 ,1, 姚方杰1, 龙黎1, 王昱琦1, 康厚扬1,2, 蒋云峰1,2, 李伟3, 邓梅1, 李豪1, 陈国跃
,1, 姚方杰1, 龙黎1, 王昱琦1, 康厚扬1,2, 蒋云峰1,2, 李伟3, 邓梅1, 李豪1, 陈国跃 ,1,2,*
,1,2,*Evaluation of resistance to stripe rust and molecular detection of resistance genes of 93 wheat landraces from the Qinghai-Tibet spring and winter wheat zones
ZHAO Xu-Yang ,1, YAO Fang-Jie1, LONG Li1, WANG Yu-Qi1, KANG Hou-Yang1,2, JIANG Yun-Feng1,2, LI Wei3, DENG Mei1, LI Hao1, CHEN Guo-Yue
,1, YAO Fang-Jie1, LONG Li1, WANG Yu-Qi1, KANG Hou-Yang1,2, JIANG Yun-Feng1,2, LI Wei3, DENG Mei1, LI Hao1, CHEN Guo-Yue ,1,2,*
,1,2,*通讯作者: *陈国跃, E-mail:gychen@sicau.edu.cn
收稿日期:2020-09-7接受日期:2021-03-20网络出版日期:2021-04-16
| 基金资助: |
Corresponding authors: *E-mail:gychen@sicau.edu.cn
Received:2020-09-7Accepted:2021-03-20Published online:2021-04-16
| Fund supported: |
作者简介 About authors
E-mail:zhaoxuyang1101@qq.com

摘要
为应对当前条锈菌强毒性小种对中国小麦生产带来的威胁, 本研究通过鉴定来自青藏春冬麦区的93份小麦地方种质对中国当前条锈菌流行小种或致病类群在苗期和成株期的抗性水平, 检测其可能携带的条锈病抗性基因, 为培育小麦抗条锈病新品种提供抗源。利用条锈菌流行小种条中32号(CYR32)和条中34号(CYR34)对93份来源于青藏春冬麦区小麦地方品种进行温室苗期抗性鉴定, 并于2015—2016、2017—2018和2018—2019年度在四川崇州和绵阳共4个田间环境下, 利用由条锈菌流行小种(CYR32、CYR33、CYR34)、水源致病类型(Su11-4、Su11-5)、贵农22致病类型(G22-14)组成的混合菌进行成株期抗性鉴定。同时利用Yr5、Yr10、Yr18、Yr24 (=Yr26)、Yr48、Yr65和Yr67共7个已知抗条锈病基因紧密连锁的侧翼分子标记或功能标记进行检测。抗性鉴定结果表明, 4份(占4.30%)种质对CYR32表现苗期抗性; 3份(占3.26%)对CYR34表现苗期抗性; 其中1份种质(白颖无芒小麦)对CYR32和CYR34均表现苗期抗性。10份种质(占10.75%)在4个田间环境中均表现成株期抗性。分子检测结果表明, 可能携带Yr18、Yr48和Yr65的种质分别有11份、40份和1份。其中, 7份可能同时携带Yr18+Yr48基因; 3份未检测出供试已知Yr基因, 推测其可能携带其他已知或未知条锈病抗性基因。上述研究结果表明, 青藏春冬麦区小麦地方种质对中国当前条锈菌流行小种或致病类群的抗性整体水平较低, 其携带抗性基因的多样性也较低; 建议对表现良好抗性且可能携带未知抗性基因的地方种质进行发掘并利用其加快育种。
关键词:
Abstract
Wheat stripe rust (yellow rust), caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most serious diseases in wheat. To address the threat of predominant Pst races to wheat production and screen resistance resources to breed new wheat cultivars in China, the resistance of 93 wheat landraces derived from Qinghai-Tibet spring and winter wheat zones to stripe rust were evaluated at seedling stage and adult plant stage, and the Yr genes that they might carry were detected. The resistance of 93 wheat landraces were evaluated at seedling stage in a greenhouse with two Pst races CYR32 and CYR34, and at adult plant stage under four field conditions with the mixture of Pst races (CYR32, CYR33, CYR34, Su11-4, Su11-5, and G22-14) in Chongzhou and Mianyang, Sichuan during 2015-2016, 2017-2018, and 2018-2019 cropping seasons, respectively. The panel of wheat landraces was detected with flanking markers closely linked to stripe rust resistance genes Yr5, Yr10, Yr18, Yr24 (=Yr26), Yr48, Yr65, and Yr67. Resistance evaluation indicated that four landraces (4.30%) were resistant to CYR32, three (3.26%) resistant to CYR34, and one was resistant to both CYR32 and CYR34 at seedling stage. Ten landraces displayed resistance to mixed races at adult plant stage under four field conditions. Molecular detection indicated that 11, 40, and 1 landrace might carry Yr18, Yr48, and Yr65, respectively. Seven landraces may carry both Yr18 and Yr48. In addition, no Yr genes were detected in the three resistant wheat landraces, indicating that these wheat landraces might carry other known or unknown stripe rust resistance genes. The wheat landraces derived from Qinghai-Tibet spring and winter wheat zones showed low levels of resistance to the current predominant Pst races in China, and they carried less resistance genes. These wheat landraces with good resistance and carrying known or unknown resistance genes should be valued to explore novel stripe rust resistance genes and accelerate their utilization in wheat breeding program.
Keywords:
PDF (1355KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵旭阳, 姚方杰, 龙黎, 王昱琦, 康厚扬, 蒋云峰, 李伟, 邓梅, 李豪, 陈国跃. 青藏春冬麦区93份小麦地方种质条锈病抗性评价及抗病基因分子鉴定[J]. 作物学报, 2021, 47(10): 2053-2063 DOI:10.3724/SP.J.1006.2021.01073
ZHAO Xu-Yang, YAO Fang-Jie, LONG Li, WANG Yu-Qi, KANG Hou-Yang, JIANG Yun-Feng, LI Wei, DENG Mei, LI Hao, CHEN Guo-Yue.
由条形柄锈菌小麦专化型(Puccinia striiformis West. f. sp. tritici Erikss., Pst)引起的小麦条锈病是世界小麦生产上最严重的叶部气传病害之一。中国是小麦条锈病最大的流行区, 也是小麦条锈病流行频率高、发生面积大、受危害最为严重的国家之一[1,2,3,4]。相比农业栽培管理、化学防治等小麦条锈病防治措施, 培育和推广抗病品种被认为是防治小麦条锈病更经济、更有效和对环境友好的举措[5,6]。目前小麦生产上使用的品种多为高抗或免疫的单基因抗病品种, 由于抗源单一使得条锈菌选择压力增加、变异频率加快, 造成所培育的抗病品种3~5年后便“丧失”抗性, 引起条锈病流行或大发生, 最终导致大幅减产。迄今, 中国先后鉴定并正式命名了34个条锈菌小种(CYR1~CYR34)及多种致病类群[7], 这些小种或致病类群的流行及发展导致在建国后全国范围内的8次小麦品种更替[8,9,10]。2009年从四川省郫县的条锈菌群体中分离的贵农22致病类型(G22-9)新菌系(avrYr10/14/26/CH42), 其对贵农22 (YrGn22)、川麦42 (YrCH42)、Moro (Yr10)和近等基因系NILS12 (Yr10)、NILS16 (Yr24)、NILS17 (Yr26)以及38个四川省小麦新品种均具有高致病性[11]。尔后, 随着新菌系G22-9的出现及扩展, 该菌系迅速发展成为优势小种[12]。鉴于此, 2016年该新菌系正式被命名为条中34号(CYR34)。目前, 该小种已成为中国小麦生产上毒性谱最宽、毒性最强的小种[8]。由于以条锈菌CYR34为代表的贵农22致病类群的出现和积累, 导致中国目前小麦育种和生产上广泛用作抗源的Yr10、Yr24 (=Yr26)基因和以川麦42、贵农系列品种、南农的92R系列为代表的重要抗病品种丧失其抗性, 这预示着又一次品种更替的开始。因此, 发掘并有效利用新抗源是控制小麦条锈病危害的重要基础性研究工作。
小麦在中国种植历史悠久, 分布极为广泛。但是由于各种植区在气候环境、耕作制度、品种种类、生产水平和栽培管理等方面存在差异, 因而形成了差异明显的自然种植区域, 据此划分为不同的小麦种植区域。金善宝[13]将中国麦小麦产区划分为10个麦区, 其中青藏春冬麦区属于中国春冬兼播麦区的亚区。该亚区位于中国西南, 包括西藏自治区全部地区、青海省除西宁市及海东地区以外的大部、甘肃省西南部的甘南州大部、四川省西部的甘孜州和阿坝州以及云南省西北部的迪庆州和怒江州部分县[14]。该麦区生态气候复杂, 以高原为主, 还有部分台地、湖盆、谷地, 是世界上种植小麦最高的地区。该麦区小麦种植面积常年在14.5万公顷左右, 是中国小麦面积较小的麦区, 其中春小麦种植面积为全部麦区面积的66%以上。由于该麦区地理环境复杂, 小麦耕作方式多样(既有春播又有冬播), 使得该麦区成为中国小麦条锈病重要的流行区, 危害严重[15,16]。
中国小麦地方品种是中国小麦的特有种质, 具有早熟性、多粒性、高度适应性和高亲和性等特点[17]。自20世纪80年代以来, 中国****利用条锈病流行小种或致病类群对中国不同地区的小麦地方种质进行了条锈病抗性鉴定, 分析了部分省区或麦区的小麦地方种质条锈病抗性特点, 筛选出一批优良抗性种质; 并结合已知条锈病抗性基因(Yr)紧密连锁分子标记进行检测, 初步掌握了中国小麦地方种质条锈病抗性基因的分布, 证实了中国小麦地方种质可能携带丰富的、尚未发掘的条锈病抗性基因[18,19,20,21,22,23,24]。自2009年CYR34被发现并逐年发展成为当前中国小麦生产上最主要的流行小种以来, 对中国青藏麦区小麦地方种质对CYR34等多小种的抗条锈性的研究, 鲜有报道。据此, 本研究利用当前小麦条锈菌流行小种CYR34和CYR32对93份来自青藏春冬麦区小麦地方种质进行室内苗期鉴定, 结合由CYR32、CYR33、CYR34小种、水源致病类型(Su11-4、Su11-5)、贵农22致病类型(G22-14)组成的混合菌进行多环境下成株期抗性鉴定, 以明确其条锈病抗性。同时, 利用已知抗条锈病性基因Yr5、Yr10、Yr18、Yr24 (=Yr26)、Yr48、Yr65和Yr67的侧翼分子标记进行检测, 分析青藏春冬麦区小麦地方种质所携带的抗条锈病基因, 旨在筛选可用于抗条锈病小麦育种的优良抗性种质, 为培育持久抗病小麦新品种提供抗源。
1 材料与方法
1.1 试验材料
供试的93份青藏春冬麦区小麦地方种质中81份来自西藏、8份来自青海、4份来自四川甘孜州, 均由四川农业大学小麦研究所收集保存(表1), 7份已知抗条锈病基因载体品种, 包括Yr5/6* Avocet S、Yr10/6* Avocet S、Yr18/6* Avocet S、Yr26/6* Avocet S、PI610750 (Yr48)、PI 480016 (Yr65)和C591 (Yr67)由美国华盛顿州立大学陈贤明教授提供。室内苗期条锈病抗性鉴定感病对照和用作诱发小麦品种铭贤169由甘肃农业科学院植物保护研究所贾秋珍研究员提供; 田间成株期条锈病抗性鉴定感病对照Avocet Susceptible (AvS)和诱发材料SY95-71由四川农业大学小麦研究所提供; 小麦条锈菌小种条中32号(CYR32)、条中34号(CYR34)及其由CYR32、CYR33、CYR34小种、水源致病类型(Su11-4、Su11-5)、贵农22致病类型(G22-14)按等比例组成的混合菌由甘肃省农业科学院植物保护研究所鉴定、繁存及提供。Table 1
表1
表193份青藏春冬麦区小麦地方品种条锈病抗性鉴定及其抗条锈病基因的分子检测结果
Table 1
| 种质 Landrace | 产地 Origin | 成株期 Adult plant stage | 苗期 Seedling stage | 抗性类型 Resistance type | 分子标记检测 Presence or absence of Yr gene based on molecular marker detection | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16CZ | 16MY | 18CZ | 19CZ | CYR32 | CYR34 | ||||||||||||||||||
| DS(%) | IT | DS(%) | IT | DS(%) | IT | IT | IT | IT | Yr18 | Yr48 | Yr65 | Yr67 | |||||||||||
| 长芒白粒麦Changmangbailimai | 西藏察雅Chaya, Tibet | 40 | 3 | 60 | 3 | 72 | 3 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 波密杂麦-5 Bomizamai-5 | 西藏波密Bomi, Tibet | 20 | 2 | 40 | 3 | 100 | 4 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 林芝杂小麦Linzhizaxiaomai | 西藏林芝Linzhi, Tibet | 80 | 4 | 80 | 3 | 80 | 3 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 无芒小麦Wumangxiaomai | 西藏昂仁Angren, Tibet | * | * | 100 | 4 | 100 | 4 | 4 | 3 | 4 | S | — | + | + | — | ||||||||
| 达孜杂小麦-3Dazizaxiaomai-3 | 西藏达孜Dazi, Tibet | 40 | 3 | 40 | 3 | 60 | 3 | 3 | 4 | 4 | S | — | + | — | — | ||||||||
| 贡吉卓Gongjizhuo | 西藏察隅Chayu, Tibet | 100 | 4 | 100 | 4 | 100 | 4 | 4 | 3 | 4 | S | — | + | — | — | ||||||||
| 翻身卓Fanshenzhuo | 西藏琼结Qiongjie, Tibet | 100 | 4 | 100 | 4 | 84 | 3 | 3 | 3 | 4 | S | — | + | — | — | ||||||||
| 苏卓卓玛Suzhuozhuoma | 西藏八宿Basu, Tibet | 20 | 2 | 20 | 3 | 72 | 3 | 1 | 3 | 4 | S | — | + | — | — | ||||||||
| 加查扎仁卓玛Jiachazharenzhuoma | 西藏加查Jiacha, Tibet | 20 | 2 | 5 | 1 | 8 | 2 | 1 | 2 | 3 | ASR | + | + | — | — | ||||||||
| 曲下小麦Quxiaxiaomai | 西藏定日Dingri, Tibet | 80 | 4 | 100 | 3 | 88 | 4 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 定日长芒Dingrichangmang | 西藏定日Dingri, Tibet | 80 | 4 | 80 | 4 | 26 | 3 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 波密短曲Bomiduanqu | 西藏波密Bomi, Tibet | 60 | 3 | 20 | 3 | 7 | 1 | 3 | 4 | 3 | S | + | + | — | — | ||||||||
| 大红麦Dahongmai | 青海平安Pingan, Qinghai | 5 | 0; | 0 | 0; | 0 | 0; | 1 | 3 | 3 | APR | + | + | — | — | ||||||||
| 朗县贡卓Langxiangongzhuo | 西藏朗县Langxian, Tibet | 5 | 0; | 5 | 1 | 10 | 2 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 丕卓Pizhuo | 西藏加查Jiacha, Tibet | * | 4 | 60 | 3 | 8 | 1 | 3 | 4 | 3 | S | + | + | — | — | ||||||||
| 广欠无芒Guangqianwumang | 西藏林芝Linzhi, Tibet | * | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 3 | ASR | + | + | — | — | ||||||||
| 加查鼠麦Jiachashumai | 西藏加查Jiacha, Tibet | * | 4 | 40 | 3 | 100 | 4 | 3 | 3 | 3 | S | + | + | — | — | ||||||||
| 准唐玉卓Huaitangyuzhuo | 西藏昂仁Angren, Tibet | 100 | 4 | 100 | 4 | 100 | 4 | 4 | 3 | 4 | S | — | + | — | — | ||||||||
| 红芒麦子Hongmangmaizi | 四川义敦Yidun, Sichuan | 20 | 2 | 5 | 1 | 4 | 1 | 1 | 3 | 3 | APR | + | — | — | — | ||||||||
| 吉雄扎嘎Jixiongzhaga | 西藏贡嘎Gongga, Tibet | 5 | 1 | 80 | 4 | 52 | 3 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 藏麦-169 Zangmai-169 | 西藏拉萨Lasa, Tibet | 100 | 4 | 100 | 4 | 88 | 4 | 4 | 4 | 3 | S | — | + | — | — | ||||||||
| 加长麦Jiachangmai | 西藏昌都Changdu, Tibet | 100 | 4 | 100 | 4 | 92 | 4 | 4 | 3 | 3 | S | — | + | — | — | ||||||||
| 踏荣卓Tarongzhuo | 西藏尼木Nimu, Tibet | 100 | 4 | 100 | 4 | 15 | 3 | 4 | 3 | 4 | S | + | — | — | — | ||||||||
| 岗吉基卓Gangjijizhuo | 西藏亚东Yadong, Tibet | 80 | 3 | 60 | 3 | 35 | 3 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 小红麦(八宝)Xiaohongmai(Babao) | 青海祁连Qilian, Qinghai | 60 | 3 | 60 | 3 | 72 | 4 | 1 | 3 | 4 | S | + | — | — | — | ||||||||
| 吉林春麦Jilinchunmai | 西藏扎囊Zhanang, Tibet | 40 | 2 | 0 | 0; | 6 | 1 | 3 | 4 | 4 | S | + | — | — | — | ||||||||
| 下康布Xiakangbu | 西藏亚东Yadong, Tibet | 20 | 2 | 5 | 1 | 60 | 4 | 2 | 4 | 3 | S | — | + | — | — | ||||||||
| 江当秃头Jiangdangtutou | 西藏乃东Naidong, Tibet | 60 | 3 | 40 | 3 | 60 | 4 | 3 | 4 | 4 | S | — | + | — | — | ||||||||
| 长芒毛颖麦Changmangmaoyingmai | 西藏察雅Chaya, Tibet | 60 | 3 | 40 | 3 | 80 | 4 | 3 | 2 | 4 | S | — | + | — | — | ||||||||
| 毛红麦Maohongmai | 青海贵德Guide, Qinghai | 100 | 4 | 100 | 4 | 100 | 4 | 3 | 3 | 3 | S | — | — | — | + | ||||||||
| 尕老汉Galaohan | 青海乐都Ledu, Qinghai | 5 | 1 | 5 | 1 | 48 | 2 | 1 | 4 | 4 | APR | — | + | — | — | ||||||||
| 兰麦Lanmai | 青海都兰Dulan, Qinghai | 100 | 4 | 100 | 4 | 100 | 4 | 4 | 4 | 3 | S | — | — | — | + | ||||||||
| 扎仁玛布Zharenmabu | 西藏昌都Changdu, Tibet | 80 | 3 | 80 | 3 | 88 | 4 | 4 | 3 | 4 | S | — | + | — | — | ||||||||
| 高山早熟小麦Gaoshanzaoshuxiaomai | 四川新龙Xinlong, Sichuan | 20 | 2 | 5 | 1 | 13 | 2 | 1 | 3 | 4 | APR | — | + | — | — | ||||||||
| 小红麦Xiaohongmai | 青海都兰Dulan, Qinghai | 60 | 3 | 10 | 3 | 40 | 3 | 3 | 3 | 4 | S | — | + | — | — | ||||||||
| 大六月黄Daliuyuehuang | 青海贵德Guide, Qinghai | 80 | 4 | 100 | 4 | 2 | 1 | 1 | 3 | 4 | S | + | — | — | — | ||||||||
| 白小麦Baixiaomai | 西藏林芝Linzhi, Tibet | 80 | 3 | 60 | 3 | 28 | 3 | 3 | 3 | 4 | S | — | + | — | — | ||||||||
| 团结基卓Tuanjiejizhuo | 西藏察雅Chaya, Tibet | 100 | 4 | 100 | 4 | 72 | 4 | 4 | 4 | 4 | S | — | + | — | — | ||||||||
| 年扎冬小麦Nianzhadongxiaomai | 西藏错那Cuona, Tibet | * | 3 | 20 | 3 | 2 | 2 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 吉古丕卓Jigupizhuo | 西藏墨竹Mozhu, Tibet | * | 3 | 0 | 3 | * | * | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 王卡麦Wangkamai | 西藏察雅Chaya, Tibet | * | 4 | 60 | 3 | 60 | 4 | 3 | 4 | 3 | S | — | + | — | — | ||||||||
| 仁布春Renbuchun | 西藏仁布Renbu, Tibet | 100 | 4 | 40 | 3 | 28 | 3 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 加查红颖Jiachahongying | 西藏加查Jiacha, Tibet | * | 4 | 80 | 3 | 76 | 4 | 4 | 3 | 1 | S | — | + | — | — | ||||||||
| 宗沙麦Zongshamai | 西藏察雅Chaya, Tibet | * | 4 | 80 | 3 | 88 | 4 | 4 | 4 | 4 | S | — | + | — | — | ||||||||
| 鲁朗白麦Lulangbaimai | 西藏林芝Linzhi, Tibet | 40 | 3 | 40 | 3 | 44 | 3 | 3 | 3 | 4 | S | — | — | + | — | ||||||||
| 红和尚头Hongheshangtou | 西藏乃东Naidong, Tibet | * | 4 | 60 | 3 | 800 | 4 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 扎国勾曲麦Zhaguogouqumai | 西藏错那Cuona, Tibet | * | 4 | 20 | 3 | 60 | 4 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 毛颖麦Maoyingmai | 西藏错那Cuona, Tibet | * | 4 | 5 | 3 | 30 | 4 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 羊日大穗Yangridasui | 西藏拉萨Lasa, Tibet | * | 4 | 0 | 0; | 56 | 4 | 3 | 3 | 3 | S | — | + | — | — | ||||||||
| 易贡卓Yigongzhuo | 西藏波密Bomi, Tibet | * | 3 | 10 | 3 | 44 | 3 | 3 | 3 | 4 | S | — | — | + | — | ||||||||
| 桑久比卓Sangjiubizhuo | 西藏察隅Chayu, Tibet | * | 3 | 40 | 3 | 60 | 3 | 3 | 4 | 3 | S | — | — | + | — | ||||||||
| 塔吉卓Tajizhuo | 西藏达孜Dazi, Tibet | * | 4 | 100 | 4 | 100 | 4 | 3 | 3 | 4 | S | — | + | — | — | ||||||||
| 曲红麦Quhongmai | 西藏拉萨Lasa, Tibet | * | 4 | 40 | 3 | 92 | 4 | 3 | 3 | 4 | S | — | + | — | — | ||||||||
| 错那白麦Cuonabaimai | 西藏错那Cuona, Tibet | * | 4 | 10 | 3 | 80 | 4 | 3 | 4 | 4 | S | — | — | + | — | ||||||||
| 白玉小麦Baiyuxiaomai | 四川白玉Baiyu, Sichuan | 20 | 2 | 0 | 0; | 0 | 0; | 2 | 4 | 4 | APR | — | — | + | — | ||||||||
| 泽当毛颖Zedangmaoying | 西藏乃东Naidong, Tibet | 10 | 2 | * | * | 37 | 4 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 扎西岗卓Zhaxigangzhuo | 西藏定日Dingri, Tibet | 100 | 4 | 100 | 4 | 100 | 4 | 4 | 4 | 4 | S | — | — | — | — | ||||||||
| 兴荣春麦Xingrongchunmai | 西藏隆子Longzi, Tibet | 20 | 3 | 10 | 2 | 10 | 3 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 中芒卓玛Zhongmangzhuoma | 西藏芒康Mangkang, Tibet | * | * | 60 | 3 | 6 | 2 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 然日无芒麦Ranriwumangmai | 四川石渠Shiqu, Sichuan | 100 | 4 | 80 | 3 | 100 | 4 | 4 | 3 | 3 | S | — | — | — | — | ||||||||
| 定结春Dingjiechun | 西藏定结Dingjie, Tibet | 5 | 1 | 5 | 1 | 0 | 0; | 1 | 3 | 1 | ASR | — | — | — | — | ||||||||
| 德阳杂麦Deyangzamai | 西藏米林Milin, Tibet | 60 | 3 | 60 | 3 | 68 | 4 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 白颖无芒小麦Baiyingwumangxiaomai | 西藏江孜Jiangzi, Tibet | 80 | 3 | 80 | 3 | 100 | 4 | 4 | 2 | 0 | S | — | — | — | — | ||||||||
| 生格小麦Shenggexiaomai | 西藏昌都Changdu, Tibet | 100 | 4 | 100 | 4 | * | 4 | 4 | 4 | 3 | S | — | — | — | — | ||||||||
| 大白麦Dabaimai | 青海化隆Hualong, Qinghai | 80 | 3 | 60 | 3 | 44 | 3 | 1 | 4 | 4 | S | — | — | — | — | ||||||||
| 红小麦Hongxiaomai | 西藏八宿Basu, Tibet | 100 | 4 | 100 | 4 | 88 | 4 | 4 | 4 | 4 | S | — | — | — | — | ||||||||
| 聂拉木无芒Nielamuwumang | 西藏聂拉木Nielamu, Tibet | 100 | 4 | 100 | 4 | 100 | 4 | 4 | 3 | 4 | S | — | — | — | — | ||||||||
| 沙马比卓Shamabizhuo | 西藏察隅Chayu, Tibet | 40 | 3 | 40 | 3 | 48 | 3 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 丁青毛颖麦Dingqingmaoyingmai | 西藏昌都Changdu, Tibet | 100 | 4 | 100 | 4 | 76 | 4 | 4 | 3 | 4 | S | — | — | — | — | ||||||||
| 无芒白麦Wumangbaimai | 西藏墨竹Mozhu, Tibet | 100 | 4 | 100 | 4 | 80 | 4 | 4 | 4 | 3 | S | — | — | — | — | ||||||||
| 泽当杂小麦Zedangzaxiaomai | 西藏乃东Naidong, Tibet | * | 4 | 80 | 4 | 26 | 4 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 扎仁卓玛Zharenzhuoma | 西藏朗县Langxian, Tibet | * | 4 | 80 | 3 | 26 | 3 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 东门秃头麦Dongmentutoumai | 西藏乃东Naidong, Tibet | * | 4 | 60 | 3 | 40 | 3 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 吉丁无芒春麦Jidingwumangchunmai | 西藏谢通门Xietongmen, Tibet | * | 4 | 100 | 4 | 100 | 4 | 4 | 4 | 4 | S | — | — | — | — | ||||||||
| 普芒红麦Pumanghongmai | 西藏普芒Pumang, Tibet | 60 | 2 | 5 | 1 | 8 | 1 | 0; | 4 | 4 | APR | — | — | — | — | ||||||||
| 曲下基卓Quxiajizhuo | 西藏拉孜Lazi, Tibet | * | 4 | 80 | 4 | 88 | 4 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 沙岗春Shagangchun | 西藏康马Kangma, Tibet | 100 | 4 | 100 | 4 | 100 | 4 | 3 | 4 | 3 | S | — | — | — | — | ||||||||
| 学隆卓Xuelongzhuo | 西藏察雅Chaya, Tibet | 100 | 4 | 5 | 0 | 61 | 3 | 3 | 4 | 3 | S | — | — | — | — | ||||||||
| 白马店卓Baimadianzhuo | 西藏林芝Linzhi, Tibet | * | 2 | 5 | 1 | 4 | 1 | 0; | 3 | 3 | APR | — | — | — | — | ||||||||
| 加查冬春麦Jiachadongchunmai | 西藏加查Jiacha, Tibet | 100 | 4 | 80 | 3 | 100 | 4 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 扎娜Zhana | 西藏曲松Qusong, Tibet | 100 | 4 | 20 | 3 | 100 | 4 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 杰果扎仁布素Jieguozharenbusu | 西藏米林Milin, Tibet | * | 3 | 0 | 1 | 17 | 3 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 拉月大穗Layuedasui | 西藏林芝Linzhi, Tibet | * | 4 | 40 | 3 | 48 | 3 | 3 | 4 | 3 | S | — | — | — | — | ||||||||
| 长芒培卓Changmangpeizhuo | 西藏堆龙Duilong, Tibet | * | 4 | 40 | 3 | 18 | 4 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 仁达长光麦Rendachangguangmai | 西藏察雅Chaya, Tibet | 100 | 4 | 100 | 4 | 96 | 4 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 东莱长红麦Donglaichanghongmai | 西藏加查Jiacha, Tibet | * | 4 | 80 | 3 | 96 | 4 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 秃毛麦Tumaomai | 西藏波密Bomi, Tibet | * | 3 | 10 | 3 | 60 | 4 | 3 | 3 | 3 | S | — | — | — | — | ||||||||
| 吉日卓Jirizhuo | 西藏林芝Linzhi, Tibet | * | 3 | 10 | 3 | 25 | 3 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 达当卓Dadangzhuo | 西藏察雅Chaya, Tibet | * | 4 | 60 | 3 | 88 | 4 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 加查红麦Jiachahongmai | 西藏加查Jiacha, Tibet | * | 4 | 100 | 4 | 72 | 4 | 3 | 3 | 4 | S | — | — | — | — | ||||||||
| 无芒毛颖麦Wumangmaoyinmai | 西藏加查Jiacha, Tibet | * | 4 | 60 | 3 | 76 | 4 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 东门红秃子Dongmenhongtuzi | 西藏乃东Naidong, Tibet | * | * | * | * | 9 | 2 | 3 | 4 | 4 | S | — | — | — | — | ||||||||
| 陇南长芒Longnanchangmang | 西藏加查Jiacha, Tibet | * | 4 | 100 | 4 | 100 | 4 | 4 | 4 | 4 | S | — | — | — | — | ||||||||
新窗口打开|下载CSV
1.2 室内苗期条锈病抗性鉴定
分别利用条锈菌小种CYR32、CYR34在甘肃省农业科学院植物保护研究所温室进行苗期接种鉴定。当供试材料生长至一叶一心期时, 采用涂抹法进行人工条锈菌接种[25]。接种后18~20 d, 待对照感病品种铭贤169充分发病后, 按0~4级标准记载各供试材料的反应型(infection type, IT) [26], 其中0~2级为抗病, 3~4级为感病。1.3 田间成株期条锈病抗性鉴定
于2015—2016、2017—2018、2018—2019年度小麦生产季, 分别在绵阳市农业科学研究院的小麦成株期条锈病鉴定圃(2016MY)和四川农业大学现代化农业(崇州)基地小麦成株期条锈病鉴定圃(2016CZ、2018CZ、2019CZ), 利用混合菌对供试材料进行鉴定。每份供试材料种植3行, 行长2 m, 行距0.3 m, 株距0.1 m, 按序播种。每间隔20行种植1行AvS, 作为诱发行和感病对照。鉴定圃四周种植2行SY95-71作诱发行。翌年1月中旬待小麦分蘖中后期, 通过涂抹法在诱发行小麦的倒数第二叶中部接种混合菌。于3月下旬至4月中旬感病对照材料AvS充分发病后, 参照NY/T 1443.1-2007《小麦抗病虫性评价技术规范第1部分: 小麦抗条锈病评价技术规范》, 调查并记录条锈病严重度和反应型数据[27]。1.4 已知条锈病抗性基因分子检测
采用改良的CTAB法[28]提取携带条锈病抗性基因的载体品种和93份小麦地方种质的叶片基因组DNA, 使用紫外分光光度计(Nanodrop 2000, Thermo Scientific, 美国)测定DNA浓度和纯度, 并稀释至100 ng μL-1作为工作液浓度, 置于冰箱4℃保存备用。利用Yr5、Yr10、Yr18、Yr24、Yr48、Yr65和Yr67紧密连锁的侧翼分子标记或基因功能标记进行检测[29,30,31,32,33,34,35]。所用引物均由擎科生物工程股份有限公司合成(表2)。扩增产物通过浓度为2% (m/v)的琼脂糖凝胶电泳或浓度为6% (v/v)的非变性聚丙烯酰胺凝胶电泳后观察并照相。Table 2
表2
表2Yr基因的侧翼标记及其序列
Table 2
| Yr 基因 Yr gene | 标记类型 Marker type | 标记名称 Marker name | 引物序列 Primer sequence (5′-3′) | 遗传距离 Genetic distance (cM) | 参考文献 Reference |
|---|---|---|---|---|---|
| Yr5 | KASP | Yr5F | GCGCCCCTTTTCGAAAAAATA | — | Marchal et al. [29] |
| Yr5H | CTAGCATCAAACAAGCTAAATA | ||||
| Yr5R | ATGTCGAAATATTGCATAACATGG | ||||
| Yr10 | Indel | Xsdauw79-F | TTGCTCTAAGCTGTGGCCT | Yuan et al. [30] | |
| Xsdauw79-R | GAGTTCAACCCCGAACACT | ||||
| Xsdauw79-NF | AGAGCCTAAGCGCCTAAGG | ||||
| Xsdauw79-NR | TTAAAATCTCCCAAGTACGCA | ||||
| Yr18 | STS | csLV34 | GTTGGTTAAGACTGGTGATGG | 0.4 | Lagudah et al. [31] |
| TGCTTGCTATTGCTGAATAGT | |||||
| Gene Marker | Cssfr5 | TTGATGAAACCAGTTTTTTTTCTA | — | Lagudah et al. [31] | |
| GGGAGCATTATTTTTTTCCATCATG | |||||
| ACTTTCCTGAAAATAATACAAGCA | |||||
| TATGCCATTTAACATAATCATGAA | |||||
| Yr24 | STS | Xwe173 | GGGACAAGGGGAGTTGAAGC | 1.4 | Wang et al. [32] |
| GAGAGTTCCAAGCAGAACAC | |||||
| STS | Xwe33 | TAAACCAAGTCCCCCAAA | — | Wang et al. [32] | |
| GGAGTCCATCTTCACCGA | |||||
| Yr48 | SSR | Xwmc727 | CATAATCAGGACAGCCGCAC | 4.4 | Lowe et al. [33] |
| TAGTGGCCTGATGTATCTAGTTGG | |||||
| Yr65 | SSR | Xgwm18 | GGTTGCTGAAGAACCTTATTTAGG | 1.2 | Cheng et al. [34] |
| TGGCGCCATGATTGCATTATCTTC | |||||
| Yr67 | SSR | Xbarc182 | CCATGGCCAACAGCTCAAGGTCTC | 0.4 | Xu et al. [35] |
| CGCAAAACCGCATCAGGGAAGCACCAAT | |||||
| SSR | Xcfa2040 | TCAAATGATTTCAGGTAACCACTA | 2.8 | Xu et al. [35] | |
| TTCCTGATCCCACCAAACAT |
新窗口打开|下载CSV
2 结果与分析
2.1 苗期条锈病抗性
苗期鉴定结果表明, 对CYR32表现抗性的材料有4份(占4.30%); 其中, 广欠无芒小麦表现为高抗。对CYR34表现抗性的材料有3份(占3.23%); 其中, 白颖无芒小麦表现为免疫。白颖无芒小麦对CYR32和CYR34均表现为抗病(表1)。综合来看, 青藏春冬麦区小麦地方种质对当前小麦条锈菌流行小种具有一定的抗病性, 但整体抗性水平较低。2.2 成株期条锈病抗性
田间成株期抗条锈的鉴定结果表明, 在绵阳农业科学院小麦成株期条锈病鉴定圃(16MY)中有17份种质(占18.28%)表现抗性; 在四川农业大学现代化农业基地(崇州)小麦成株期条锈病鉴定圃(2016CZ、2018CZ、2019CZ), 分别有17份(占18.28%)、18份(占19.35%)和15份(占16.13%)表现抗性。综合4个鉴定圃的田间成株期抗性鉴定结果, 在供试材料中有10份种质(占10.75%)在所有鉴定圃均表现抗性, 其中3份表现为低反应型(ITs 0~1)、低严重度(DS<20%)的高抗病性(表1)。结合苗期及成株期抗性鉴定结果, 小麦加查扎仁卓玛和广欠无芒对CYR32具有全生育期条锈病抗性; 定结春对CYR34具有全生育期条锈病抗性。2.3 抗条锈病基因的分子检测
93份供试小麦地方种质的分子检测结果显示, 17份(占18.28%)可能携带Yr18 (图1); 40份(占43.01%)可能携带Yr48; 6份(占6.45%)可能携带Yr65; 2份(占2.82%)可能携带Yr67。同时发现, 12份可能同时携带Yr18+Yr48, 1份可能同时携带Yr48+Yr65 (表1)。所有供试材料均为未检测到Yr5、Yr10和Yr24。结合成株期抗性鉴定发现, 10份表现成株期条锈病抗性地方种质中, 3份(定结春、普芒红麦和白马店卓)均未检测到所有供试Yr基因, 推测其可能携带其他已知或未知抗条锈病基因。图1
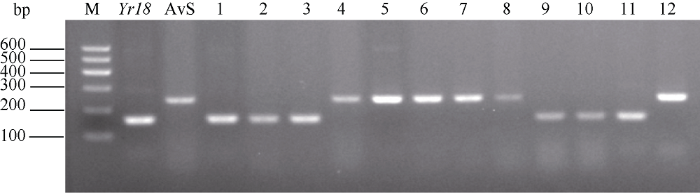 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1青藏春冬麦区部分小麦地方种质Yr18的分子检测
M: 分子量; Yr18: Yr18/6* Avocet S; AvS: Avocet Susceptible; 1: 加查扎仁卓玛; 2: 大红麦; 3: 广欠无芒; 4: 尕老汉; 5: 高山早熟小麦; 6: 白玉小麦; 7: 普芒红麦; 8: 白马店卓; 9: 长芒白粒麦; 10: 波密杂麦-5; 11: 林芝杂小麦; 12: 踏荣卓。
Fig. 1Molecular detection of Yr18 in part of wheat landraces collected from the Qinghai-Tibet spring and winter wheat zones in China
M: molecular weight standard; Yr18: Yr18/6* Avocet S; AvS: Avocet Susceptible; 1: Jiachazharenzhuoma; 2: Dahongmai; 3: Guangqianwumang; 4: Galaohan; 5: Gaoshanzaoshuxiaomai; 6: Baiyuxiaomai; 7: Pumanghongmai; 8: Baimadianzhuo; 9: Changmangbailimai; 10: Bomizamai-5; 11: Linzhizaxiaomai; 12: Tarongzhuo.
3 讨论
青藏春冬麦区地处青藏高原, 属于冬春麦交替种植区, 条锈病常年流行, 与其他麦区相比, 具有更为独特的条锈病流行环境。因此, 在这一麦区的条锈病流行体系中经长期自然选择而得以保留下来的小麦地方种质可能蕴含丰富的条锈病抗性基因[36], 有待进一步开发与利用。前人对来自此麦区的特有亚种和育成品种(系)进行了条锈病抗性鉴定。李菁等[37]对117份西藏半野生小麦进行苗期和成株期条锈病抗性鉴定发现, 仅有17份(占14.53%)具有苗期抗性, 4份(占3.42%)具有成株期抗性, 表明来自西藏的半野生小麦对当前中国的条锈菌流行小种抗性水平较低。彭岳林等[38]对48份西藏小麦种质进行成株期条锈病抗性鉴定发现, 有19份(占14.53%)具有成株期抗性, 5份(占10.42%)具有成株期抗性但易变化, 24份(占50%)表现感病, 表明多数西藏小麦品种(系)感条锈病。袁飞敏等[16]利用小种CYR29、CYR32和CYR34对青海高原的197份小麦品种(系)进行苗期抗性鉴定发现, 苗期对CYR29、CYR32和CYR34小种表现抗性的种质分别占88.3%、43.6%和61.9%, 对三者均表现抗性的种质占37.5%, 整体上来看, 当前青海高原春小麦品种(系)的条锈病抗性整体水平较低。本研究93份青藏春冬麦区的小麦地方种质的条锈病抗性较低。筛选后的6份具有苗期条锈病抗性、10份具有成株期条锈病抗性的优良地方种质为丰富条锈病抗性基因库及抗条锈病育种利用提供了材料。3.1 Yr5、Yr10和Yr24的分子检测
近年来, 国内****利用中国不同时期条锈病流行小种对小麦地方种质、主栽品种、后备品种及高代育种材料进行了抗性评价, 并结合已知Yr基因紧密连锁的侧翼分子标记或功能标记检测其可能携带的抗病基因, 为开展抗病品种的合理布局、发掘抗条锈病新基因和培育小麦抗条锈病新品种提供了参考依据[11,16,18-23,36]。Yr5来源于六倍体小麦斯卑尔脱(Triticum aestivum subsp. spelta var. album), Macer等[39]将其定位在2B染色体上。随后, Law等[40]进一步将Yr5定位于2BL上, 离着丝粒的遗传距离为21 cM。2018年Marchal等[29]开发了与Yr5共分离的KASP标记。除了澳大利亚和印度于20世纪分别报道对Yr5致病的条锈菌小种外, 目前中国也已发现对Yr5致病的新菌系[41]。但是由于含Yr5的小麦品种没有大面积种植, 其抗性的持久度还没有真正接受生产考验[42]。Yr10来源于一个土耳其普通小麦PI 178383, 被定位在1BS上, Crest、Jacman和Moro等品种携带此基因[43]。2018年Yuan等[30]开发了与Yr10共分离的Indel标记, 可用于该基因的分子检测。目前, Yr10基因在中国小麦抗病育种中利用较少。李峰奇等[44]利用Yr10紧密连锁标记SC200和Xpsp3000对黄淮麦区126个小麦品种(系)抗条锈病基因检测显示, 仅陕麦139、周麦98165、西农739和普冰202共4份材料可能含有Yr10。韩德俊等[17]采用Yr10的STS (sequence-tagged site)分子标记S26M47, 对1980份小麦地方品种和国外种质抗条锈性鉴定和分子标记检测表明, 仅有Spanish D188和红茧儿麦可能含有Yr10。伍玲等[45]利用SCAR (sequence characterized amplified region)标记SC200, 对四川小麦区试品系72份材料检测显示, 仅R146检测出Yr10。在中国, 强毒性小种CYR34的出现, 致使Yr10丧失抗性[46]。Yr24 (=Yr26)来源于硬粒小麦(T. durum), 位于小麦染色体1BS上, Yr26来源于圆锥小麦(T. turgidum), 其载体品种为普通小麦-簇毛麦易位系92R89、92R90、92R149、92R178和92R137, 也被定位于1BS染色体上。2006年Li等[47]通过等位性验证抗谱分析, 认为Yr26、Yr24和来自于川麦42的抗条锈病基因YrCH42可能是同一基因。Yr24被转移到普通小麦后, 由于其优良的抗病性和较好的农艺性状, 在育种中被广泛利用。韩德俊等[48]对中国“西北-华北长江中下游”条锈病流行区内500余份小麦主栽品种和后备品系进行抗条锈病鉴定和评价, 认为抗源主要是携带Yr24 (=Yr26)的种质。张培禹等[49]对四川盆地67个小麦品种(系)的条锈病抗性进行鉴定, 并结合分子检测、抗谱测定和系谱追踪, 显示有26份材料可能携带Yr26。但是由于对Yr26有毒性的致病类型avrYr10/14/26/CH42的出现, 有27份材料的苗期抗性丧失。李北等[50]对来自重庆麦区的89份高代品系和18份当地主栽小麦品种进行抗条锈病基因分子检测, 推测可能有39份材料携带Yr26。黄苗苗等[20]通过分子检测发现, 来自甘肃麦区的223份小麦地方品种携带Yr24 (=Yr26)的材料比例达50.22%。但由于对Yr26有毒性的致病类型V26出现, 该基因目前已在中国麦区丧失抗性。本研究分子检测发现来自青藏春冬麦区的93份小麦地方种质均未检测到上述Yr5、Yr10和Yr24 (=Yr26)基因。3.2 携带Yr48、Yr65和Yr67的地方种质
Yr48来源于北美普通小麦PI 610750, 对北美条锈菌流行小种(PST-78、PST-79、PST-80、PST-90和PST-98等)具有成株期抗性。Lowe等[33]利用SSR分子标记Xwmc727和Xgwm291将该基因定位在小麦染色体5AL上。Yr65来源于硬粒小麦PI 480016, 对多个北美条锈菌流行小种具有全生育期抗性, 并将该基因定位于染色体1BS上[34]。已有报道显示, 中国小麦育成品种及地方种质中携带Yr48和Yr65, 抗性基因有效性分析发现, Yr48对中国当前流行小种CYR32、CYR33及CYR34已丧失抗性, 而Yr65则具有成株期抗性[51]。本研究利用Yr48特异分子标记检测, 发现40份地方种质可能携带Yr48。结合抗性鉴定发现, 5份材料(加查扎仁卓玛、大红麦、广欠无芒、尕老汉和高山早熟小麦)对条锈病表现为成株期抗性(ITs 0~2), 推测其可能还携带有其他已知或未知的抗性基因或组合。基于分子检测获得的6份可能携带Yr65的种质中, 仅白玉小麦表现为成株期抗性(ITs 0~2), 其余5份材料均表现感病(ITs 3~4), 表明在供试的青藏春冬麦区地方种质中, 仅有白玉小麦可能携带了Yr65。Yr67来源于印度的普通小麦C591, 对中国条锈菌小种CYR32具有全生育期抗性, 该基因被定位于小麦染色体7BL上[35,52]。近年来研究表明, Yr67对CYR33和CYR34等小种表现感病[46]。利用Yr67特异分子标记检测发现, 来自青藏春冬麦区的2份材料(毛红麦和兰麦)可能携带Yr67, 但苗期抗性鉴定发现, 毛红麦和兰麦均对CYR32表现为感病(ITs 3~4), 基因型和表型的不一致说明这两份材料可能并不携带Yr67。3.3 携带Yr18的地方种质
Yr18是成株期抗性基因, 来源于普通小麦, 位于染色体7DS上, 其载体品种有Thatcher、Jupeteco 73R和Anza等[53], 具有慢锈性。已有研究证实, Yr18广泛存在于中国主栽品种、后备品种及高代育种材料, 尤其是小麦地方种质中。杨文雄等[22]利用Yr18的特异分子标记csLV34检测了422份中国地方品种, 发现在被测地方品种中约有85%含有Yr18。黄苗苗等[20]发现, 来自甘肃的小麦农家品种, 近10%的材料携带Yr18。伍玲[54]利用Yr18紧密连锁的分子标记csLV34及6个功能标记cssfr1~cssfr6, 并结合成株期抗性鉴定, 对中国150份小麦地方品种进行Yr18分子检测, 发现82份含有Yr18。本研究利用STS标记csLV34及功能标记cssfr5对青藏春冬麦区的小麦地方种质进行分子检测, 发现17份材料可能携带Yr18。已有研究证实仅当Yr18单独存在时, 该基因控制的抗性水平往往不高, 而当其和具有加性效应的微效基因结合在一起时能产生较高水平的持久抗性[55]。曾庆东等[46]利用CYR32、CYR33及CYR34对Yr18单基因系AvSYr18NIL和Anza进行成株期抗性鉴定发现其在部分环境中表现抗病而部分环境中表现感病, 即在各环境下的成株期抗性表现不一致。本研究利用Yr18连锁的侧翼分子标记和功能标记检测发现, 共有17份地方种质可能携带Yr18。其中, 波密杂麦-5等7份种质苗期对CYR32和CYR34均表现为感病(ITs 3~4), 且在多个田间环境下成株期抗性也表现不一致, 这与曾庆东等人研究结果类似[46]。加查扎仁卓玛等4份地方种质在成株期均表现抗病(ITs 0~2), 推测这些种质除携带Yr18外, 可能还聚合了其他已知或未知的抗条锈病基因。长芒白粒麦等6份种质在成株期均表现为感病(ITs 3~4), 而伍玲等[56]的研究中也发现携带Yr18的部分小麦地方种质在成株期均感病, 其推测这些地方种质中Yr18位点可能存在新的等位变异, 也可能存在类似抑制基因等调控机制导致Yr18对条锈病等病害的抗性丧失。总体看来, 通过对青藏春冬麦区小麦地方种质进行已知条锈病抗性基因检测后发现其所携带抗性基因的多样性较低, 但对具有优异抗性且可能携带未知抗性基因的地方种质应加以重视, 并加快其条锈病抗性基因的发掘与育种利用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Stripe (or yellow) rust caused by Puccinia striiformis f. sp. tritici is the most destructive foliar disease of wheat in China. The pathogen populations were analyzed for virulence evolution, complexity, phenotypic dynamics, and diversity on temporal and spatial bases. A total of 41 races were identified and characterized from 4,714 stripe rust isolates collected during 2003 through 2007 from wheat growing areas in 15 provinces in China. The races were based on avirulence/virulence patterns to 19 differential host genotypes. Chinese stripe rust population exhibited high diversity with a complex virulence structure. Comparisons using the relative Shannon's index indicated that some differences in the richness and evenness of races were present in pathogen populations within years and between regions despite a national tendency to reduced diversity over time. A noticeably increased frequency of race CYR33 (Chinese yellow rust 33) with virulence for YrSu was the major virulence change recorded in this study compared to the results on an annual basis. Isolates of Puccinia striiformis f. sp. tritici from different regions showed differences in the composition of races, distribution frequency, and diversity. The uneven distribution of major races and comparatively greater diversity in the Northwest and Southwest regions than that in the Huang-Huai-Hai region suggest that long-distance migrations of the pathogen occur from one or more over-summering areas eastward into over-wintering areas. This supports the hypothesis that southern Gansu and northwestern Sichuan comprises a "center of origin for virulence". Mutation of virulence or avirulence for host resistance in the stripe rust fungus may be the basic cause of the occurrence of new virulent types. The subsequent dominance of certain races will vary with parasitic fitness and the opportunities to be selected through large-scale cultivation of varieties with matching resistance genes. Implications of the center of origin for virulence variation and diversity in the pathogen population and an alternative strategy for limiting virulence evolution are discussed.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Over time, many single, all-stage resistance genes to stripe rust (Puccinia striiformis f. sp. tritici) in wheat (Triticum aestivum L.) are circumvented by race changes in the pathogen. In contrast, high-temperature, adult-plant resistance (HTAP), which only is expressed during the adult-plant stage and when air temperatures are warm, provides durable protection against stripe rust. Our objective was to identify major quantitative trait loci (QTL) for HTAP resistance to stripe rust in the spring wheat cultivar 'Louise'. The mapping population consisted of 188 recombinant inbred lines (RIL) from a Louise (resistant) by 'Penawawa' (susceptible) cross. F(5:6) lines were evaluated for stripe rust reaction under natural infection in replicated field trials at five locations in the US Pacific Northwest in 2007 and 2008. Infection type (IT) and disease severity were recorded for each RIL 2-4 times per location. In all environments, Penawawa, the susceptible parent, was rated with an IT ranging from 6 to 8 at all growth stages evaluated. In contrast, Louise, the resistant parent, was rated with an IT of 2 or 3 across growth stages. Distribution of IT values was bimodal, indicating a single major gene was affecting the trait. The parents and RIL population were evaluated with 295 polymorphic simple sequence repeat and one single nucleotide polymorphism markers. One major QTL, designated QYrlo.wpg-2BS, associated with HTAP resistance in Louise, was detected on chromosome 2BS (LOD scores ranging from 5.5 to 62.3 across locations and years) within a 16.9 cM region flanked by Xwmc474 and Xgwm148. SSR markers associated with QYrlo.wpg-2BS are currently being used in marker-based forward breeding strategies to transfer the target region into adapted germplasm to improve the durability of resistance in resulting cultivars.
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 3]
DOIURL [本文引用: 3]
DOIPMID [本文引用: 3]

The locus Lr34/Yr18/Pm38 confers partial and durable resistance against the devastating fungal pathogens leaf rust, stripe rust, and powdery mildew. In previous studies, this broad-spectrum resistance was shown to be controlled by a single gene which encodes a putative ATP-binding cassette transporter. Alleles of resistant and susceptible cultivars differed by only three sequence polymorphisms and the same resistance haplotype was found in the three independent breeding lineages of Lr34/Yr18/Pm38. Hence, we used these conserved sequence polymorphisms as templates to develop diagnostic molecular markers that will assist selection for durable multi-pathogen resistance in breeding programs. Five allele-specific markers (cssfr1-cssfr5) were developed based on a 3 bp deletion in exon 11 of the Lr34-gene, and one marker (cssfr6) was derived from a single nucleotide polymorphism in exon 12. Validation of reference genotypes, well characterized for the presence or absence of the Lr34/Yr18/Pm38 resistance locus, demonstrated perfect diagnostic values for the newly developed markers. By testing the new markers on a larger set of wheat cultivars, a third Lr34 haplotype, not described so far, was discovered in some European winter wheat and spelt material. Some cultivars with uncertain Lr34 status were re-assessed using the newly derived markers. Unambiguous identification of the Lr34 gene aided by the new markers has revealed that some wheat cultivars incorrectly postulated as having Lr34 may possess as yet uncharacterised loci for adult plant leaf and stripe rust resistance.
DOIURL [本文引用: 3]
DOIURL [本文引用: 3]
DOIPMID [本文引用: 3]

This manuscript reports two new genes ( Yr64 and Yr65 ) for effective resistance to stripe rust and usefulness of their flanking SSR markers for marker-assisted selection. Stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most important diseases of wheat worldwide and resistance is the best control strategy. Durum wheat accessions PI 331260 and PI 480016 were resistant to all tested Pst races. To transfer the resistance genes to common wheat and map them to wheat chromosomes, both accessions were crossed with the stripe rust-susceptible spring wheat 'Avocet S'. Resistant F3 plants with 42 chromosomes were selected cytologically and by rust phenotype. A single dominant gene for resistance was identified in segregating F4 lines from each cross. F6 populations for each cross were developed from single F5 plants and used for genetic mapping. Different genes from PI 331260 and PI 480016 were mapped to different loci in chromosome 1BS using simple sequence repeat markers. The gene from PI 331260 was flanked by Xgwm413 and Xgdm33 in bin 1BS9-0.84-1.06 at genetic distances of 3.5 and 2.0 cM; and the gene from PI 480016 was flanked by Xgwm18 and Xgwm11 in chromosome bin C-1BS10-0.50 at 1.2 and 2.1 cM, respectively. Chromosomal locations and race and allelism tests indicated that the two genes are different from previously reported stripe rust resistance genes, and therefore are named as Yr64 from PI 331260 and Yr65 from PI 480016. These genes and their flanking markers, and selected common wheat lines with the genes should be valuable for diversifying resistance genes used in breeding wheat cultivars with stripe rust resistance.
DOIURL [本文引用: 4]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Stripe rust is one of the most destructive diseases of wheat. Breeding for resistance is the most economical and environmentally acceptable means to control stripe rust. Genetic studies on resistance sources are very important. Previous inheritance studies on Triticum aestivum subsp. spelta cv. album and wheat cultivar Lee showed that each possessed a single dominant gene for stripe rust resistance, i.e., Yr5 and Yr7, respectively. Both were located on the long arm of chromosome 2B, but due to the complexities caused by genetic background effects there was no clear evidence on the allelism or linkage status of these genes. Our study, involving an intercross of Avocet S near-isogenic lines possessing the genes, provided clear evidence for allelism or extremely close linkage of Yr5 and Yr7 based on phenotypic and molecular studies.
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
PMID [本文引用: 1]

Stripe rust, caused by Puccinia striiformis f. sp. tritici (PST), is one of the most devastating diseases in common wheat (Triticum aestivum L.) worldwide. The objectives of this study were to map a stripe rust resistance gene in Chinese wheat cultivar Chuanmai 42 using molecular markers and to investigate its allelism with Yr24 and Yr26. A total of 787 F2 plants and 186 F3 lines derived from a cross between resistant cultivar Chuanmai 42 and susceptible line Taichung 29 were used for resistance gene tagging. Also 197 F2 plants from the cross Chuanmai 42xYr24/3*Avocet S and 726 F2 plants from Chuanmai 42xYr26/3*Avocet S were employed for allelic test of the resistance genes. In all, 819 pairs of wheat SSR primers were used to test the two parents, as well as resistant and susceptible bulks. Subsequently, nine polymorphic markers were employed for genotyping the F2 and F3 populations. Results indicated that the stripe rust resistance in Chuanmai 42 was conferred by a single dominant gene, temporarily designated YrCH42, located close to the centromere of chromosome 1B and flanked by nine SSR markers Xwmc626, Xgwm273, Xgwm11, Xgwm18, Xbarc137, Xbarc187, Xgwm498, Xbarc240 and Xwmc216. The resistance gene was closely linked to Xgwm498 and Xbarc187 with genetic distances of 1.6 and 2.3 cM, respectively. The seedling tests with 26 PST isolates and allelic tests indicated that YrCH42, Yr24 and Yr26 are likely to be the same gene.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Stripe rust, caused by Puccinia striiformis Westend. f. sp. tritici (PST), is one of the most destructive diseases of common wheat (Triticum aestivum L.). To determine inheritance of stripe rust resistance and map the resistance gene(s) in wheat variety C591, F(1), F(2,) and F(3) progenies derived from the Taichung 29 x C591 cross were inoculated with Chinese PST race CY32 in the greenhouse. Genetic analysis identified a single dominant gene, temporarily designated YrC591. A total of 178 SSR and 130 AFLP markers were used to test the parents and resistant and susceptible bulks. From the bulk segregant analysis, seven polymorphic SSR and two AFLP markers were selected for genotyping the F(2) population. SSR marker Xcfa2040-7B, and SCAR marker SC-P35M48 derived from AFLP marker P35M48 ( 373 ) were identified to be closely linked to the resistance gene with genetic distances of 8.0 and 11.7 cM, respectively. The SSR markers mapped the resistance gene on chromosome arm 7BL. In the seedling test with five PST races, the reaction patterns of C591 were different from wheat cultivars or lines carrying Yr2 or Yr6 that also are found on chromosome 7B. The results indicate that YrC591 is probably a novel stripe rust resistance gene.
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
