 ,, 谢赛, 王超智, 李焱龙, 张献龙, 闵玲
,, 谢赛, 王超智, 李焱龙, 张献龙, 闵玲 ,*华中农业大学植物科学技术学院 / 作物遗传改良国家重点实验室, 湖北武汉 430070
,*华中农业大学植物科学技术学院 / 作物遗传改良国家重点实验室, 湖北武汉 430070Mechanism of GhPIF4 regulating anther abortion under high temperature stress in cotton
CHEN Miao ,, XIE Sai, WANG Chao-Zhi, LI Yan-Long, ZHANG Xian-Long, MIN Ling
,, XIE Sai, WANG Chao-Zhi, LI Yan-Long, ZHANG Xian-Long, MIN Ling ,*National Key Laboratory of Crop Genetic Improvement / College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei, China
,*National Key Laboratory of Crop Genetic Improvement / College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei, China通讯作者:
收稿日期:2019-11-30接受日期:2020-03-24网络出版日期:2020-09-12
| 基金资助: |
Received:2019-11-30Accepted:2020-03-24Online:2020-09-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (5422KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈淼, 谢赛, 王超智, 李焱龙, 张献龙, 闵玲. 棉花GhPIF4调控高温下花药败育机制初探[J]. 作物学报, 2020, 46(9): 1368-1379. doi:10.3724/SP.J.1006.2020.94188
CHEN Miao, XIE Sai, WANG Chao-Zhi, LI Yan-Long, ZHANG Xian-Long, MIN Ling.
棉花、玉米、小麦和水稻等许多作物的价值部位均与果实和种子密切相关, 其产量均建立于生殖发育的基础上, 这些作物的生殖发育过程除受作物本身的基因型决定外, 环境因素也会影响这一进程, 并最终决定作物产量。一方面, 利用环境因素可以开展特殊育种, 如水稻光敏和光温敏核不育系的选育[1,2,3]; 另一方面, 高温、干旱等环境因素也会导致雄性败育进而造成作物减产[4,5,6,7], 因而环境因素特别是高温造成雄性败育的机制值得深入探究。
环境因素影响下的植物生理生化改变涉及复杂的分子网络, 在模式植物拟南芥中, PIF4 (Phytochrome-interacting factor 4)是响应光信号的一个关键因子, 其隶属于PIF家族, PIF蛋白是一类受光调控的bHLH类型转录因子[8,9,10,11,12], 同时PIF4也是一个温度响应的关键因子, 拟南芥热形态建成中的下胚轴伸长、气孔数量减少和花期提前等过程均受PIF4的调控[13,14]。PIF4对温度变化的响应来自多方面, 首先, 红光受体phyB也具有温度感应的功能, 高温能促进phyB由Pfr状态向Pr状态转换, Pfr状态phyB丰度的下降减缓了其对PIF4的抑制作用[15,16]; 在表达量水平上, 一方面, 高温可能会直接降低PIF4转录抑制因子ELF3 (Early flowering 3)的活性从而促进PIF4的表达[17], 另一方面, 高温会影响PIF4启动子区域的一些表观遗传修饰, 如促进H3K9去乙酰化等来提升PIF4的表达水平[18]。同时, PIF4、PIF7和phyB能随着日照变短减缓对CBF (C-repeat binding factor)途径的抑制作用从而提高拟南芥耐冷性。PIF4也参与调控拟南芥昼夜节律等同时受到温度和光调控的过程[19], 以及抑制拟南芥在高温下的免疫力和抑制红光下花青素合成等过程[20,21]。PIF4对拟南芥生理过程的调控在一定程度上与激素途径密切相关, PIF4可以直接促进生长素合成[22,23,24], 并可与BZR1 (Brassinazole resistant 1)和ARF6 (Auxin response factor 6)形成BAP复合体协同调控一些共有靶标基因的转录[25,26]。
本实验室前期研究表明高温胁迫会扰乱棉花花药中的糖代谢途径和生长素途径并导致花药败育[27], 而GhCKI同时参与糖代谢和生长素途径, 是高温胁迫下高温敏感型材料‘H05’花药败育的重要因子[27,28]。GhCKI可以磷酸化GhTCP15, 磷酸化的GhTCP15与GhPIF4的启动子结合增强, 从而促进早期体胚发育中生长素的合成进而影响细胞增殖[29]。因此我们推测棉花中GhPIF4可能是参与高温响应并影响生长素稳态的一个关键因子。本研究从陆地棉‘YZ1’中克隆了GhPIF4基因和启动子, 研究了GhPIF4在多个棉花材料中的组织表达情况, 确定了GhPIF4蛋白作用于细胞核, 并对GhPIF4进行了遗传转化, 旨在进一步了解高温胁迫下棉花花药败育的机制, 为耐高温棉花分子育种提供理论基础。
1 材料与方法
1.1 植物材料
本研究中用来进行高温处理的材料为陆地棉(Gossypium hirsutum)‘YZ1’和高温敏感型陆地棉品系‘H05’。用于组织表达分析的材料为陆地棉‘H05’, 以陆地棉材料‘YZ1’作为转化目的基因GhPIF4的受体材料。用于亚细胞定位试验的材料为本氏烟草(Nicotiana benthamiana)。温室种植‘H05’, 于盛蕾时期设定白天28~35℃持续12 h, 晚上20~25℃持续12 h为正常条件, 高温条件为白天37~39℃持续12 h, 晚上29~31℃持续12 h, 高温处理周期为7 d。本氏烟草生长温度设定为光照时25℃, 黑暗时20℃; 光周期为16 h光照和8 h黑暗。1.2 载体与菌株
pGEM-T Easy载体用于TA克隆; pDONOR221为BP-LR反应入门载体; 用于GhPIF4遗传转化的35S:GhPIF4:His 载体基于 pGBW409载体构建; 用于亚细胞定位的载体为pMDC43; pGWB433为启动子表达模式分析所用载体, 用于构建 proGhPIF4:GUS (表1)。载体转化的大肠杆菌受体为TOP10; 棉花下胚轴的转化及烟草的侵染载体为农杆菌菌株GV3101 (表1)。Table 1
表1
表1实验中用到的载体和菌株
Table 1
| 载体/菌株 Vector/strain | 用途 Purpose |
|---|---|
| pGEM-T Easy载体 pGEM-T Easy vector | TA克隆 TA clone |
| pDONOR221载体 pDONOR221 vector | BP反应 BP reaction |
| pGBW409载体 pGBW409 vector | 35S:GhPIF4:His载体的构建 Construction of 35S:GhPIF4:His vector |
| pMDC43载体 pMDC43 vector | GhPIF4亚细胞定位 Subcelluar localization of GhPIF4 |
| pGWB433载体 pGWB433 vector | GhPIF4启动子表达模式分析 Analysis of GhPIF4 promoter expression pattern |
| 大肠杆菌TOP10 E. coli TOP10 | 载体转化 Vector transformation |
| 农杆菌GV3101 Agrobacterium GV3101 | 棉花下胚轴的转化及烟草的侵染 Transformation of cotton hypocotyl and tobacco infection |
新窗口打开|下载CSV
1.3 载体的构建
使用引物GhPIF4-CDS-F和GhPIF4-CDS-R (表2)在‘YZ1’花药cDNA中扩增GhPIF4的编码序列, 通过BP反应将带有BP接头的GhPIF4编码序列(表2)重组至入门载体pDONOR221上, 然后LR反应将GhPIF4编码序列重组至目的载体pMDC43上, 从而获得35S:GhPIF4:GFP 和35S:GFP (不含GhPIF4编码序列)的重组载体。Table 2
表2
表2本实验中所用的引物及用途
Table 2
| 引物名称Primer name | 引物序列Primer sequence (5°-3°) | 用途Purpose |
|---|---|---|
| GhPIF4-CDS-F | ATGGATCACCAACATGAACAACA | Gene amplification |
| GhPIF4-CDS-R | TCAGTTAAATCCCGGATTGGCAG | Gene amplification |
| GhPIF4-CDS-BP-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTGGATGGAT CACCAACATGAACAACA | BP reaction |
| GhPIF4-CDS-BP-R | GGGGACCACTTTGTACAAGAAAGCTGGGTGTCAGTTA AATCCCGGATTGGCAG | BP reaction |
| proGhPIF4-F | TTTTTGTCTCCCATTACAGTATC | Gene promoter amplification |
| proGhPIF4-R | GGGTCATAAACTGGAAATTCAGA | Gene promoter amplification |
| GhUB7-qRT-F | CTTGACCTTCTTCTTCTTGTGCTTG | qRT-PCR |
| GhUB7-qRT-R | GAAGGCATTCCACCTGACCAAC | qRT-PCR |
| GhPIF4-CDS-qRT-F | AACTACACCTCAAAGTCCCACGG | qRT-PCR |
| GhPIF4-CDS-qRT-R | CCCGGATTGGCAGTGGTC | qRT-PCR |
| GhTAA1-qRT-F | GGTCTTAAAAAGGTTGGGGCTTA | qRT-PCR |
| GhTAA1-qRT-R | TTAGCTTGGGTATGTGTTTGATTTG | qRT-PCR |
| GhYUC2-qRT-F | ACCGATGTGGGTTTTGGCGAAT | qRT-PCR |
| GhYUC2-qRT-R | CTCAGCATTTTCCCCGGTAGCA | qRT-PCR |
| GhCYP71A13-qRT-F | CGTAAACAGACCGAAACGCAGC | qRT-PCR |
| GhCYP71A13-qRT-R | GTGGTTGCGGAAAAGAGTTCGC | qRT-PCR |
新窗口打开|下载CSV
扩增GhPIF4的编码序列, 通过TA克隆将扩增到的编码序列连接至pGEM-T Easy载体上, 经含有氨苄抗性固体LB培养基筛选后, 挑取阳性克隆进行菌体 PCR 检验并送测序, 以测序后无突变的pGEM-T Easy载体质粒为模板, 通过BP反应将带有BP接头的GhPIF4编码序列(表2)重组至入门载体pDONOR221上, 然后LR反应将GhPIF4编码序列重组至目的载体pGWB409上, 从而获得35S: GhPIF4:His重组载体。
proGhPIF4:GUS载体的构建与35S:GhPIF4:His构建方法一致, 不同的是扩增proGhPIF4:GUS载体的是GhPIF4启动子序列, 目的载体为pGWB433。
1.4 主要酶类与试剂
DNA提取、质粒抽提及PCR纯化试剂盒购自天根公司, qRT-PCR试剂购自ABI公司, 反转录酶M-MLV Reverse Transcriptase购自 Promega公司。载体构建过程中所用的抗生素氨苄青霉素、卡那霉素、利福平等均购自Sigma公司。1.5 DNA的精提取与Southern杂交
1.5.1 DNA的精提取 取棉花幼嫩叶片, 参照DNA提取试剂盒(天根生化科技DP305-03)方法提取DNA。1.5.2 Southern杂交 取15~20 μg质量较好的棉花叶片基因组DNA用Hind III (NEB, USA), 37℃充分酶切72 h, 并在0.8%琼脂糖凝胶上电泳分离, 通过碱转移方法转移到Hybond-N+尼龙膜上, 固定DNA。探针标记的方法采用地高辛标记(Roche), 具体实验操作方法见胡海燕博士学位论文[30]。
1.6 棉花RNA的提取
采用天根RNAprep Pure多糖多酚植物总RNA提取试剂盒(天根生化科技DP441) 提取棉花不同组织RNA。1.7 RNA反转录
(1) 取3 μg RNA于0.5 mL无RNA酶离心管中, 加入1 μL oligo(dT), 用DEPC水补充体积至15 μL, 并简单离心。(2) 将上述混合体系置PCR仪中, 设置程序为70℃ 10 min, 之后冰上冷却10 min。(3) 向混合物中加入: 5 μL 5×MLV buffer, 1 μL RNasin, 1 μL M-MLV RTase, 1.25 μL 10 mmol L-1 dNTP和1.75 μL DEPC水。充分混合后, 于PCR仪中42℃反应60 min, 然后70℃反应7 min, 反应结束后将产物放置于-20℃保存。1.8 实时荧光定量PCR (quantitative real-time PCR, qRT-PCR)
根据基因序列设计特异性定量PCR引物(表2), 将反转的cDNA模板稀释100倍, 反应体系为: 6.5 μL稀释的cDNA模板, 6.5 μL SYBR Green Master Mix Reagent (Bio-Rad公司), 正反引物各0.5 μL, 混匀。用ABI 7500实时荧光定量 PCR仪(Applied Biosystems, USA)设95℃ 30 s, 1个循环; 95℃ 5 s, 60℃ 35 s, 40个循环。内参基因为GhUB7。1.9 GUS染色
取proGhPIF4:GUS转基因阳性植株的不同组织, 浸入GUS染液于37℃染色约6 h, 然后用75%的酒精在常温条件下进行3~5次脱色, 最后用体视显微镜(LEICA MZFL III)及单反数码相机观察及照相。GUS染液(100 mL)含90 mg X-Gluc (5-溴-4-氯- 3-吲哚-β-葡萄糖苷酸), 10 mg氯霉素, 20 mL甲醇, 50 mmol L-1磷酸钾缓冲液(pH 7.0)。
1.10 TTC花粉活性染色
用镊子将开花当天的花药夹到装有TTC染液的离心管中, 在37℃培养箱中反应30 min后, 取出在显微镜下观察。TTC染液(1 L)含8 g TTC (2,3,5-三苯基氯化四氮唑, 分子量334.8), 1 L磷酸缓冲液(三水合磷酸氢二钾26.6 g, 磷酸二氢钾10.2 g)。1.11 亚细胞定位
将35S:GhPIF4:GFP和35S:GFP载体质粒转化农杆菌后, 于28℃摇床中活化菌液, 同时活化等体积的能表达番茄丛矮病毒P19的农杆菌(P19能有效抑制植物对外源导入载体表达的沉默效应, 提高表达量)。待菌液摇至浑浊, 室温3000×g离心10 min弃上清液, 收集菌体, 用适量悬浮液(100 mL悬浊液中含有1 mL 10 mmol L-1 MgCl2、1 mL 10 mmol L-1 MES、200 μL 150 μmol L-1 AS)重悬管底菌体。将P19菌液分别与重悬后的35S:GhPIF4:GFP和35S: GFP菌液等体积混合, 混合后菌液的OD值大致在0.4~0.6, 将混合菌液置28℃摇床活化30 min, 选取本氏烟草壮硕的叶片背面注射菌液, 暗培养24 h后, 光照12 h, 选取注射区域叶肉部分, 叶片背面朝上置激光共聚焦显微镜(Zeiss HAL 100/HBO)下观察荧光。1.12 生长素含量测定
称取0.05 g左右液氮研磨后的花药粉末于2 mL离心管中, 在冰上避光加入750 μL的抽提液(组分为甲醇:ddH2O:乙酸 = 80:19:1, 并添加浓度为10 ng mL-1的2H5-IAA作为IAA内标), 样品于4℃避光抽提12 h后, 4℃、13,000×g离心15 min, 取上清液, 然后向沉淀中加入450 μL抽提缓冲液, 4℃避光振荡抽提3 h后, 4℃、13,000×g离心15 min, 合并2次上清液于新的离心管中, 在氮吹仪中将上清液吹干, 再加入300 μL 80%甲醇充分溶解后, 用0.22 μm滤膜(津腾公司尼龙66)过滤上清液, 然后用高效液相色谱-质谱联用技术(HPLC-MS)检测花药中的IAA含量。1.13 棉花转基因株系遗传转化
将已构建好的35S:GhPIF4:His载体, proGhPIF4:GUS载体利用农杆菌侵染法侵染‘YZ1’的下胚轴, 具体遗传转化操作步骤参照Jin等[31]的方法。2 结果与分析
2.1 GhPIF4的鉴定与基因结构分析
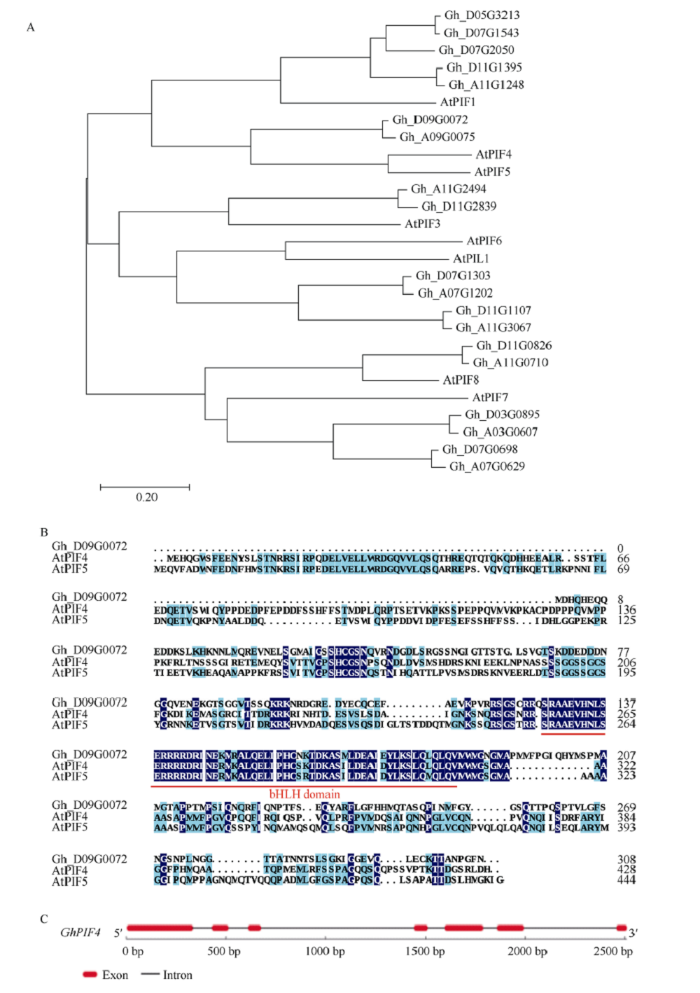
从拟南芥基因组数据库Araport (图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1陆地棉中GhPIFs和 GhPIL1s的进化树及GhPIF4基因结构特征
A: 陆地棉中GhPIFs和 GhPIL1s的进化树分析; B: GhPIF4与AtPIF4和AtPIF5的氨基酸序列比对, 红线表示bHLH domain; C: GhPIF4的基因结构。红色方框表示外显子, 黑线表示内含子。
Fig. 1Phylogenetic tree analysis of GhPIFs and GhPIL1s in Gossypium hirsutum and structural characteristics of GhPIF4 gene
A: the phylogenetic tree analysis of GhPIFs and GhPIL1s in Gossypium hirsutum; B: comparison of amino acid sequences between GhPIF4, AtPIF4 and AtPIF5. Red line indicates bHLH domain; C: gene structure of GhPIF4. Red box and black line indicate exon and intron, respectively.
根据进化分析, 相对于Gh_A09G0075, Gh_ D09G0072与AtPIF4具有更近的亲缘关系, 因而我们将Gh_D09G0072命名为GhPIF4。通过SMART (
2.2 GhPIF4的亚细胞定位
GhPIF4、AtPIF4和AtPIF5具有保守的bHLH结构域(图1-B), 属于bHLH类转录因子。前人研究中发现AtPIF4定位于细胞核[11], 为了检测GhPIF4是否也定位于细胞核, 本研究构建35S:GhPIF4: GFP载体并转化烟草叶片, 通过GFP (green fluorescent protein)融合蛋白的荧光显示目的蛋白的亚细胞定位, 35S:GFP 作为对照。转化36 h后取烟草叶片于激光共聚焦显微镜下观察, 结果35S:GFP在烟草叶肉细胞的细胞核和细胞膜中均有GFP信号, 而35S:GhPIF4:GFP只在细胞核中有绿色荧光信号(图2), 与在拟南芥中报道过的亚细胞定位结果一致。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2GhPIF4蛋白的亚细胞定位
35S:GFP: GFP空载在烟草中的亚细胞定位; 35S:GhPIF4:GFP: GFP 标记的GhPIF4蛋白在烟草中的亚细胞定位。Bar = 20 μm。
Fig. 2Subcelluar localization of GhPIF4
35S:GFP: subcellular localization of the GFP in Nicotiana benthamiana; 35S:GhPIF4:GFP: subcellular localization of the GhPIF4:GFP fusion protein in Nicotiana benthamiana. Bar = 20 μm.
2.3 GhPIF4组织表达及高温诱导表达分析
为了检测GhPIF4的组织表达模式, 选取高温敏感材料‘H05’的根、茎、叶、苞片、萼片、花瓣、开花前一天的花药、柱头和胚珠等组织进行表达量检测。表明GhPIF4在不同的组织中表达相差较大, 在陆地棉‘H05’中, GhPIF4在开花前一天的花药中表达量最高, 约为柱头的3.3倍, 其他组织表达量均低于柱头, 根和花瓣中几乎没有表达(图3-A)。同时在用于转化的陆地棉‘YZ1’的根、下胚轴、茎、叶、花瓣、开花前一天的花药以及发育5 d胚珠中进行组织表达模式分析, 表明在陆地棉‘YZ1’中, GhPIF4也在开花前一天的花药中优势表达(图3-B)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3GhPIF4 组织表达及高温诱导表达分析
A: GhPIF4在‘H05’中的组织表达分析。B: GhPIF4在‘YZ1’中的组织表达分析。C: proGhPIF4:GUS转基因棉花组织表达情况; a: 开花当天的整体花药; b: 开花当天的单个花药; c: 萼片; d: 花瓣; e: 幼嫩的叶子。D: GhPIF4在‘H05’不同发育时期的花药中高温诱导表达分析。a、c、d、e图, bar = 1 cm; b图bar = 100 μm; HN和HH分别表示正常温度和高温胁迫条件下的‘H05’; < 7 mm表示花蕾长度小于7 mm花药的混合; **P < 0.01。
Fig. 3Expression of GhPIF4 in different tissue and induced by high temperature in Gossypium hirsutum
A and B: the expression analysis of GhPIF4 in H05 (A) and YZ1 (B), respectively. C: the expression pattern of proGhPIF4:GUS transgenic cotton; a: whole anther from the blooming flower; b: single anther from the blooming flower; c: sepal; d: petal; e: young leaf. D: the heat-induced expression analysis of GhPIF4 in ‘H05’ anthers at different development stages. Figures a, c, d, e, bar = 1 cm; figure b, bar = 100 μm; HN and HH refer to ‘H05’ under normal temperature and high temperature stress conditions, respectively; < 7 mm represents a mixture of flower buds less than 7 mm in length; **P < 0.01.
与此同时, 我们扩增了GhPIF4起始密码子上游1803 bp的启动子区域, 并以pGWB433为目的载体构建proGhPIF4:GUS重组载体, 以陆地棉‘YZ1’为受体进行转化, 最终获得T2代转基因植株, 分别取T2代植株的不同组织, 进行GUS染色, 观察GUS信号在转基因的不同组织中表达情况。结果开花当天的花药中出现了明显的GUS染色信号, 而在叶片、萼片和花瓣中均未检测到GUS染色信号。与GhPIF4在‘YZ1’不同组织中的表达情况基本一致, 说明GhPIF4在棉花花药中高量表达(图3-C)。
进一步详细分析GhPIF4在棉花花药不同发育时期的表达, 本研究参照吴元龙等[32]对棉花花药发育时期与花蕾长度相关性的划分方式, 将高温敏感材料‘H05’不同发育时期的花药按照花蕾长度分为<7 mm (四分体之前)、7~9 mm (小孢子释放期)、9~14 mm (绒毡层降解期)、14~19 mm (有丝分裂I期)、19~24 mm (有丝分裂II期)和>24 mm (花药开裂期) 6个时期, 并从温室获得正常温度和高温处理后这6个时期的花药, 之后提取这6个时期花药的RNA, 并反转录成cDNA模板进行高温诱导表达分析。结果表明, 常温条件下, GhPIF4的表达在‘H05’>24 mm时期表达量最高, 约为14~19 mm时期的2倍, 其他时期的表达量均比14~19 mm时期低, 此结果与组织表达及GUS染色结果一致。高温后, GhPIF4的表达在‘H05’的14~19 mm、19~24 mm和>24 mm三个发育后期花药中受到显著诱导, 而前期花药中GhPIF4的表达受高温诱导不显著(图3-D)。
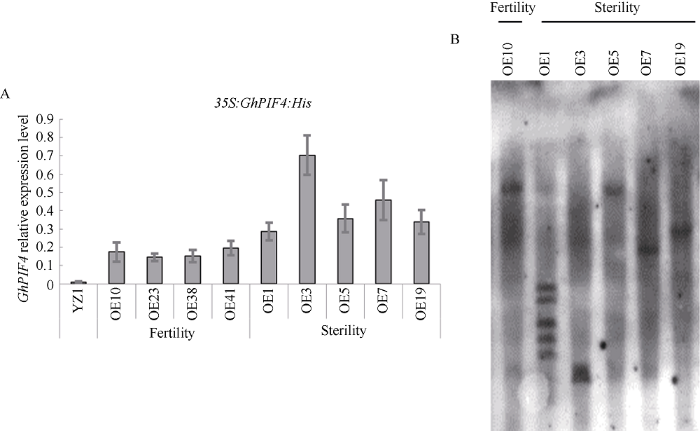
2.4 超表达GhPIF4导致雄性败育且存在剂量效应
构建3'端融合His标签的超表达载体35S: GhPIF4:His, 并以陆地棉‘YZ1’为受体进行转化。获得101个转化单株, 其中89个单株是阳性的, 因T0代超表达转基因株系中既有不育材料也有可育材料出现。因此取超表达材料中的可育材料OE10、OE23、OE38和OE41以及不育材料OE1、OE3、OE5、OE7、OE19开花前一天的花药进行表达量分析。结果表明, 不育材料和可育材料中GhPIF4表达量均比对照‘YZ1’高, 并且出现败育的材料中GhPIF4的表达量均明显的高于可育的材料, 说明GhPIF4导致花药败育可能存在剂量效应(图4-A)。进一步对T0代可育材料OE10以及败育材料OE1、OE3、OE5、OE7、OE19进行Southern检测, 结果显示可育材料OE10和败育材料OE5、OE7和OE19为单拷贝材料(图4-B)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图435S:GhPIF4:His转基因棉花GhPIF4表达量检测与拷贝数检测
A: T0代可育和败育材料中GhPIF4的表达量分析; B: T0代转基因材料的拷贝数检测。
Fig. 4Expression of GhPIF4 and Southern blotting in 35S:GhPIF4:His transgenic cottons
A: the expression level of GhPIF4 in both T0 fertility plants and T0 sterility plants; B: southern blotting of T0 transgenic plants.
2.5 高量超表达GhPIF4导致花粉缺失花药不开裂
3个转基因超表达系整个花药形态与‘YZ1’有明显的差别, OE5和OE19的花药小而干瘪并丛生于柱头基部, 花柱突出, OE7柱头畸形, 三者的花药均皱缩, 不能正常开裂(图5-A)。对开花当天的花药进行TTC染色, 结果OE5、OE7和OE19的花药着色均比‘YZ1’浅, 说明OE5、OE7和OE19的花药中花粉的活力很低或是几乎没有生成花粉(图5-B)。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图535S:GhPIF4:His转基因棉花表型
A: 整体花药, bar = 1 cm; B: TTC染色单个花药, bar = 500 μm。
Fig. 5Phenotype of 35S:GhPIF4:His transgenic cotton lines
A: the whole anthers, bar = 1 cm; B: the single anther stained by TTC and observed under stereo microscope, bar = 500 μm.
2.6 超表达GhPIF4扰乱生长素稳态导致棉花花药败育
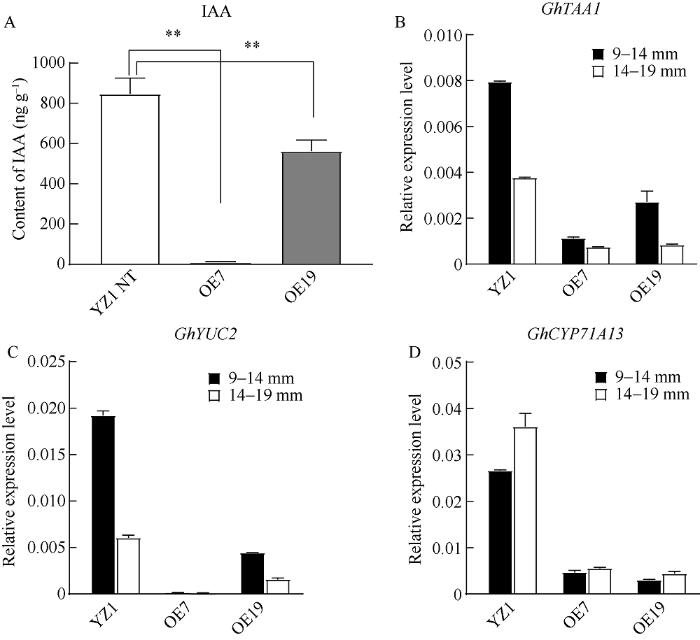
在拟南芥中, PIF4可以促进生长素的合成[22,23,24]。而本实验室前期研究表明高温胁迫会导致高温敏感材料 ‘H05’花药发育后期IAA含量升高, 从而造成花药不开裂[27]。本研究中GhPIF4超表达材料出现败育的表型, 为探究其原因是否与花药中生长素的含量有关, 选取GhPIF4超表达败育材料14~19 mm的花药提取IAA, 并用高效液相色谱-质谱联用技术(HPLC-MS)检测花药中的IAA含量。结果表明, 常温条件下, 超表达材料OE7和OE19相比对照‘YZ1’花药中生长素的含量显著下降。‘YZ1’生长素的含量约是OE7的79倍, OE19的1.5倍(图6-A)。为了探究生长素含量的下降是否与生长素合成相关基因的表达有关, 以‘YZ1’, OE7和OE19 绒毡层降解期(9~14 mm)、有丝分裂I期(14~19 mm)的cDNA为模板, 检测生长素合成关键基因TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1)、YUCCA2 (YUC2)、CYTOCHROME P450, FAMILY 71, SUBFAMILY A, POLYPEPTIDE 13 (CYP71A13)的表达情况。结果表明, 绒毡层降解期(9~14 mm)、有丝分裂I期(14~19 mm)中OE7和OE19花药中GhTAA1、GhYUC2、GhCYP71A13的表达均比对照‘YZ1’低(图6-B~D)。前人研究表明拟南芥中yuc2yuc6双突变体的花药无法形成正常有活力的花粉[33,34,35,36], 高温胁迫下拟南芥和大麦的花药中生长素含量下降以及YUC2和YUC6基因表达降低会导致花药败育[37], 因此我们猜测棉花中生长素过低也会导致花药败育。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6超表达GhPIF4败育棉花花药中生长素含量及生长素合成相关基因表达量
A: 超表达GhPIF4败育棉花花药中生长素含量降低; B~D: 超表达GhPIF4败育棉花花药中生长素合成相关基因表达分析; **P < 0.01。
Fig. 6Content of IAA and the expression level of auxin synthesis genes in sterile anthers of GhPIF4 overexpression transgenic cottons
A: the content of IAA was decreased in sterile anthers of GhPIF4 overexpression transgenic cottons; B-D: the expression level of auxin synthesis genes in sterile GhPIF4 overexpression transgenic cottons; GhTAA1 (B), GhYUC2 (C), GhCYP71A13 (D); **P < 0.01.
3 讨论
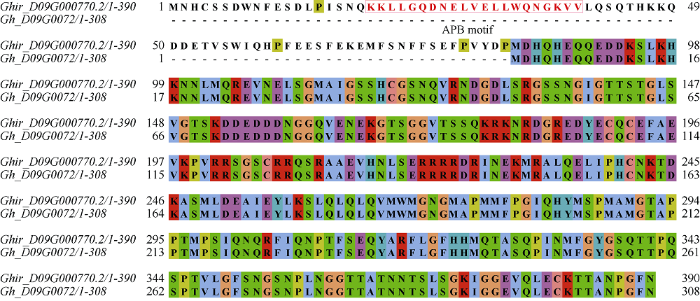
GhPIF4在棉花花药中优势表达, 并在棉花花药发育的后期明显受高温诱导表达。本实验室前期的研究表明, 棉花花药在四分体时期、绒毡层降解期和花药开裂期对高温敏感, 而绒毡层降解期和花药开裂期这2个时期均处于棉花花药发育的后期。在高温敏感材料‘H05’中, 高温导致的绒毡层降解期和花药开裂期的花药中糖代谢和生长素稳态的扰乱是高温导致雄性败育的重要原因之一[27]。生长素参与调控花药的开裂[38,39], 花药开裂主要由药室内壁的次生加厚、裂口的开裂以及隔膜细胞的降解3个过程构成[40], 在花药发育后期, 生长素通过抑制木质素合成基因MYB26的表达来调控花药药室内壁的次生加厚[38,39,40,41], 也会通过抑制茉莉酸的合成来调控裂口开裂发生的时间[38]; 生长素也参与后期花药中花粉的发育, 生长素的缺失会影响小孢子的有丝分裂过程, 造成可育花粉数量的减少[33,34]。作为拟南芥PIF4的同源基因, GhPIF4在棉花中可能也参与生长素的调控。为验证GhPIF4参与高温胁迫导致的棉花雄性败育过程, 扩增了陆地棉‘YZ1’中的GhPIF4基因, 转入由组成型启动子35S驱动GhPIF4表达的超表达载体会造成转基因植株的雄性败育, 并且这种败育现象发生在GhPIF4表达量相对较高的转基因植株中, 即具有剂量效应。超表达GhPIF4导致棉花花药败育可能与扰乱早花药中的激素稳态有关, 在拟南芥中, PIF4参与调控或响应生长素、BR和GA途径, PIF4能够在高温环境下促进生长素合成基因TAA1 (Tryptophan aminotransferase of arabidopsis 1)、YUC8 (YUCCA 8)和CYP79B2 (Cytochrome p450, family 79, subfamily b, polypeptide 2)的表达进而促进生长素合成[22,23], 也能够直接调控IAA19和IAA29等生长素响应基因的表达[35]。本研究表明, 在OE7和OE9这2个超表达GhPIF4导致败育的转基因材料花药中, 生长素含量显著下降, 表达量分析也显示, GhTAA1、GhYUC2和GhCYP71A13三个依赖色氨酸的生长素合成途径相关基因的表达量也显著下降。YUC2和YUC6是拟南芥花药中最主要的生长素合成基因, YUC2和YUC6同时突变会造成拟南芥雄性败育[33,34,35,36]; 高温胁迫下拟南芥和大麦的花药中YUC2和YUC6基因表达下降和生长素含量的降低会导致花药败育[37]; TAA1催化初步的生长素合成[42], 而CYP71A13是催化生长素合成中间产物由吲哚-3-乙醛肟(indole-3-acetaldoxime, IAOx)向吲哚-3-乙腈(indole-3-acetonitrile, IAN)转化的关键基因[43], 这些基因表达量的下调可能是OE7和OE9花药中生长素含量下降的直接原因。在花药发育过程中, 生长素途径的精确调控对于花药发育至关重要, 以生长素响应因子ARF6为例, arf6-2突变体表现出花药开裂延迟的特征[44], 而免疫miR167调控的转基因拟南芥则具有花药败育的表型[45], 因而超表达GhPIF4可能在花药发育的某一时期改变花药内部的生长素稳态进而影响花药发育。在拟南芥中, PIF4可以促进生长素合成, 而在OE7和OE9花药中则出现生长素含量下降的现象, 根据棉花的三代基因组, 本研究发现棉花二代基因组中GhPIF4缺失一个APB motif, 导致GhPIF4序列不完整, 因此在GhPIF4超表达株系中GhPIF4的功能可能会受到影响, 推测APB结构域的缺失可能是造成生长素含量下降的原因之一(图7)。其次, OE7和OE19转基因株系中可以明显观察到开花当天的花药小而干瘪, 花药的长度不一定准确地对应发育时期, 导致生长素含量的测定存在误差。拟南芥中高温胁迫会导致花药生长素合成降低进而导致花药败育, 而棉花花药中生长素过多的积累则会导致花药不开裂, 因此我们猜测在棉花花药中是否生长素过低和过高均会导致花药败育, 关于超表达GhPIF4抑制生长素基因的表达, 原因尚不清楚, 其分子机制有待进一步研究。
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7GhirPIF4和GhPIF4的氨基酸序列比对
红色方框表示APB motif。
Fig. 7Comparison of amino acid sequences between GhirPIF4 and GhPIF4
Red box indicates APB motif.
4 结论
克隆了一个棉花bHLH转录因子基因GhPIF4, 其蛋白定位于细胞核; GhPIF4主要在棉花花药中表达, 且在棉花花药发育后期明显受高温诱导表达; 以‘YZ1’为遗传背景超表达GhPIF4后导致转基因植株花药败育。超表达GhPIF4株系0E7和OE19生长素含量下降与生长素合成关键基因的表达下调一致, 然而探究生长素下降是由于棉花二代基因组中GhPIF4缺失一个APB motif或是超表达材料花蕾长度比对照小, 导致生长素测定的花药长度与花药发育时期不能对应, 亦或是棉花花药中生长素过多和过低均会导致花药败育, 其分子机制还需进一步研究。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:7915844 [本文引用: 1]
DOI:10.1146/annurev-arplant-050213-040119URLPMID:24313845 [本文引用: 1]

In plants, male sterility can be caused either by mitochondrial genes with coupled nuclear genes or by nuclear genes alone; the resulting conditions are known as cytoplasmic male sterility (CMS) and genic male sterility (GMS), respectively. CMS and GMS facilitate hybrid seed production for many crops and thus allow breeders to harness yield gains associated with hybrid vigor (heterosis). In CMS, layers of interaction between mitochondrial and nuclear genes control its male specificity, occurrence, and restoration of fertility. Environment-sensitive GMS (EGMS) mutants may involve epigenetic control by noncoding RNAs and can revert to fertility under different growth conditions, making them useful breeding materials in the hybrid seed industry. Here, we review recent research on CMS and EGMS systems in crops, summarize general models of male sterility and fertility restoration, and discuss the evolutionary significance of these reproductive systems.
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/pcp/pcp135URLPMID:19808807 [本文引用: 1]

Plant male reproductive development is highly organized and sensitive to various environmental stressors, including high temperature. We have established an experimental procedure to evaluate high temperature injury in japonica rice plants. High temperature treatment (39 degrees C/30 degrees C) starting at the microspore stage repeatedly reduced spikelet fertility in our system. Morphological observations revealed that pollen viability in plants exposed to high temperatures was lower than that in control plants. Most pollen grains in high temperature-treated plants displayed a normal round shape and stained reddish purple with Alexander's reagent; however, the pollen grains were very poorly attached and displayed limited germination on the stigma. To investigate gene regulatory mechanisms in the anther in high temperature environments, DNA microarray analysis was performed by comparing non-treated samples with samples treated with 2-4 d of high heat. Genes responsive to high temperatures were identified from clustering of microarray data. Among these, at least 13 were designated as high temperature-repressed genes in the anther. Expression analyses revealed that these genes were expressed specifically in the immature anther mainly in the tapetum at the microspore stage and down-regulated after 1 d of high temperature. The expression levels of Osc6, OsRAFTIN and TDR, which are tapetum-specific genes, were unaffected by high temperatures. These results suggest that not all tapetal genes are inhibited by increased temperatures and the tapetum itself is not degraded in such an environment. However, high temperatures may disrupt some of the tapetum functions required for pollen adhesion and germination on the stigma.
URLPMID:20351019 [本文引用: 1]
URLPMID:28676806 [本文引用: 1]
DOI:10.1111/pce.12142URLPMID:23731015 [本文引用: 1]

In plants, male reproductive development is extremely sensitive to adverse climatic environments and (a)biotic stress. Upon exposure to stress, male gametophytic organs often show morphological, structural and metabolic alterations that typically lead to meiotic defects or premature spore abortion and male reproductive sterility. Depending on the type of stress involved (e.g. heat, cold, drought) and the duration of stress exposure, the underlying cellular defect is highly variable and either involves cytoskeletal alterations, tapetal irregularities, altered sugar utilization, aberrations in auxin metabolism, accumulation of reactive oxygen species (ROS; oxidative stress) or the ectopic induction of programmed cell death (PCD). In this review, we present the critically stress-sensitive stages of male sporogenesis (meiosis) and male gametogenesis (microspore development), and discuss the corresponding biological processes involved and the resulting alterations in male reproduction. In addition, this review also provides insights into the molecular and/or hormonal regulation of the environmental stress sensitivity of male reproduction and outlines putative interaction(s) between the different processes involved.
DOI:10.1105/tpc.104.025643URLPMID:15486100 [本文引用: 1]

The phytochrome (phy) family of sensory photoreceptors (phyA to phyE) in Arabidopsis thaliana control plant developmental transitions in response to informational light signals throughout the life cycle. The photoactivated conformer of the photoreceptor Pfr has been shown to translocate into the nucleus where it induces changes in gene expression by an unknown mechanism. Here, we have identified two basic helix-loop-helix (bHLH) transcription factors, designated PHYTOCHROME-INTERACTING FACTOR5 (PIF5) and PIF6, which interact specifically with the Pfr form of phyB. These two factors cluster tightly with PIF3 and two other phy-interacting bHLH proteins in a phylogenetic subfamily within the large Arabidopsis bHLH (AtbHLH) family. We have identified a novel sequence motif (designated the active phytochrome binding [APB] motif) that is conserved in these phy-interacting AtbHLHs but not in other noninteractors. Using the isolated domain and site-directed mutagenesis, we have shown that this motif is both necessary and sufficient for binding to phyB. Transgenic expression of the native APB-containing AtbHLH protein, PIF4, in a pif4 null mutant, rescued the photoresponse defect in this mutant, whereas mutated PIF4 constructs with site-directed substitutions in conserved APB residues did not. These data indicate that the APB motif is necessary for PIF4 function in light-regulated seedling development and suggest that conformer-specific binding of phyB to PIF4 via the APB motif is necessary for this function in vivo. Binding assays with the isolated APB domain detected interaction with phyB, but none of the other four Arabidopsis phys. Collectively, the data suggest that the APB domain provides a phyB-specific recognition module within the AtbHLH family, thereby conferring photoreceptor target specificity on a subset of these transcription factors and, thus, the potential for selective signal channeling to segments of the transcriptional network.
DOI:10.1038/23500URLPMID:10466729 [本文引用: 1]

The phytochrome photoreceptor family directs plant gene expression by switching between biologically inactive and active conformers in response to the sequential absorption of red and farred photons. Several intermediates that act late in the phytochrome signalling pathway have been identified, but fewer have been identified that act early in the pathway. We have cloned a nuclear basic helix-loop-helix protein, PIF3, which can bind to non-photoactive carboxy-terminal fragments of phytochromes A and B and functions in phytochrome signalling in vivo. Here we show that full-length photoactive phytochrome B binds PIF3 in vitro only upon light-induced conversion to its active form, and that photoconversion back to its inactive form causes dissociation from PIF3. We conclude that photosensory signalling by phytochrome B involves light-induced, conformer-specific recognition of the putative transcriptional regulator PIF3, providing a potential mechanism for direct photoregulation of gene expression.
URLPMID:15448264 [本文引用: 1]
DOI:10.1093/emboj/21.10.2441URLPMID:12006496 [本文引用: 2]

Plants sense and respond to red and far-red light using the phytochrome (phy) family of photoreceptors. However, the mechanism of light signal transduction is not well defined. Here, we report the identification of a new mutant Arabidopsis locus, srl2 (short under red-light 2), which confers selective hypersensitivity to continuous red, but not far-red, light. This hypersensitivity is eliminated in srl2phyB, but not srl2phyA, double mutants, indicating that this locus functions selectively and negatively in phyB signaling. The SRL2 gene encodes a bHLH factor, designated PIF4 (phytochrome-interacting factor 4), which binds selectively to the biologically active Pfr form of phyB, but has little affinity for phyA. Despite its hypersensitive morphological phenotype, the srl2 mutant displays no perturbation of light-induced expression of marker genes for chloroplast development. These data suggest that PIF4 may function specifically in a branch of the phyB signaling network that regulates a subset of genes involved in cell expansion. Consistent with this proposal, PIF4 localizes to the nucleus and can bind to a G-box DNA sequence motif found in various light-regulated promoters.
URLPMID:18252845 [本文引用: 1]
DOI:10.1038/nplants.2015.190URLPMID:27250752 [本文引用: 1]

Temperature is a major factor governing the distribution and seasonal behaviour of plants. Being sessile, plants are highly responsive to small differences in temperature and adjust their growth and development accordingly. The suite of morphological and architectural changes induced by high ambient temperatures, below the heat-stress range, is collectively called thermomorphogenesis. Understanding the molecular genetic circuitries underlying thermomorphogenesis is particularly relevant in the context of climate change, as this knowledge will be key to rational breeding for thermo-tolerant crop varieties. Until recently, the fundamental mechanisms of temperature perception and signalling remained unknown. Our understanding of temperature signalling is now progressing, mainly by exploiting the model plant Arabidopsis thaliana. The transcription factor PHYTOCHROME INTERACTING FACTOR 4 (PIF4) has emerged as a critical player in regulating phytohormone levels and their activity. To control thermomorphogenesis, multiple regulatory circuits are in place to modulate PIF4 levels, activity and downstream mechanisms. Thermomorphogenesis is integrally governed by various light signalling pathways, the circadian clock, epigenetic mechanisms and chromatin-level regulation. In this Review, we summarize recent progress in the field and discuss how the emerging knowledge in Arabidopsis may be transferred to relevant crop systems.
URLPMID:30786235 [本文引用: 1]
URLPMID:27789798 [本文引用: 1]
URLPMID:27789797 [本文引用: 1]
DOI:10.1016/j.cub.2014.10.076URLPMID:25557663 [本文引用: 1]

Plant development is highly responsive to ambient temperature, and this trait has been linked to the ability of plants to adapt to climate change. The mechanisms by which natural populations modulate their thermoresponsiveness are not known. To address this, we surveyed Arabidopsis accessions for variation in thermal responsiveness of elongation growth and mapped the corresponding loci. We find that the transcriptional regulator EARLY FLOWERING3 (ELF3) controls elongation growth in response to temperature. Through a combination of modeling and experiments, we show that high temperature relieves the gating of growth at night, highlighting the importance of temperature-dependent repressors of growth. ELF3 gating of transcriptional targets responds rapidly and reversibly to changes in temperature. We show that the binding of ELF3 to target promoters is temperature dependent, suggesting a mechanism where temperature directly controls ELF3 activity.
URLPMID:29547672 [本文引用: 1]
URLPMID:22927419 [本文引用: 1]
URLPMID:26259175 [本文引用: 1]
DOI:10.1016/j.cub.2016.11.012URLPMID:28041792 [本文引用: 1]

Temperature is a key seasonal signal that shapes plant growth. Elevated ambient temperature accelerates growth and developmental transitions [1] while compromising plant defenses, leading to increased susceptibility [2, 3]. Suppression of immunity at elevated temperature is at the interface of trade-off between growth and defense [2, 4]. Climate change and the increase in average growth-season temperatures threaten biodiversity and food security [5, 6]. Despite its significance, the molecular mechanisms that link thermosensory growth and defense responses are not known. Here we show that PHYTOCHROME INTERACTING FACTOR 4 (PIF4)-mediated thermosensory growth and architecture adaptations are directly linked to suppression of immunity at elevated temperature. PIF4 positively regulates growth and development and negatively regulates immunity. We also show that natural variation of PIF4-mediated temperature response underlies variation in the balance between growth and defense among Arabidopsis natural strains. Importantly, we find that modulation of PIF4 function alters temperature sensitivity of defense. Perturbation of PIF4-mediated growth has resulted in temperature-resilient disease resistance. This study reveals a molecular link between thermosensory growth and immunity in plants. Elucidation of the molecular mechanisms that define environmental signal integration is key to the development of novel strategies for breeding temperature-resilient disease resistance in crops.
URLPMID:22479194 [本文引用: 3]
URLPMID:22123947 [本文引用: 3]
URLPMID:22536829 [本文引用: 2]
[本文引用: 1]
DOI:10.1016/j.tplants.2019.04.002URLPMID:31076166 [本文引用: 1]

Coordination of cell proliferation, cell expansion, and differentiation underpins plant growth. To maximise reproductive success, growth needs to be fine-tuned in response to endogenous and environmental cues. This developmental plasticity relies on a cellular machinery that integrates diverse signals and coordinates the downstream responses. In arabidopsis, the BAP regulatory module, which includes the BRASSINAZOLE RESISTANT 1 (BZR1), AUXIN RESPONSE FACTOR 6 (ARF6), and PHYTOCHROME INTERACTING FACTOR 4 (PIF4) transcription factors (TFs), has been shown to coordinate growth in response to multiple growth-regulating signals. In this Opinion article, we provide an integrative view on the BAP module control of cell expansion and discuss whether its function is conserved or diversified, thus providing new insights into the molecular control of growth.
URLPMID:24481135 [本文引用: 4]
DOI:10.1111/tpj.12245URLPMID:23662698 [本文引用: 1]

Anther infertility under high temperature (HT) conditions is a critical factor contributing to yield loss in cotton (Gossypium hirsutum). Using large-scale expression profile sequencing, we studied the effect of HT on cotton anther development. Our analysis revealed that altered carbohydrate metabolism or disrupted tapetal programmed cell death (PCD) underlie anther sterility. Expression of the Gossypium hirsutum casein kinase I (GhCKI) gene, which encodes a homolog of casein kinase I (CKI), was induced in an HT-sensitive cotton line after exposure to HT. As mammalian homologs of GhCKI are involved in inactivation of glycogen synthase and the regulation of apoptosis, GhCKI may be considered a target gene for improving anther fertility under HT conditions. Our studies suggest that GhCKI exhibits starch synthase kinase activity, increases glucose content in early-stage buds and activates the accumulation of abscisic acid, thereby disturbing the balance of reactive oxygen species and eventually disrupting tapetal PCD, leading to anther abortion or indehiscence. These results indicate that GhCKI may be a key regulator of tapetal PCD and anther dehiscence, with the potential to facilitate regulation of HT tolerance in crops.
[本文引用: 1]
华中农业大学博士学位论文,
[本文引用: 1]
PhD Dissertation of Huazhong Agricultural University,
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/srep09608URLPMID:26043720 [本文引用: 1]

To understand the mechanisms of male sterility in cotton (Gossypium spp.), combined histological, biochemical and transcription analysis using RNA-Seq was carried out in the anther of the single-gene recessive genic male sterility system of male sterile line 1355A and male fertile line 1355B, which are near-isogenic lines (NILs) differing only in the fertility trait. A total of 2,446 differentially expressed genes were identified between the anthers of 1355AB lines, at three different stages of development. Cluster analysis and functional assignment of differentially expressed genes revealed differences in transcription associated with pollen wall and anther development, including the metabolism of fatty acids, glucose, pectin and cellulose. Histological and biochemical analysis revealed that a major cellular defect in the 1355A was a thicker nexine, consistent with the RNA-seq data, and further gene expression studies implicated differences in fatty acids synthesis and metabolism. This study provides insight into the phenotypic characteristics and gene regulatory network of the genic male sterile line 1355A in upland cotton.
DOI:10.1371/journal.pgen.1007397URLPMID:29813066 [本文引用: 3]

Gametophytic development in Arabidopsis depends on nutrients and cell wall materials from sporophytic cells. However, it is not clear whether hormones and signaling molecules from sporophytic tissues are also required for gametophytic development. Herein, we show that auxin produced by the flavin monooxygenases YUC2 and YUC6 in the sporophytic microsporocytes is essential for early stages of pollen development. The first asymmetric mitotic division (PMI) of haploid microspores is the earliest event in male gametophyte development. Microspore development in yuc2yuc6 double mutants arrests before PMI and consequently yuc2yuc6 fail to produce viable pollens. Our genetic analyses reveal that YUC2 and YUC6 act as sporophytic genes for pollen formation. We further show that ectopic production of auxin in tapetum, which provides nutrients for pollen development, fails to rescue the sterile phenotypes of yuc2yuc6. In contrast, production of auxin in either microsporocytes or microspores rescued the defects of pollen development in yuc2yuc6 double mutants. Our results demonstrate that local auxin biosynthesis in sporophytic microsporocytic cells and microspore controls male gametophyte development during the generation transition from sporophyte to male gametophyte.
DOI:10.1007/s11103-006-0005-zURLPMID:16786302 [本文引用: 3]

It was well known that auxin is critical for anther/pollen grain development, however, the clear distribution and detailed effects of auxin during floral development are still unclear. We have shown here that, through analyzing GUS activities of Arabidopsis lines harboring auxin response elements DR5-GUS, auxin was mainly accumulated in the anther during flower stages 10-12. Further studies employing the indoleacetic acid-lysine synthetase (iaaL) coding gene from Pseudomonas syringae subsp. savastanoi under control of the promoter region of Arabidopsis phosphatidylinositol monophosphate 5-kinase 1 gene, which conducts the anther filament-specific expression, showed that block of auxin flow of filaments resulted in shortened filaments and significantly defective pollen grains. Similar phenotype was observed in tobacco plants transformed with the same construct, confirming the effects of auxin flow in filaments on anther development. Detailed studies further revealed that the meiosis process of pollen grain was normal while the mitosis at later stage was significantly defected, indicating the effects of auxin flow in filaments on pollen grain mitosis process. Analysis employing [(14)C]IAA, as well as the observation on the expression of AtPIN1, coding for auxin efflux carrier, demonstrated the presence of polar auxin transport in anther filaments and pollen grains.
DOI:10.1105/tpc.113.112417URLPMID:23757399 [本文引用: 3]

Both blue light (BL) and auxin are essential for phototropism in Arabidopsis thaliana. However, the mechanisms by which light is molecularly linked to auxin during phototropism remain elusive. Here, we report that phytochrome interacting factoR4 (PIF4) and PIF5 act downstream of the BL sensor phototropin1 (PHOT1) to negatively modulate phototropism in Arabidopsis. We also reveal that PIF4 and PIF5 negatively regulate auxin signaling. Furthermore, we demonstrate that PIF4 directly activates the expression of the auxin/indole-3-acetic acid (IAA) genes IAA19 and IAA29 by binding to the G-box (CACGTG) motifs in their promoters. Our genetic assays demonstrate that IAA19 and IAA29, which physically interact with auxin response factor7 (ARF7), are sufficient for PIF4 to negatively regulate auxin signaling and phototropism. This study identifies a key step of phototropic signaling in Arabidopsis by showing that PIF4 and PIF5 link light and auxin.
URLPMID:16818609 [本文引用: 2]
DOI:10.1073/pnas.1000869107URLPMID:20421476 [本文引用: 2]

With global warming, plant high temperature injury is becoming an increasingly serious problem. In wheat, barley, and various other commercially important crops, the early phase of anther development is especially susceptible to high temperatures. Activation of auxin biosynthesis with increased temperatures has been reported in certain plant tissues. In contrast, we here found that under high temperature conditions, endogenous auxin levels specifically decreased in the developing anthers of barley and Arabidopsis. In addition, expression of the YUCCA auxin biosynthesis genes was repressed by increasing temperatures. Application of auxin completely reversed male sterility in both plant species. These findings suggest that tissue-specific auxin reduction is the primary cause of high temperature injury, which leads to the abortion of pollen development. Thus, the application of auxin may help sustain steady yields of crops despite future climate change.
URLPMID:23410518 [本文引用: 3]
DOI:10.1105/tpc.107.057570URLPMID:18628351 [本文引用: 2]

We provide evidence on the localization, synthesis, transport, and effects of auxin on the processes occurring late in Arabidopsis thaliana stamen development: anther dehiscence, pollen maturation, and preanthesis filament elongation. Expression of auxin-sensitive reporter constructs suggests that auxin effects begin in anthers between the end of meiosis and the bilocular stage in the somatic tissues involved in the first step of dehiscence as well as in the microspores and in the junction region between anther and filament. In situ hybridizations of the auxin biosynthetic genes YUC2 and YUC6 suggest that auxin is synthesized in anthers. In agreement with the timing of auxin effects, the TIR1, AFB1, AFB2, and AFB3 auxin receptor-encoding genes are transcribed in anthers only during late stages of development starting at the end of meiosis. We found that in tir1 afb triple and quadruple mutants, anther dehiscence and pollen maturation occur earlier than in the wild type, causing the release of mature pollen grains before the completion of filament elongation. We also assessed the contribution of auxin transport to late stamen developmental processes. Our results suggest that auxin synthesized in anthers plays a major role in coordinating anther dehiscence and pollen maturation, while auxin transport contributes to the independent regulation of preanthesis filament elongation.
DOI:10.1105/tpc.5.10.1217URLPMID:8281038 [本文引用: 2]
DOI:10.1105/tpc.106.046391URLPMID:17329564 [本文引用: 1]

The Arabidopsis thaliana MYB26/MALE STERILE35 (MS35) gene is critical for the development of secondary thickening in the anther endothecium and subsequent dehiscence. MYB26 is localized to the nucleus and regulates endothecial development and secondary thickening in a cell-specific manner in the anther. MYB26 expression is seen in anthers and also in the style and nectaries, although there is no effect on female fertility in the ms35 mutant. MYB26 expression in anthers occurs early during endothecial development, with maximal expression during pollen mitosis I and bicellular stages, indicating a regulatory role in specifying early endothecial cell development. Overexpression of MYB26 results in ectopic secondary thickening in both Arabidopsis and tobacco (Nicotiana tabacum) plants, predominantly within the epidermal tissues. MYB26 regulates a number of genes linked to secondary thickening, including IRREGULAR XYLEM1 (IRX1), IRX3, IRX8, and IRX12. Changes in expression were also detected in two NAC domain genes, NAC SECONDARY WALL-PROMOTING FACTOR1 (NST1) and NST2, which have been linked to secondary thickening in the anther endothecium. These data indicate that MYB26 regulates NST1 and NST2 expression and in turn controls the process of secondary thickening. Therefore, MYB26 appears to function in a regulatory role involved in determining endothecial cell development within the anther and acts upstream of the lignin biosynthesis pathway.
DOI:10.1016/j.cell.2008.01.047URLPMID:18394997 [本文引用: 1]

Plants have evolved a tremendous ability to respond to environmental changes by adapting their growth and development. The interaction between hormonal and developmental signals is a critical mechanism in the generation of this enormous plasticity. A good example is the response to the hormone ethylene that depends on tissue type, developmental stage, and environmental conditions. By characterizing the Arabidopsis wei8 mutant, we have found that a small family of genes mediates tissue-specific responses to ethylene. Biochemical studies revealed that WEI8 encodes a long-anticipated tryptophan aminotransferase, TAA1, in the essential, yet genetically uncharacterized, indole-3-pyruvic acid (IPA) branch of the auxin biosynthetic pathway. Analysis of TAA1 and its paralogues revealed a link between local auxin production, tissue-specific ethylene effects, and organ development. Thus, the IPA route of auxin production is key to generating robust auxin gradients in response to environmental and developmental cues.
DOI:10.1104/pp.15.00481URLPMID:25953104 [本文引用: 1]

In Arabidopsis (Arabidopsis thaliana), a number of defense-related metabolites are synthesized via indole-3-acetonitrile (IAN), including camalexin and indole-3-carboxylic acid (ICOOH) derivatives. Cytochrome P450 71A13 (CYP71A13) is a key enzyme for camalexin biosynthesis and catalyzes the conversion of indole-3-acetaldoxime (IAOx) to IAN. The CYP71A13 gene is located in tandem with its close homolog CYP71A12, also encoding an IAOx dehydratase. However, for CYP71A12, indole-3-carbaldehyde and cyanide were identified as major reaction products. To clarify CYP71A12 function in vivo and to better understand IAN metabolism, we generated two cyp71a12 cyp71a13 double knockout mutant lines. CYP71A12-specific transcription activator-like effector nucleases were introduced into the cyp71a13 background, and very efficient somatic mutagenesis was achieved. We observed stable transmission of the cyp71a12 mutation to the following generations, which is a major challenge for targeted mutagenesis in Arabidopsis. In contrast to cyp71a13 plants, in which camalexin accumulation is partially reduced, double mutants synthesized only traces of camalexin, demonstrating that CYP71A12 contributes to camalexin biosynthesis in leaf tissue. A major role of CYP71A12 was identified for the inducible biosynthesis of ICOOH. Specifically, the ICOOH methyl ester was reduced to 12% of the wild-type level in AgNO3-challenged cyp71a12 leaves. In contrast, indole-3-carbaldehyde derivatives apparently are synthesized via alternative pathways, such as the degradation of indole glucosinolates. Based on these results, we present a model for this surprisingly complex metabolic network with multiple IAN sources and channeling of IAOx-derived IAN into camalexin biosynthesis. In conclusion, transcription activator-like effector nuclease-mediated mutation is a powerful tool for functional analysis of tandem genes in secondary metabolism.
DOI:10.1242/dev.01955URLPMID:16107481 [本文引用: 1]

Pollination in flowering plants requires that anthers release pollen when the gynoecium is competent to support fertilization. We show that in Arabidopsis thaliana, two paralogous auxin response transcription factors, ARF6 and ARF8, regulate both stamen and gynoecium maturation. arf6 arf8 double-null mutant flowers arrested as infertile closed buds with short petals, short stamen filaments, undehisced anthers that did not release pollen and immature gynoecia. Numerous developmentally regulated genes failed to be induced. ARF6 and ARF8 thus coordinate the transition from immature to mature fertile flowers. Jasmonic acid (JA) measurements and JA feeding experiments showed that decreased jasmonate production caused the block in pollen release, but not the gynoecium arrest. The double mutant had altered auxin responsive gene expression. However, whole flower auxin levels did not change during flower maturation, suggesting that auxin might regulate flower maturation only under specific environmental conditions, or in localized organs or tissues of flowers. arf6 and arf8 single mutants and sesquimutants (homozygous for one mutation and heterozygous for the other) had delayed stamen development and decreased fecundity, indicating that ARF6 and ARF8 gene dosage affects timing of flower maturation quantitatively.
URLPMID:17021043 [本文引用: 1]
