 ,1,2, 李德芳
,1,2, 李德芳 ,2,*, 邓勇2, 潘根2, 陈安国2, 赵立宁2, 唐慧娟2
,2,*, 邓勇2, 潘根2, 陈安国2, 赵立宁2, 唐慧娟2Cloning of the key enzyme gene HcTPPJ in trehalose biosynthesis of kenaf and its expression in response to abiotic stress in kenaf
LI Hui ,1,2, LI De-Fang
,1,2, LI De-Fang ,2,*, DENG Yong2, PAN Gen2, CHEN An-Guo2, ZHAO Li-Ning2, TANG Hui-Juan2
,2,*, DENG Yong2, PAN Gen2, CHEN An-Guo2, ZHAO Li-Ning2, TANG Hui-Juan2通讯作者:
收稿日期:2020-01-10接受日期:2020-08-19网络出版日期:2020-09-08
| 基金资助: |
Received:2020-01-10Accepted:2020-08-19Online:2020-09-08
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (797KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李辉, 李德芳, 邓勇, 潘根, 陈安国, 赵立宁, 唐慧娟. 红麻海藻糖生物合成关键酶基因HcTPPJ的克隆及响应逆境的表达分析 [J]. 作物学报, 2020, 46(12): 1914-1922. doi:10.3724/SP.J.1006.2020.04006
LI Hui, LI De-Fang, DENG Yong, PAN Gen, CHEN An-Guo, ZHAO Li-Ning, TANG Hui-Juan.
海藻糖是由2个葡萄糖分子以1,1糖苷键构成的非还原性糖, 广泛存在于微生物、真菌、酵母、无脊椎动物和植物体内, 不仅可以作为生物体内的能源、碳源, 还可以作为信号物质调控生物体内的物质代谢和生长发育[1,2]。海藻糖对多种生物活性物质具有非特异性保护作用, 在抵御高温、干旱、高盐碱等非生物逆境胁迫过程中具有重要作用[3,4]。谢冬微等[5]的研究表明, 在低温胁迫条件下, 施加一定浓度的外源海藻糖能够促进冬小麦胚芽的生长, 提高其抗寒性。施加一定浓度的外源海藻糖不仅能够提高玉米幼苗的抗寒性还能够提高其对盐胁迫的抗性[6,7]。
海藻糖的合成在不同生物体内、不同外界环境下具有不同的途径, 迄今为止, 已经报道了5条生物合成途径[8]。在植物体内海藻糖的合成途径是TPS/TPP途径: 海藻糖-6-磷酸合成酶(trehalose- 6-phosphate synthase, TPS)催化葡萄糖从UDP-葡糖糖向葡萄糖-6-磷酸转化形成海藻糖-6-磷酸和UDP, 然后海藻糖-6-磷酸磷酸酶(trehalose-6-phosphate phosphatase, TPP)催化海藻糖-6-磷酸脱磷酸化形成海藻糖。由此可知, TPP是植物体内海藻糖合成途径的最后一步关键酶。
植物体内TPP基因是以基因家族的形式存在。在拟南芥中发现了10个TPP基因(AtTPPA~AtTPPJ), 共线性分析发现, 这10个基因都是由全基因组复制产生的, 且都具有酵母异源表达活性[9]。通过酵母功能互补试验, Vogel等[10]首次鉴定并克隆了2个拟南芥TPP基因家族成员AtTPPA和AtTPPB。在酵母TPS2突变体中表达这2个基因能够产生海藻糖, 实现功能互补, 证明AtTPPA和AtTPPB基因具有编码海藻糖-6-磷酸磷酸酶的活性。过表达AtTPPD基因能够提高拟南芥植株淀粉和可溶性糖的含量, 从而提高拟南芥的耐盐性[11]。水稻中有13个OsTPP基因位点, 其中OsTPP1基因的表达受低温胁迫、盐胁迫、渗透胁迫和ABA处理的诱导, 在12℃低温胁迫下, 水稻根系TPP酶活性和海藻糖含量瞬时升高, 推测OsTPP1基因参与水稻早期的低温胁迫响应过程; 超表达分析表明, OsTPP1基因能够激活一系列下游基因的表达, 从而提高转基因植株的耐盐性和抗寒性[12,13]。以上研究表明, TPP基因在植物响应逆境胁迫过程中具有重要的作用, 可以作为作物抗逆遗传改良的候选基因。
脱落酸(abscisic acid, ABA)是植物体内响应逆境胁迫的重要信号分子。大量研究表明, 9-顺式-环氧类胡萝卜素双加氧酶(9-cis-epoxycarotenoid, NCED)是ABA合成途径的关键酶, NCED基因的表达量能够反应植物内源ABA含量的变化。Satoshi等[14]的研究表明, 缺失ATNCED3基因的拟南芥突变体, 体内的ABA含量降低, 同时突变体对干旱胁迫敏感, 而提高ATNCED3基因的表达量, 能够增加拟南芥体内ABA的含量[15]。在拟南芥中, 超表达水稻OsNCED3基因能够增加转基因拟南芥的ABA含量, 且与野生型拟南芥相比更加耐旱[16]。
红麻(Hibiscus cannabinus L.)是锦葵科木槿属一年生草本植物。红麻的栽培主要以收获韧皮纤维为主要目的。红麻纤维是重要的纺织原料, 主要用于麻绳、麻袋、地毯等[17,18]。随着全球人口的增加, 人口、粮食、耕地之间的矛盾日益加剧, 红麻的种植必须转向盐碱地、干旱坡地、不能生产粮食的重金属污染土地。因此, 研究红麻的耐逆机理、选育红麻耐逆品种对维持红麻产业的可持续发展具有重要意义。
TPP基因参与植物响应逆境胁迫过程, 且具有多种生物功能。迄今为止, 有关红麻HcTPP基因参与逆境调控的研究还未见报道。本课题组前期通过对红麻转录组序列(CL541.Contig2)分析发现, TPP基因表达受盐胁迫的诱导[19]。因此, 本研究通过PCR扩增获得了CL541.Contig2 cDNA序列, 并通过荧光定量PCR分析了其在盐、干旱、脱落酸不同逆境胁迫下的时空表达特征。
1 材料与方法
1.1 试验材料的种植和处理
红麻品种K215由中国农业科学院麻类研究所红麻育种课题组提供。挑选籽粒饱满, 大小均匀的红麻种子, 用0.1%的氯化汞消毒10 min, 蒸馏水冲洗3次, 然后播种于发芽盒, 光照培养箱培养(温度25~28℃, 光照13 h d-1)。培养10 d后, 选取植株健壮、长势均匀的红麻幼苗, 移植到1/2 Hoagland营养液中培养, 直到红麻幼苗植株长到四叶一心(14 d)。1.1.1 红麻植株的盐胁迫处理 选取健壮的红麻幼苗进行NaCl胁迫处理。在1/2 Hoagland营养液中加入NaCl, 使其最终浓度分别为50 mmol L-1 NaCl、100 mmol L-1 NaCl、150 mmol L-1 NaCl, 处理3 d, 每个处理5株, 每个处理重复3次。同时在150 mmol L-1 NaCl条件下, 进行不同时间胁迫处理, 处理时间分别为0.5、1、2、4、8 h。对照组按正常模式进行培养。
1.1.2 红麻植株的模拟干旱胁迫处理 选取健壮的红麻幼苗进行模拟干旱处理。在1/2 Hoagland营养液中加入PEG-6000, 使其最终浓度为10%, 胁迫处理时间分别为0.5、1、2、4、8 h; 对照组按正常模式进行培养。每个处理5株, 每个处理重复3次。
1.1.3 红麻植株的脱落酸喷施处理 对红麻幼苗叶片喷施浓度为200 μmol L-1 ABA溶液, 处理时间分别为0 (喷施清水)、1、2、4、6 h。每个处理5株, 每个处理重复3次。
1.2 红麻HcTPPJ基因的克隆及测序
参照多糖多酚植物总RNA提取试剂盒[天根生化科技(北京)有限公司]说明书提取红麻叶片总RNA, 然后逆转录合成cDNA作为基因克隆的模板。根据转录组Unigene序列(编号: CL541.Contig2), 利用Primer Premier 5.0设计HcTPPJ基因全长扩增引物HcTPPJ-F/R (表1)。HcTPPJ基因全长扩增PCR反应体系包含2.5 μL 10×LA PCR buffer (Mg2+ plus)、0.25 μL LA Taq酶(TaKaRa)、1 μL 2.5 mmol L-1 dNTP、0.25 μL 10 μmol L-1 HcTPPJ-F、0.25 μL 10 μmol L-1 HcTPPJ-R、20 ng cDNA, ddH2O补足25 μL。HcTPPJ基因全长扩增PCR反应程序为94℃预变性2 min; 94℃变性30 s, 55℃退火30 s, 72℃延伸90 s, 30个循环; 72℃延伸3 min, 4℃终止反应。反应产物经1.2%琼脂糖凝胶电泳检测。参照琼脂糖凝胶DNA回收试剂盒[天根生化科技(北京)有限公司]的说明书切胶回收, 然后将回收片段连接到pMD19-T载体上, 转化大肠杆菌DH5α感受态细胞, 挑取阳性菌落送生工生物工程(上海)有限公司测序。Table 1
表1
表1本研究所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequences (5°-3°) | 引物用途 Primer usage |
|---|---|---|
| HcTPPJ-F | ATGGTGAGTTTCTTTGAA | 基因克隆 |
| HcTPPJ-R | TTACATTTTAGATTGCCCT | Gene cloning |
| HcTPPJ-QF | AACCTTTCTGCCTTGAGT | 实时荧光定量PCR |
| HcTPPJ-QR | AAATTGGCTGAGCTGTAC | qRT-PCR |
| HcNCED3-QR | AGGCGGTCGTCGGACTCGTT | 实时荧光定量PCR |
| HcNCED3-QF | GACTGCTTCTGCTTCCACCTCTG | qRT-PCR |
| Actin-QF | CAGGCAGTTCTTTCTTTGT | 内参基因 |
| Actin- QR | ATCCTCCAATCCAGACACT | Reference gene |
新窗口打开|下载CSV
1.3 红麻HcTPPJ基因的生物信息学分析
利用NCBI在线程序ORF finder (1.4 不同器官红麻HcTPPJ基因的表达特征
培养3 d后, 分别提取对照组红麻根、茎、叶的总RNA, 反转录合成cDNA作为荧光定量PCR的模板, 荧光定量PCR引物序列HcTPPJ-QF/QR见表1, 内参基因为β-actin, 内参引物为Actin-QF/QR (表1) [15]。荧光定量PCR的反应体系为10 μL, 包含Power 2×SYBR PCR Premixture 5 μL、上下游引物各0.3 μL、cDNA 2 μL, ddH2O补足10 μL。荧光定量PCR的反应程为95℃预变性2 min; 95℃变性15 s, 61℃退火15 s, 72℃延伸20 s, 40个循环。每个反应3个生物学重复, 每个生物学重复3个技术重复。1.5 盐、干旱、ABA胁迫下红麻HcTPPJ基因的表达特征
分别提取对照组和150 mmol L-1 NaCl处理组第3天植株的根、茎、叶总RNA, 150 mmol L-1 NaCl处理组0.5、1、2、4、8 h不同胁迫时间以及50 mmol L-1 NaCl、100 mmol L-1 NaCl处理组植株叶片的总RNA; 分别提取对照组和干旱胁迫处理8 h植株的根、茎、叶总RNA以及0.5、1、2、4 h不同胁迫处理时间植株叶片的总RNA; 分别提取喷施ABA 0 (喷施清水)、1、2、4、6 h的植株叶片总RNA, 并反转录成cDNA作为荧光定量PCR的模板, 荧光定量PCR引物序列HcTPPJ-QF/QR见表1, 内参基因为β-actin, 内参引物为Actin-QF/QR (表1)。荧光定量PCR的反应体系和反应程序同1.4。1.6 盐和干旱胁迫下红麻脱落酸合成途径关键酶基因HcNCED3的表达特征
分别提取对照组和150 mmol L-1 NaCl胁迫下0.5、1、2、4 h不同时间植株叶片的总RNA; 分别提取对照组和干旱胁迫下0.5、1、2、4 h不同处理时间植株叶片的总RNA, 并反转录成cDNA作为荧光定量PCR的模板, 荧光定量PCR引物序列HcNCED3-QF/QR见表1, 内参基因为β-actin, 内参引物为Actin-QF/QR (表1)。荧光定量PCR的反应体系和反应程序同1.4。2 结果与分析
2.1 红麻HcTPPJ基因的克隆及测序
根据转录组CL541.Contig2Unigene序列设计特异性引物, 以叶片总RNA反转录的cDNA为模板进行PCR扩增, 反应产物经1.2%琼脂糖凝胶电泳检测, 结果如图1所示。对PCR产物进行切胶回收, 然后连接到pMD19-T载体上, 送生工生物工程(上海)股份有限公司测序。图 1
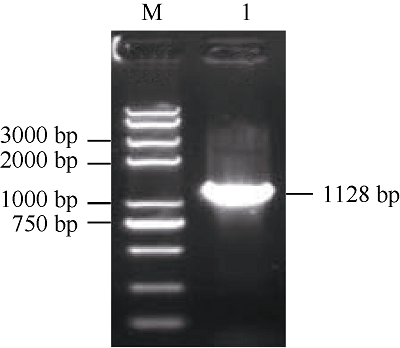 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图 1HcTPPJ cDNA全长琼脂糖凝胶电泳
M: DNA marker 2K Plus II; 1: PCR产物。
Fig. 1Agarose gel electrophoresis of cDNA full length of HcTPPJ
M: DNA marker 2K Plus II; 1: PCR product.
2.2 红麻HcTPPJ基因的生物信息学分析
红麻HcTPPJ基因的PCR扩增产物经上海生工sanger测序后发现, HcTPPJ基因cDNA序列全长1128 bp, 经ORF finder在线预测, 该基因最大开放读码框为1128 bp, 编码一个含有375个氨基酸的多肽蛋白, 其理论分子量为42.5 kD, 等电点为9.23。经NCBI在线工具BLASTP序列比对后发现, HcTPPJ编码的氨基酸序列与可可树(XP007047993.1)、木槿(XP021300935.1)、榴莲(XP022752277.1)、雷蒙德氏棉(XP012469771.1)、橡胶树(XP021678420.1)、毛果杨(XP002307096.2)、石榴(XP031395344.1)中TPPJ氨基酸序列的相似性分别为92.53%、92.53%、91.73%、88.80%、80.05%、79.95%、79.09%。利用DNAMAN软件将以上物种的TPPJ氨基酸序列同水稻、拟南芥的TPPJ氨基酸序列进行一致性比对分析发现, 其氨基酸序列的一致性为71.18%, TPPJ氨基酸序列在不同的物种间具有高度的相似性(图2)。推测该基因可能在不同的物种具有相似的生物学功能, 故命名该基因为HcTPPJ。利用MEGA6.0软件构建HcTPPJ基因系统进化树, 结果如图3所示。红麻与木槿的亲缘关系最近, 这可能是因为两者同属锦葵科木槿属。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2红麻HcTPPJ与其他植物TPPJ蛋白氨基酸序列一致性比对
OsTPP1: 水稻; AtTPPA、AtTPPB、AtTPPD: 拟南芥; TPPJ: 可可树、木槿、榴莲、雷蒙德氏棉、毛果杨、红麻、橡胶树、石榴。不同颜色代表不同氨基酸残基的保守性。蓝色表示氨基酸完全保守; 粉红色、青色、黄色分别表示氨基酸的保守性为75%以上、50%以上及33%以上; 白色表示氨基酸的保守性不足33%。
Fig. 2Amino acid sequence alignment of HcTPPJ and TPPJ proteins from other plants
OsTPP1: Oryza sativa; AtTPPA, AtTPPB, AtTPPD: Arabidopsis thaliana; TPPJ: Theobroma cacao, Hibiscus syriacus, Durio zibethinus, Gossypium raimondii, Populus trichocarpa, Hibiscus cannabinus, Hevea brasiliensis, Punica granatum. The different colors indicate that conservatism of different amino acid residues. The blue means amino acids are complete conservation, the pink, cyan, yellow mean that the conservation of amino acids is no less than 75%, no less than 50%, no less than 33%, respectively, and white mean no more than 33%.
图3
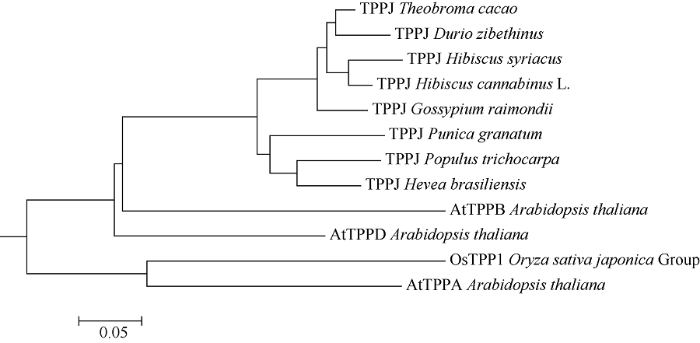 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3红麻HcTPPJ基因与其他植物TPPJ基因系统进化树
TPPJ: 可可树、木槿、榴莲、雷蒙德氏棉、毛果杨、红麻、橡胶树、石榴; AtTPPA、AtTPPB、AtTPPD: 拟南芥; OsTPP1: 水稻。
Fig. 3Phylogenetic tree of HcTPPJ and TPPJ from other plants
TPPJ: Theobroma cacao, Hibiscus syriacus, Durio zibethinus, Gossypium raimondii, Populus trichocarpa, Hibiscus cannabinus, Hevea brasiliensis, Punica granatum; AtTPPA, AtTPPB, AtTPPD: Arabidopsis thaliana; OsTPP1: Oryza sativa.
2.3 红麻HcTPPJ基因在不同器官的表达特征
由图4可知, HcTPPJ基因在红麻植株的根、茎、叶中均有表达, 且其在根中的表达量高于茎、叶中。说明HcTPPJ是一个组成型表达基因。图4
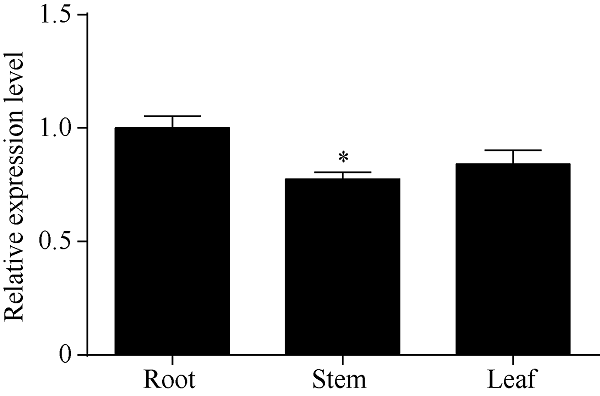 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4HcTPPJ基因在不同器官的表达
* 表示在0.05水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 4Expression of HcTPPJ gene in different organs
* indicates significantly different at the 0.05 probability level. Error bars represent the standard error of each treating group (n = 3).
2.4 盐胁迫下红麻HcTPPJ基因的表达特征
由图5可知, 150 mmol L-1 NaCl溶液胁迫3 d后, 根、茎、叶中HcTPPJ基因的表达显著上调, 与对照相比, 该基因在根、叶中的上调表达量达到了极显著差异水平(P<0.01)。由图6可知, 叶片中HcTPPJ基因的表达量随着NaCl溶液浓度的增加先降低后升高, 当NaCl溶液浓度为150 mmol L-1时, HcTPPJ基因的表达量与对照(0 mmol L-1 NaCl)差异达极显著水平(P<0.01)。由图7可知, 在150 mmol L-1 NaCl溶液胁迫下, 随着时间的增加, HcTPPJ基因在叶片中的表达量呈现先下降后上升然后再下降的变化趋势, 当NaCl溶液胁迫4 h时, HcTPPJ基因的表达量达到最高, 且与对照相比达极显著差异水平(P<0.01)。这可能是因为低浓度的NaCl胁迫或短时间的NaCl胁迫能够促进红麻的生长, 而高浓度或者长时间的胁迫会抑制红麻的生长。表明HcTPPJ基因的表达受盐胁迫的诱导, 可以进一步研究其在红麻响应盐胁迫过程的作用。图5
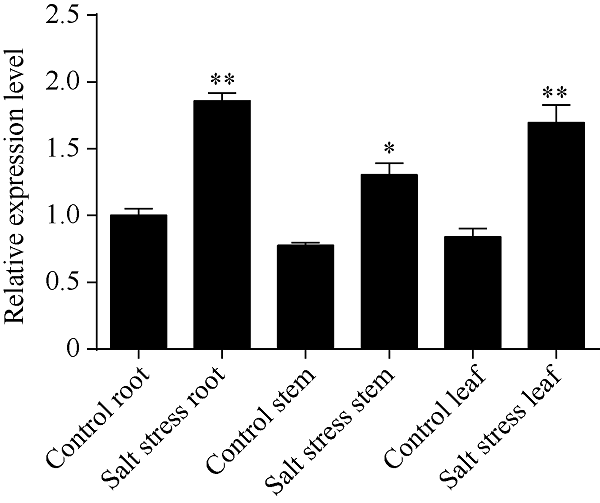 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5150 mmol L-1 NaCl胁迫3 d后HcTPPJ基因在不同器官的表达
*与**分别表示在0.05和0.01水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 5Expression of HcTPPJ in different organs after three days under 150 mmol L-1 NaCl stress
* and ** indicate significantly different at the 0.05 and 0.01 probability levels, respectively. Error bars represent the standard error of each treating group (n = 3).
图6
 新窗口打开|下载原图ZIP|生成PPT
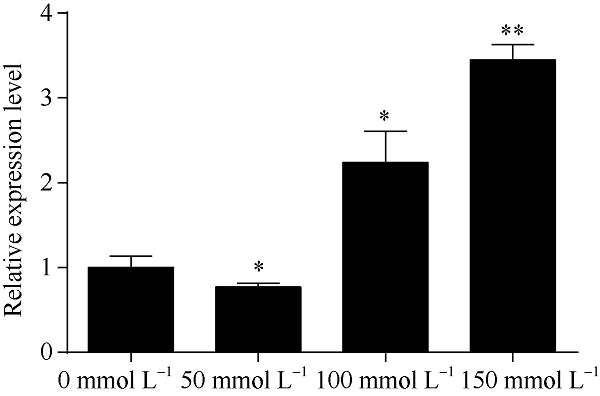
新窗口打开|下载原图ZIP|生成PPT图6不同NaCl浓度胁迫下HcTPPJ基因在叶片的表达
*与**分别表示在0.05和0.01水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 6Expression of HcTPPJ in leaves under different NaCl concentration stress
* and ** indicate significantly different at the 0.05 and 0.01 probability levels, respectively. Error bars represent the standard error of each treating group (n = 3).
图7
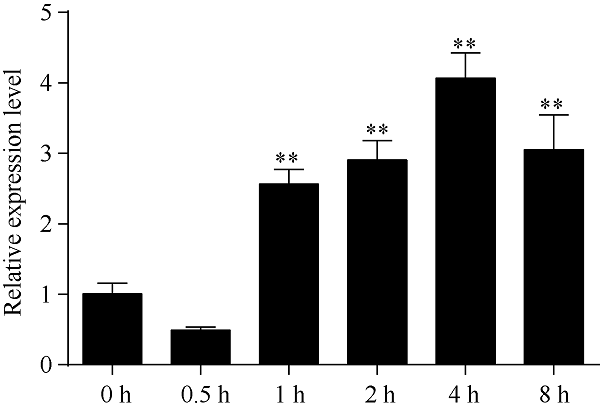 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7150 mmol L-1 NaCl胁迫下不同时间HcTPPJ基因在叶片的表达
**表示在0.01水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 7Expression of HcTPPJ in leaves under 150 mmol L-1 NaCl stress at different times
** indicate significantly different at the 0.01 probability level. Error bars represent the standard error of each treating group (n = 3).
2.5 干旱胁迫下红麻HcTPPJ基因的表达特征
干旱胁迫8 h时, 红麻植株根、茎、叶中HcTPPJ基因均上调表达, 且在干旱胁迫下, HcTPPJ基因在根、茎、叶的表达量与对照相比差异显著(P<0.05)(图8-A)。随着干旱胁迫时间的增加, HcTPPJ基因在叶片中的表达量呈先下降再上升然后再下降的变化趋势(图8-B), 在干旱胁迫4 h时, HcTPPJ基因的表达量最高, 且与对照相比差异极显著(P<0.01)。这可能是因为短期的干旱胁迫有利于红麻的生长, 而随着干旱胁迫时间的延长, 红麻的生长逐渐受到抑制。表明HcTPPJ基因的表达受干旱胁迫的诱导。图8
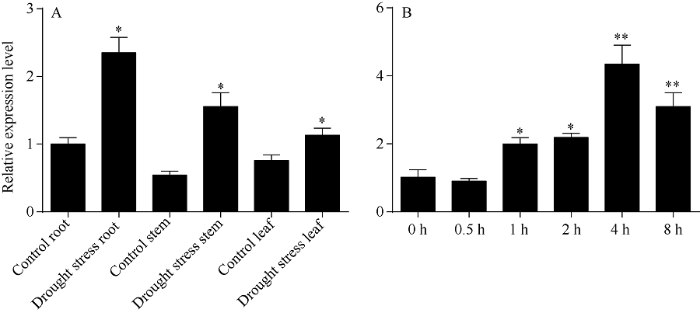 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8干旱胁迫8 h HcTPPJ在不同器官的表达(A)和干旱胁迫下不同时间HcTPPJ基因在叶片的表达(B)
*与**分别表示在0.05和0.01水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 8Expression of HcTPPJ in different organs under drought stress 8 h (A) and expression of HcTPPJ in leaves under drought stress at different times (B)
* and ** indicate significantly different at the 0.05 and 0.01 probability levels, respectively. Error bars represent the standard error of each treating group (n = 3).
2.6 盐、干旱胁迫下红麻脱落酸(ABA)合成途径关键基因的表达特征
由图9可知, 随着盐胁迫时间的延长, HcNCED3基因的表达量先下降后上升然后再下降后上升; 随着干旱胁迫时间的延长, HcNCED3基因的表达量先上升再下降。在盐、干旱胁迫0.5 h后, HcNCED3基因表达量总体呈现上调趋势, 其表达量与对照相比差异达极显著水平(P<0.01)。表明, 在盐、干旱胁迫下, 红麻植株体内ABA的含量增加, 且ABA可能在不同的时间诱导不同的基因表达。图9
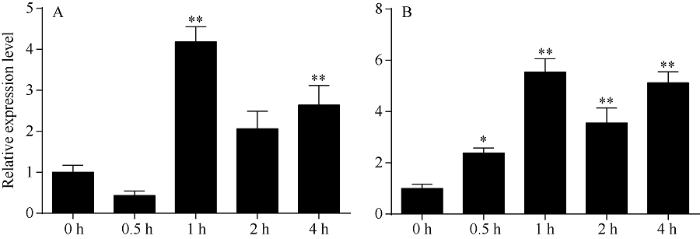 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9盐(A)、干旱(B)胁迫下HcNCED3基因在不同时间的表达
*与**分别表示在0.05和0.01水平下差异显著。误差线为每组处理的标准误差(n = 3)。
Fig. 9Expression of HcNCED3 at different times under salt (A) or drought (B) stress
* and ** indicate significantly different at the 0.05 and 0.01 probability levels, respectively. Error bars represent the standard error of each treating group (n = 3).
2.7 外源激素ABA调控红麻HcTPPJ基因的表达特征
ABA在植物响应逆境胁迫过程中具有重要的调控作用。为了明确HcTPPJ基因是否受ABA的调控, 本研究利用荧光定量PCR技术分析了喷施200 μmol L-1 ABA溶液后, 红麻叶片HcTPPJ基因的表达特征。由图10可知, 喷施ABA后, 随着时间的延长, HcTPPJ基因在叶片中的表达量先下降后上升, 但是同对照相比, 其下调和上升的表达量都不明显。由此推测, HcTPPJ基因的表达可能不受ABA的诱导。图10
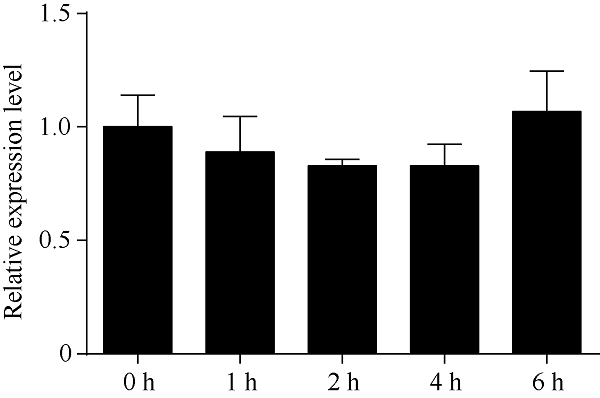 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10ABA胁迫下HcTPPJ基因在不同时间的表达
误差线为每组处理的标准误差(n = 3)。
Fig. 10Expression of HcTPPJ at different times under ABA stress
Error bars represent the standard error of each treating group (n = 3).
3 讨论
海藻糖是一种稳定的可溶性非还原糖, 能够作为渗透调节物质来提高植物适应非生物逆境胁迫的能力。李昉峻等[20]研究表明, 镉胁迫下海藻糖能够显著抑制水稻幼苗对镉离子的吸附和积累。庞椿朋等[21]研究表明, 高温胁迫下, 对番茄叶片喷施1.0%的海藻糖溶液能够显著促进番茄叶片的光合作用。在植物体内, TPP是海藻糖合成的关键酶, 因此, 研究TPP基因的生物功能对提高植物的抗逆性具有重要意义。TPP基因在植物响应非生物逆境胁迫过程中具有重要作用。杨雪等[22]的研究表明, 同对照相比, 干旱和盐胁迫下OsTPP3基因在水稻幼苗根和芽中的表达量显著提高。丁泽红等[23]研究表明, 在干旱胁迫下, MeTPP6基因在木薯根和叶片中的表达量显著上升。本研究通过荧光定量PCR分析发现, 在盐、干旱胁迫下, HcTPPJ基因在红麻植株根、茎、叶中的表达量显著上升, 随着盐胁迫浓度的提高, 盐胁迫、干旱胁迫时间的延长, 叶片中HcTPPJ基因的表达量显著上升。表明HcTPPJ基因的表达受盐、干旱胁迫的诱导, 推测其在红麻响应盐、干旱胁迫过程中具有重要作用。
脱落酸是广泛分布在植物体内的天然激素, 同时作为重要的信号分子能够诱导某些抗逆基因的表达, 提高植物的耐逆性。在高盐、干旱、低温等逆境胁迫下具有重要作用, 且受ABA诱导表达的基因已陆续被克隆, 如红麻HcWD40[18]、番茄SINAC41[24]等。本研究通过荧光定量分析发现, 在红麻叶片中HcNCED3基因的表达受盐、干旱胁迫诱导, 且随着胁迫时间的延长, HcNCED3的表达量显著上升, 这表明红麻植株体内ABA的含量在不断增加。
在盐、干旱胁迫下, 红麻叶片HcTPPJ基因与ABA合成途径关键酶基因HcNCED3都呈现上调表达趋势。为了研究HcTPPJ基因的表达是否受ABA信号分子的诱导, 本研究以ABA作为信号分子, 对叶片喷施200 μmol L-1 ABA溶液。通过荧光定量分析发现, 喷施ABA溶液后随着时间的延长, HcTPPJ基因在红麻叶片中的表达量变化不明显, 这表明HcTPPJ基因的表达不受ABA的诱导。
4 结论
HcTPPJ基因可能在红麻响应盐、干旱胁迫过程中具有重要作用。在红麻响应盐、干旱胁迫过程中, HcTPPJ基因表达可能不受ABA的信号调控。关于HcTPPJ基因如何在红麻响应盐、干旱胁迫过程中发挥作用还需要进一步的研究。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1093/glycob/cwg047URLPMID:12626396 [本文引用: 1]

Trehalose is a nonreducing disaccharide in which the two glucose units are linked in an alpha,alpha-1,1-glycosidic linkage. This sugar is present in a wide variety of organisms, including bacteria, yeast, fungi, insects, invertebrates, and lower and higher plants, where it may serve as a source of energy and carbon. In yeast and plants, it may also serve as a signaling molecule to direct or control certain metabolic pathways or even to affect growth. In addition, it has been shown that trehalose can protect proteins and cellular membranes from inactivation or denaturation caused by a variety of stress conditions, including desiccation, dehydration, heat, cold, and oxidation. Finally, in mycobacteria and corynebacteria, trehalose is an integral component of various glycolipids that are important cell wall structures. There are now at least three different pathways described for the biosynthesis of trehalose. The best known and most widely distributed pathway involves the transfer of glucose from UDP-glucose (or GDP-glucose in some cases) to glucose 6-phosphate to form trehalose-6-phosphate and UDP. This reaction is catalyzed by the trehalose-P synthase (TPS here, or OtsA in Escherichia coli ). Organisms that use this pathway usually also have a trehalose-P phosphatase (TPP here, or OtsB in E. coli) that converts the trehalose-P to free trehalose. A second pathway that has been reported in a few unusual bacteria involves the intramolecular rearrangement of maltose (glucosyl-alpha1,4-glucopyranoside) to convert the 1,4-linkage to the 1,1-bond of trehalose. This reaction is catalyzed by the enzyme called trehalose synthase and gives rise to free trehalose as the initial product. A third pathway involves several different enzymes, the first of which rearranges the glucose at the reducing end of a glycogen chain to convert the alpha1,4-linkage to an alpha,alpha1,1-bond. A second enzyme then releases the trehalose disaccharide from the reducing end of the glycogen molecule. Finally, in mushrooms there is a trehalose phosphorylase that catalyzes the phosphorolysis of trehalose to produce glucose-1-phosphate and glucose. This reaction is reversible in vitro and could theoretically give rise to trehalose from glucose-1-P and glucose. Another important enzyme in trehalose metabolism is trehalase (T), which may be involved in energy metabolism and also have a regulatory role in controlling the levels of trehalose in cells. This enzyme may be important in lowering trehalose concentrations once the stress is alleviated. Recent studies in yeast indicate that the enzymes involved in trehalose synthesis (TPS, TPP) exist together in a complex that is highly regulated at the activity level as well as at the genetic level.
DOI:10.1104/pp.17.01634URLPMID:29592862 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.112.201400URLPMID:22855938 [本文引用: 1]

Trehalose is a nonreducing sugar used as a reserve carbohydrate and stress protectant in a variety of organisms. While higher plants typically do not accumulate high levels of trehalose, they encode large families of putative trehalose biosynthesis genes. Trehalose biosynthesis in plants involves a two-step reaction in which trehalose-6-phosphate (T6P) is synthesized from UDP-glucose and glucose-6-phosphate (catalyzed by T6P synthase [TPS]), and subsequently dephosphorylated to produce the disaccharide trehalose (catalyzed by T6P phosphatase [TPP]). In Arabidopsis (Arabidopsis thaliana), 11 genes encode proteins with both TPS- and TPP-like domains but only one of these (AtTPS1) appears to be an active (TPS) enzyme. In addition, plants contain a large family of smaller proteins with a conserved TPP domain. Here, we present an in-depth analysis of the 10 TPP genes and gene products in Arabidopsis (TPPA-TPPJ). Collinearity analysis revealed that all of these genes originate from whole-genome duplication events. Heterologous expression in yeast (Saccharomyces cerevisiae) showed that all encode active TPP enzymes with an essential role for some conserved residues in the catalytic domain. These results suggest that the TPP genes function in the regulation of T6P levels, with T6P emerging as a novel key regulator of growth and development in higher plants. Extensive gene expression analyses using a complete set of promoter-beta-glucuronidase/green fluorescent protein reporter lines further uncovered cell- and tissue-specific expression patterns, conferring spatiotemporal control of trehalose metabolism. Consistently, phenotypic characterization of knockdown and overexpression lines of a single TPP, AtTPPG, points to unique properties of individual TPPs in Arabidopsis, and underlines the intimate connection between trehalose metabolism and abscisic acid signaling.
DOI:10.1046/j.1365-313x.1998.00064.xURLPMID:9681009 [本文引用: 1]

It is currently thought that most flowering plants lack the capacity to synthesize trehalose, a common disaccharide of bacteria, fungi and invertebrates that appears to play a major role in desiccation tolerance. Attempts have therefore been made to render plants more drought-resistant by the expression of microbial genes for trehalose synthesis. It is demonstrated here that Arabidopsis thaliana itself possesses genes for at least one of the enzymes required for trehalose synthesis, trehalose-6-phosphate phosphatase. The yeast tps2 mutant, which lacks this enzyme, is heat-sensitive, and Arabidopsis cDNA able to complement this effect has been screened for. Half of the yeast transformants that grew at 38.6 degrees C were also able to produce trehalose. All of these expressed one of two Arabidopsis cDNA, either AtTPPA or AtTPPB, which are both homologous to the C-terminal part of the yeast TPS2 gene and other microbial trehalose-6-phosphate phosphatases. Yeast tps2 mutants expressing AtTPPA or AtTPPB contained trehalose-6-phosphate phosphatase activity that could be measured both in vivo and in vitro. The enzyme dephosphorylated trehalose-6-phosphate but not glucose-6-phosphate or sucrose-6-phosphate. Both genes are expressed in flowers and young developing tissue of Arabidopsis. The finding of these novel Arabidopsis genes for trehalose-6-phosphate phosphatase strongly indicates that a pathway for trehalose biosynthesis exists in plants.
DOI:10.1089/ars.2013.5693URL [本文引用: 1]
DOI:10.1007/s11103-005-7404-4URL [本文引用: 1]

Trehalose serves as a stress protectant and/or reserve carbohydrate in a variety of organisms including bacteria, yeast, and invertebrates. Recently, trace amounts of trehalose have been detected in higher plants, although the function of trehalose in plants remains unknown. A cDNA clone (OsTPP1) encoding a putative trehalose-6-phosphate phosphatase (TPP) for trehalose biosynthesis was isolated from rice. Functionality of the clone was demonstrated by complementation of a yeast mutant and enzymatic activity of the recombinant protein. Northern blots revealed that the OsTPP1 transcript levels were fairly low or under detectable limits in most of the tissues under ambient conditions but were highly induced within 1–2h of chilling stress (12°C) in both root and shoot tissues of seedlings. This induction was transient and disappeared after 6h of the chilling stress. Transient expression of OsTPP1 was also induced under severe chilling stress (4°C) as well as salinity and drought stresses at ambient temperatures. Application of exogenous ABA (50μM) resulted in a transient increase of OsTPP1 expression within 20min of the treatment, thereby suggesting involvement of ABA in OsTPP1 gene regulation. Measurements of total cellular TPP activity and trehalose content in roots indicated that both TPP activity and trehalose levels were transiently increased after chilling (12°C) stress. Collectively, the data indicate that transient activation of trehalose biosynthesis is involved in early chilling stress response in rice. Possible functions of trehalose in the early stages of chilling stress response are discussed.]]>
DOI:10.1007/s00425-008-0729-xURL [本文引用: 1]

Trehalose plays a protective role in yeast and microorganisms under abiotic stresses. However, little is known about its role in higher plants when subjected to environmental challenges. A systematic search of rice databases discovered a large TPS/TPP gene family in the rice genome, which is similar to that found in Arabidopsis thaliana, especially in the gene family structure. Expression analysis demonstrated that OsTPP1 was initially and transiently up-regulated after salt, osmotic and abscisic acid (ABA) treatments but slowly up-regulated under cold stress. OsTPP1 overexpression in rice enhanced tolerance to salt and cold stress. Analysis of the overexpression lines revealed that OsTPP1 triggered abiotic stress response genes, which suggests a possible transcriptional regulation pathway in stress induced reprogramming initiated by OsTPP1. The current study revealed the mechanism of an OsTPP gene involved in stress tolerance in rice and also suggested the use of OsTPP1 in abiotic stress engineering of crops.]]>
DOI:10.1046/j.1365-313x.2001.01096.xURLPMID:11532178 [本文引用: 1]

Abscisic acid (ABA), a plant hormone, is involved in responses to environmental stresses such as drought and high salinity, and is required for stress tolerance. ABA is synthesized de novo in response to dehydration. 9-cis-epoxycarotenoid dioxygenase (NCED) is thought to be a key enzyme in ABA biosynthesis. Here we demonstrate that the expression of an NCED gene of Arabidopsis, AtNCED3, is induced by drought stress and controls the level of endogenous ABA under drought-stressed conditions. Overexpression of AtNCED3 in transgenic Arabidopsis caused an increase in endogenous ABA level, and promoted transcription of drought- and ABA-inducible genes. Plants overexpressing AtNCED3 showed a reduction in transpiration rate from leaves and an improvement in drought tolerance. By contrast, antisense suppression and disruption of AtNCED3 gave a drought-sensitive phenotype. These results indicate that the expression of AtNCED3 plays a key role in ABA biosynthesis under drought-stressed conditions in Arabidopsis. We improved drought tolerance by gene manipulation of AtNCED3 causing the accumulation of endogenous ABA.
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.plantsci.2009.09.014URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1007/s10265-016-0898-9URLPMID:27999968 [本文引用: 1]

Kenaf (Hibiscus cannabinus L.) is an economically important global natural fiber crop. As a consequence of the increased demand for food crops and the reduction of available arable land, kenaf cultivation has increasingly shifted to saline and alkaline land. To investigate the molecular mechanism of salinity tolerance in kenaf, we performed Illumina high-throughput RNA sequencing on shoot tips of kenaf and identified 71,318 unigenes, which were annotated using four different protein databases. In total, 2,384 differentially expressed genes (DEGs) were identified between the salt-stressed and the control plants, 1,702 of these transcripts were up-regulated and 683 transcripts were down-regulated. Thirty-seven transcripts belonging to 15 transcription-factor families that respond to salt stress were identified. Gene ontology function enrichment analysis revealed that the genes encoding antioxidant enzymes were up-regulated. The amino acid metabolism and carbohydrate metabolism pathways were highly enriched among these DEGs under salt stress conditions. In order to confirm the RNA-seq data, we randomly selected 20 unigenes for analysis using a quntitative real-time polymerase chain reaction. Our study not only provided the large-scale assessment of transcriptome resources of kenaf but also guidelines for understanding the mechanism underlying salt stress responses in kenaf.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
