鞘和茎秆的SS、淀粉和NSC含量的相关性
Table 5
| 器官 Organ | 部位 Site | 可溶性糖 SS | 淀粉 Starch | 非结构性碳水化合物 NSC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | P | R2 | F | P | R2 | F | P | ||
| 叶片Leaf | 上部 Upper | 0.0684 | 0.8077 | 0.3881 | 0.1077 | 1.3276 | 0.2737 | 0.1593 | 2.0851 | 0.1766 |
| 下部 Lower | 0.0150 | 0.1678 | 0.6900 | 0.1692 | 2.2408 | 0.1625 | 0.1029 | 1.2623 | 0.2851 | |
| 叶鞘Sheath | 上部 Upper | 0.0002 | 0.0026 | 0.9603 | 0.1917 | 2.6093 | 0.1345 | 0.0706 | 0.8362 | 0.3801 |
| 下部 Lower | 0.2005 | 2.7586 | 0.1249 | 0.0784 | 0.9356 | 0.3542 | 0.1709 | 2.2681 | 0.1602 | |
| 茎秆Stem | 上部 Upper | 0.3241 | 5.2743 | 0.0423 | 0.2771 | 4.2160 | 0.0646 | 0.3317 | 5.4589 | 0.0394 |
| 下部 Lower | 0.4442* | 8.7913 | 0.0129 | 0.9000** | 98.9956 | 0.0000 | 0.8303** | 53.8256 | 0.0000 | |
新窗口打开|下载CSV
2.3 头季稻收获前不同节位的叶片、叶鞘和茎秆的全氮含量
图4显示了13个水稻品种头季收获前植株上、下不同节位叶片、叶鞘和茎秆的全氮含量。品种间以及上下节位间的全氮含量除了上下部茎秆之间差异不显著(P=0.257)外, 均存在极显著的差异(P值均远小于0.001)。上、下部节位叶片, 叶鞘和茎秆中全氮的变化范围分别为1.842%~3.508%和11.171%~2.757%, 0.601%~1.051%和0.452%~1.028%, 以及0.379%~0.830%和0.260%~0.947%。13个品种上部叶片的氮素含量均显著大于下部叶片的氮素含量, 而在叶鞘和茎秆中, 则有部分品种表现出植株下部组织的氮素含量更高。例如, 再生率最高的品种谷优676茎秆和叶鞘中全氮含量植株下部比上部分别高17.22%和47.18%。相关性分析表明各部位氮素含量与再生率的相关性均不显著(R2范围为0.0056~0.1864)。图4
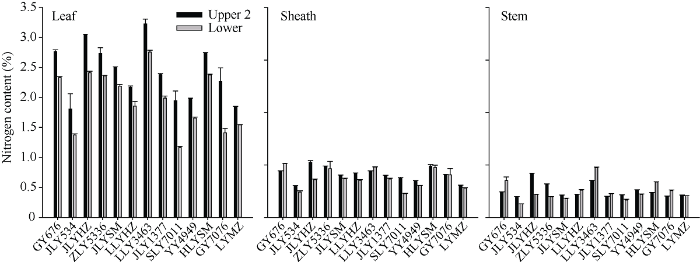 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4头季收获前水稻植株上部与下部节位叶片(左)、叶鞘(中)和茎秆(右)的全氮含量
缩略词同
Fig. 4Total nitrogen content of the upper and lower leaves (left), sheaths (mid), and stems (right) before main crop harvest in rice
Abbreviations are the same as those given in
2.4 BR合成及信号途径关键基因在头季稻收获前再生腋芽中的表达
选择3个再生率高的品种谷优676, 晶两优534及智两优5336和1个再生率较低但再生季产量高的品种甬优4949, 对其腋芽中BR合成、代谢及信号转导途径的关键基因表达进行了qRT-PCR分析。如图5所示, BR合成上游基因CYP90B在再生率高的3个品种中表达量相对低于甬优4949, 而在BR合成中、下游的CYP90A、D2、CYP85A2等基因的表达量则显著高于甬优4949; 参与BR羟基化代谢的CYP734A1基因则是再生力强的品种普遍低于甬优4949。可见强再生力品种具有较强的BR合成能力, 而且BR代谢的速率也慢, 这样可能维持细胞内较高的BR含量。图5
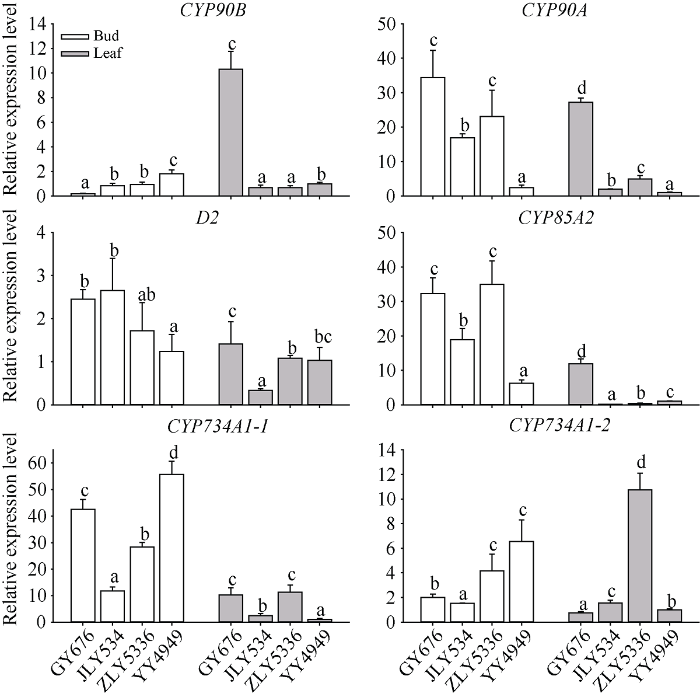 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5头季收获前水稻腋芽和叶片BR合成与代谢关键基因的相对表达量
缩略词同
Fig. 5Relative expression levels of key genes in BR synthesis and metabolism in rice buds and leaves before main crop harvest
Abbreviations are the same as those given in
对于BR信号转导过程中的关键基因表达分析发现(图6), 3个高再生率品种腋芽中BR受体BRI基因的表达量均显著高于甬优4949; 同样, 信号通路中的BSK表达也具有相同的特征。而抑制BR与受体BRI结合的BKI基因的表达情况则正好相反, 反馈抑制BR合成途径基因BZR基因在甬优4949中表达量也显著高于其他3个高再生率品种。BR信号通路末端调控细胞分裂的转录因子CYCD3的表达量在3个高再生率品种中也显著高于甬优4949。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6头季收获前水稻腋芽和叶片BRs信号转导途径关键基因的相对表达量
缩略词同
Fig. 6Relative expression levels of key genes in BR signalling pathway in rice buds and leaves before main crop harvest
Abbreviations are the same as those given in
叶片中, BR合成、代谢和信号转导途径的基因表达量一般都低于腋芽中。而且其表达量与再生率没有相关性。
2.5 SL合成及信号转导途径关键基因在头季稻收获前再生腋芽中的表达
如图7所示, 水稻中参与SL合成的D27、D17和D10中, 合成途径上游的基因D27在甬优4949腋芽中具有极高的表达量, 是其他3个高再生率品种中表达量的30倍以上, 而合成途径中间和下游的D17和D10的表达量与再生率没有明显的相关特征。SL信号转导途径中的D3、D14、D53、SPL14和TB1基因, 除D14在4个品种中的表达特征比较特异外, 其他4个基因在晶两优534腋芽中的表达明显高于另外3个品种。而在叶片中, TB1和SPL14表达量极低, 几乎检测不到; D3、D14和D53的表达在再生率高的3个品种中高于甬优4949。图7
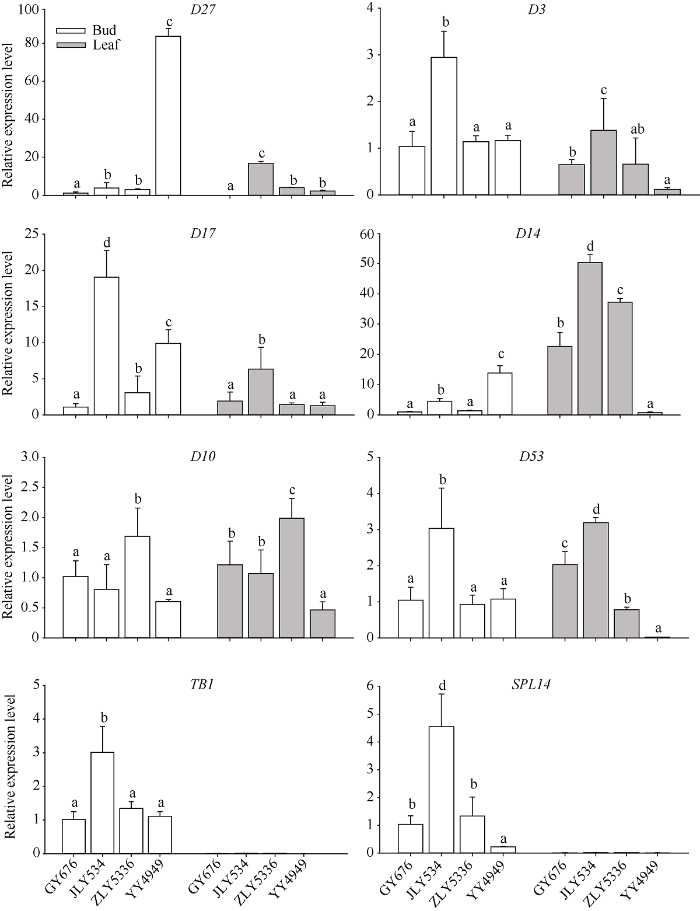 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7头季收获前水稻腋芽和叶片SL合成和信号转导途径关键基因的相对表达量
缩略词同
Fig. 7Relative expression levels of key genes in SL synthesis and signalling pathway in rice buds and leaves before main crop harvest
Abbreviations are the same as those given in
3 讨论
再生季产量不高和不稳定, 制约着再生稻大面积推广和应用, 增大再生季亩穗数是提高和稳定再生季产量的主导栽培措施。再生力与再生季产量存在显著的相关性[17], 再生力是再生稻品种选育和筛选的关键指标之一[1-2,18]。而本研究中, 13个品种的再生力与再生季及全年产量均无显著的相关性。再生季产量不仅取决于穗数,还决定于穗粒数。如图1所示, 本研究中两个偏离直线回归较大的品种为晶两优534和甬优4949, 它们的再生季产量为所测试品种中最大, 均超过6 t hm-2, 而它们的再生力则相差极大, 分别为1.99和1.57; 穗粒数分别为92.6和123.3, 甬优4949再生季较多的穗粒数弥补了其再生率的不足。何爱斌等[19]的研究中也表明籼粳杂交稻甬优4949的再生率为1.45, 再生季穗粒数为120粒, 其穗粒数远高于另一籼型杂交稻两优6326。可见, 大穗型和多穗型品种类型的差异会对再生率和再生季产量相关性分析产生影响, 将再生力作为关键筛选指标可能仅适用于多穗型的品种。早期的再生稻生产注重高留桩, 以快速建立再生季高产群体, 保障再生季能安全齐穗; 所选择的品种高位腋芽萌发快[19,20]; 然而, 在当前的机械化生产过程中, 高位腋芽容易受到碾压而难以恢复, 导致再生季产量受到影响。因此, 低位节腋芽萌发快的品种成为机械化再生稻生产的关键之一。本研究利用的江西南部再生稻筛选平台, 也是通过低留桩进行品种再生力测试。采用低留桩方式, 基部节位萌发的芽比例增加, 低节位芽发育时期长于高位芽, 穗粒数明细增多, 这也可能是导致所测试品种的再生力与再生季产量相关性较弱的原因。
研究表明强再生力品种头季具有更高的茎鞘的干物重[7], 近年来进一步研究证明收获后稻桩的干物重[9]以及稻桩的NSC[10]含量均与再生力显著相关。本试验对13个品种的上部2节位和下部其他节位的叶片、叶鞘和茎秆的NSC及氮素含量进行了详细地分析, 结果表明水稻下部节位茎秆的淀粉和NSC含量与再生力具有极显著的相关性, 而与上部节位茎秆的淀粉和NSC含量没有显著的相关性, 而且茎秆主要储藏物质淀粉含量与再生率相关性最大。同时, 上下不同节位的叶鞘及叶片中的淀粉和NSC含量也与再生力没有相关性。可见, 直接为腋芽萌发提供物质和能量的下部节位的茎秆对水稻腋芽生长具有重要意义, 可以将水稻下部节位茎秆的淀粉含量作为强再生力品种筛选的关键指标。再生力强的品种在下部节位茎秆淀粉含量高, 而上部节位茎秆含量并不突出, 反而一些再生力居中的品种如隆两优3446上部节位茎秆的含量较多(图2-C), 暗示其可能高位节腋芽萌发快。下一步需要细化研究下部不同节位在抽穗后不同阶段的淀粉含量与再生芽萌发的关系, 为量化茎秆中淀粉含量作为再生率的筛选指标提供依据。本研究中不同器官的氮素含量与再生力均无相关性, 然而头季齐穗后10~20 d增施氮肥是再生季高产的重要调控措施[11-12,21], 表明氮素本身不直接促进再生芽的萌发, 它可能通过延缓叶片衰老, 维持头季稻花后光合物质生产能力[1], 促进多余的光合产物以淀粉形式储存在茎秆中。
BR和SL参与植株分枝的调控[22,23]。在水稻中, 也陆续鉴定到一批参与分蘖形成的基因, 相当一部分属于BR和SL生物合成和信号转导途径中的关键基因[24,25,26,27,28,29]。本研究对3个强再生力品种和一个再生力一般的品种的相关基因表达进行了分析, 也证明了这些基因同样能调控水稻再生腋芽的发育。再生力强的3个品种中, 促进分蘖的激素BR的合成和信号转导基因表达量显著高于再生力低的品种甬优4949, 而抑制分蘖的激素SL的合成和信号途径的基因则表达量低。Xu等[30]对汕优63腋芽萌发不同阶段的蛋白质组分析也发现了参与BR合成的4个蛋白含量在腋芽萌发过程中含量逐渐增加。我们在头季收获前15 d对晶两优534喷施芸薹素, 发现收割后4 d稻桩茎秆的可溶性糖和淀粉含量比对照均增加50%以上, 其再生率和再生季产量也显著提高(未发表结果), 表明激素信号可能调控头季稻的碳水化合物代谢及其流向。本研究中再生力强的品种中BR合成和信号转导基因表达水平高, 下部节位茎秆淀粉积累量也高, 两者之间是否存在相互调控关系尚需要进一步的深入研究。同样, 不同节位的萌发腋芽BR和SL合成和信号转导相关基因的表达水平是否存在差异也需要作进一步研究。
近些年, 谷优676在福建尤溪屡破年最高产量, 最高年次产量已达19.5 t hm-2, 它具有穗大粒多, 再生力强的特点[31]。本研究发现谷优676的下部节位茎秆的淀粉和NSC含量显著高于其他品种, 同时, 促进腋芽发育的BR合成和信号转导途径基因表达量高, 而抑制腋芽萌发的SL合成和信号转导途径基因表达量低。这为我们利用该品种解析再生芽萌发机制提供了良好的材料基础。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 4]
[本文引用: 4]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Brassinosteroids (BRs) are a group of steroid phytohormones with wide-ranging biological activity. Genetic, genomic and proteomic studies have greatly advanced our understanding of BR signaling in Arabidopsis and revealed a connected signal transduction pathway from the cell surface receptor kinase BRASSINOSTEROID-INSENSITIVE1 (BRI1) and BRI1-ASSOCIATED RECEPTOR KINASE 1 (BAK1) to the BRASSINAZOLE-RESISTANT1 (BZR1) family of transcription factors and their targets mediating physiological functions. However, compared with the dicot model plant Arabidopsis, much less is known about BR signaling in rice, which is a monocot. In this review, we provide an update on the progress made by BR studies in rice and discuss how BR regulates various important agronomic traits to determine rice grain yield. Specifically, we discuss the function of novel components including LEAF AND TILLER ANGLE INCREASED CONTROLLER (LIC), DWARF and LOW-TILLERING (DLT), DWARF1 (D1) and TAIHU DWARF1 (TUD1) in rice BR signaling, and provide a rice BR-signaling pathway model that involves a BRI1-dependent pathway as well as a G-protein α subunit-mediated signaling pathway. The recent significant advances in our understanding of BR-mediated molecular mechanisms underlying agronomic traits will be of great help for rice molecular breeding.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

We characterized a rice dwarf mutant, ebisu dwarf (d2). It showed the pleiotropic abnormal phenotype similar to that of the rice brassinosteroid (BR)-insensitive mutant, d61. The dwarf phenotype of d2 was rescued by exogenous brassinolide treatment. The accumulation profile of BR intermediates in the d2 mutants confirmed that these plants are deficient in late BR biosynthesis. We cloned the D2 gene by map-based cloning. The D2 gene encoded a novel cytochrome P450 classified in CYP90D that is highly similar to the reported BR synthesis enzymes. Introduction of the wild D2 gene into d2-1 rescued the abnormal phenotype of the mutants. In feeding experiments, 3-dehydro-6-deoxoteasterone, 3-dehydroteasterone, and brassinolide effectively caused the lamina joints of the d2 plants to bend, whereas more upstream compounds did not cause bending. Based on these results, we conclude that D2/CYP90D2 catalyzes the steps from 6-deoxoteasterone to 3-dehydro-6-deoxoteasterone and from teasterone to 3-dehydroteasterone in the late BR biosynthesis pathway.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
