 ,*, 杨彦明, 赵洲, 徐忠山, 海霞, 韩宇婷内蒙古农业大学/内蒙古杂粮工程技术研究中心/全国农业科研杰出人才及其创新团队, 内蒙古呼和浩特 010019
,*, 杨彦明, 赵洲, 徐忠山, 海霞, 韩宇婷内蒙古农业大学/内蒙古杂粮工程技术研究中心/全国农业科研杰出人才及其创新团队, 内蒙古呼和浩特 010019Effects of salt stress on physiological indexes and differential proteomics of oat leaf
CHEN Xiao-Jing, LIU Jing-Hui ,*, YANG Yan-Ming, ZHAO Zhou, XU Zhong-Shan, HAI Xia, HAN Yu-TingInner Mongolia Agricultural University/Cereal Engineering Technology Research Center, Inner Mongolia Autonomous Region/ National Agricultural Research Outstanding Talents and Innovation Team, Huhhot 010019, Inner Mongolia, China
,*, YANG Yan-Ming, ZHAO Zhou, XU Zhong-Shan, HAI Xia, HAN Yu-TingInner Mongolia Agricultural University/Cereal Engineering Technology Research Center, Inner Mongolia Autonomous Region/ National Agricultural Research Outstanding Talents and Innovation Team, Huhhot 010019, Inner Mongolia, China通讯作者:
收稿日期:2018-12-13接受日期:2019-05-12网络出版日期:2019-06-06
| 基金资助: |
Received:2018-12-13Accepted:2019-05-12Online:2019-06-06
| Fund supported: |
作者简介 About authors
E-mail:1131036201@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (1133KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈晓晶, 刘景辉, 杨彦明, 赵洲, 徐忠山, 海霞, 韩宇婷. 盐胁迫对燕麦叶片生理指标和差异蛋白组学的影响[J]. 作物学报, 2019, 45(9): 1431-1439. doi:10.3724/SP.J.1006.2019.81088
CHEN Xiao-Jing, LIU Jing-Hui, YANG Yan-Ming, ZHAO Zhou, XU Zhong-Shan, HAI Xia, HAN Yu-Ting.
中国盐渍土总面积9913万公顷, 约占国土面积的1.03%[1], 全球大约20%的陆地面积和近一半的灌溉土地都受到盐影响[2]。盐渍化是全球面临的严峻问题之一,也是危害农业生产的主要因素, 如何提高植物耐盐性, 充分利用盐渍土已成为目前研究的重点。目前, 蛋白组学分析已经成为揭示盐胁迫下表达差异的最佳策略之一。前人分析了盐胁迫条件下水稻、小麦、玉米、大麦等植物不同组织器官细胞和亚细胞结构蛋白质组变化特征[3], 并已经在水稻[4,5,6]、小麦[7,8]等叶片中鉴定出404种与盐胁迫相关蛋白质。燕麦作为粮饲兼用作物, 具有耐盐碱、耐贫瘠、抗寒等特性, 已成为改良盐碱地的先锋作物[9]。目前有关燕麦耐盐性的研究主要集中在生理生化指标、离子吸收及气孔排盐机制上[9,10,11], 关于盐胁迫对燕麦蛋白质组学的影响鲜有报道[12]。在燕麦响应逆境胁迫领域开展蛋白质组学相关研究, 为新的抗逆基因(或蛋白)的鉴定奠定了基础。非标定量法(Label-Free)是近年来重要的质谱定量方法, 通过比较质谱分析次数或质谱峰强度, 分析不同来源样品蛋白的数量变化来提高低丰度蛋白质的检测效率和蛋白质定量的准确性。本试验运用Label-Free技术, 对比研究对照与处理间差异蛋白的变化及涉及的功能, 旨在挖掘响应盐胁迫相关的蛋白, 明确参与燕麦耐盐过程的相关代谢通路, 为作物抗盐育种提供更有效地科学依据和理论基础。
1 材料与方法
1.1 试验设计
2017—2018年, 在内蒙古农业大学燕麦产业研究中心温室, 选用吉林省白城市农业科学院选育的白燕5号开展盆栽试验。设对照(BYC)及盐胁迫(BYS) 2个处理, 每10桶为一次重复, 每个处理重复3次, 共60桶。每桶播30粒种子至装满配置基质(沙土∶蛭石∶陶粒 = 3∶1∶2)的塑料桶(桶上径24 cm、下径22 cm、高25 cm), 出苗后间苗至20株。每桶底部钻5个直径4 mm圆孔渗水透气。每周浇Hogland 营养液3次, 每次250 mL。于燕麦三叶期对燕麦进行150 mmol L-1盐胁迫处理(按摩尔浓度NaCl∶Na2SO4 = 1∶1混合溶于营养液) 6 d, 对照浇相同体积营养液, 在燕麦分蘖期分别取BYC与BYS处理各重复倒三叶叶片共2 g至1.5 mL冻存管中, 液氮速冻, -80℃保存用于蛋白质组学分析。1.2 常规指标测定与方法
采用硫代巴比妥酸法[13] 测定丙二醛(MDA)含量; 用氮蓝四唑(NBT)光还原法测定[14] 超氧化物歧化酶(SOD)活性; 用愈创木酚法测定[13]过氧化物酶(POD)活性; 用磺基水杨酸提取法[15]测定游离脯氨酸(Pro)含量。1.3 燕麦叶片总蛋白的提取
将冻存待测的样本用液氮预冷的粉碎器粉碎。用液氮研磨粉碎后的粉末。将粉末按照1︰10 (w/v)加入Lysis buffer涡旋混匀。以0.2 s on、2 s off, 振幅22%超声60 s。室温提取30 min。15,000 × g 10℃离心1 h, 取上清液, 分装后冻存于-80℃。1.4 蛋白定量
采用Bradford法[16]测定样本所提取的蛋白浓度。根据曲线公式计算各样品蛋白浓度(μg μL-1)。具体操作步骤见程德金等[17]的研究。1.5 蛋白酶解(filter aided sample preparation, FASP)
蛋白定量后取200 μg蛋白溶液置于离心管。加入DTT, 使终浓度为25 mmol L-1, 60℃反应1 h。加入碘乙酰胺, 使终浓度为50 mmol L-1, 室温10 min。将还原烷基化后的蛋白溶液加入10 K超滤管, 12,000 ×g离心20 min, 弃掉收集管底部溶液。加入Dissolution buffer 100 μL, 12,000 × g离心20 min, 弃掉收集管底部溶液, 重复3次。更换新的收集管, 在超滤管中加入胰蛋白酶, 总量4 μg (与蛋白质量比为1︰50), 体积50 μL, 37℃反应过夜。次日, 12,000 × g离心20 min, 酶解消化后的肽段溶液离心收集于管底部。在超滤管中加入50 μL Dissolution buffer, 12,000 × g再次离心20 min, 与上步合并, 收集管底部共得到100 μL酶解后的样品。冻干待上样。1.6 纳升级反相色谱-Q Exactive进行蛋白质分析
将高pH反相分离得到的组份用20 μL 2%甲醇和0.1%甲酸复溶。12,000 ×g离心10 min, 吸取上清液上样。上样体积10 μL, 采取夹心法上样。Loading Pump流速350 nL min-1, 15 min。分离流速300 nL min-1。1.7 质谱数据分析
使用数据库uniprot-Pooideae361804_20170619.fasta. fasta (362,934 sequences)。质谱分析是由Thermo Q Exactive型质谱完成, 可信度在95%以上的谱肽(Peptide Spectrum Matches, 简称PSMs)为可信PSMs, 至少包含一个unique肽段(特有肽段)的蛋白为可信蛋白, 只保留可信的谱肽和蛋白, 并做FDR验证, 去除FDR大于1%的肽段和蛋白。在比较的样品对之间, 将蛋白在不同重复组中差异倍数的均值作为2个样品的差异倍数, 并做T-test检验得到P-value, 以此作为显著性指标。1.8 数据处理及生物信息学分析
采用Microsoft Excel 2003和SAS 9.0软件统计分析数据。对鉴定到的蛋白进行常见功能数据库注释, 包括COG、GO和KEGG数据库; 最后针对筛选出来的差异蛋白进行GO、KEGG功能富集分析等一系列的差异蛋白功能分析。2 结果与分析
2.1 盐胁迫对游离脯氨酸、抗氧化酶活性、及丙二醛的影响
MDA是脂质过氧化的产物, 是细胞膜稳定性的标志物; SOD和POD在活性氧(ROS)清除中起重要作用; 植物体内Pro含量在一定程度上反映了植物的抗逆性, 由图1可知, BYS处理的MDA、SOD活性均呈下降趋势, 分别较BYC降低16.7%和23.4%, 但无显著差异。POD较BYC显著降低21.2%, Pro含量变化不明显, 较BYC升高了1.12%。图1
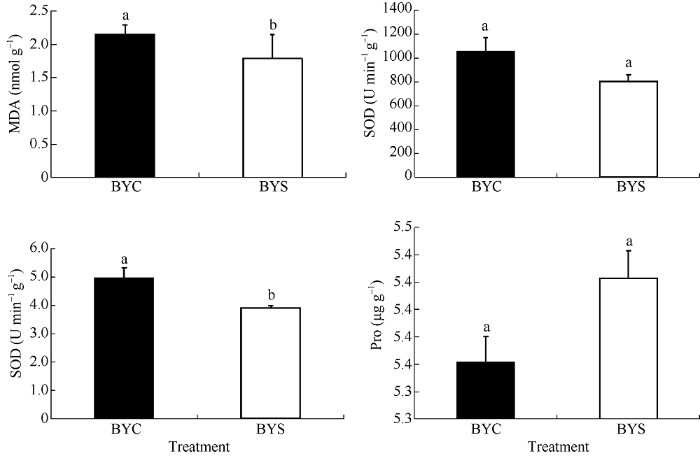 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1盐胁迫对抗氧化酶活性的影响
标明不同小写字母的柱值在处理间差异显著(P < 0.05)。
Fig. 1Effect of salt stress on antioxidant enzyme activity
Bars superscripted by different letters are significantly different between treatments (P < 0.05).
2.2 盐胁迫下差异表达蛋白质筛选
在相对定量时, 将两个样品蛋白丰度比定义为差异倍数。本研究以蛋白质差异倍数大于2且经统计检验其P-value值小于0.05时, 视为上调蛋白; 以差异倍数小于0.5且经统计检验其P-value值小于0.05时, 视为下调蛋白。将BYS和BYC比较, 得到差异表达蛋白76个, 其中51个蛋白上调表达, 25个蛋白下调表达。对每个蛋白差异倍数以2为底取对数后作出分布如图2, 表达量上调的蛋白居于横坐标0位置的右侧, 表达量下调的蛋白居于横坐标0位置的左侧。图2
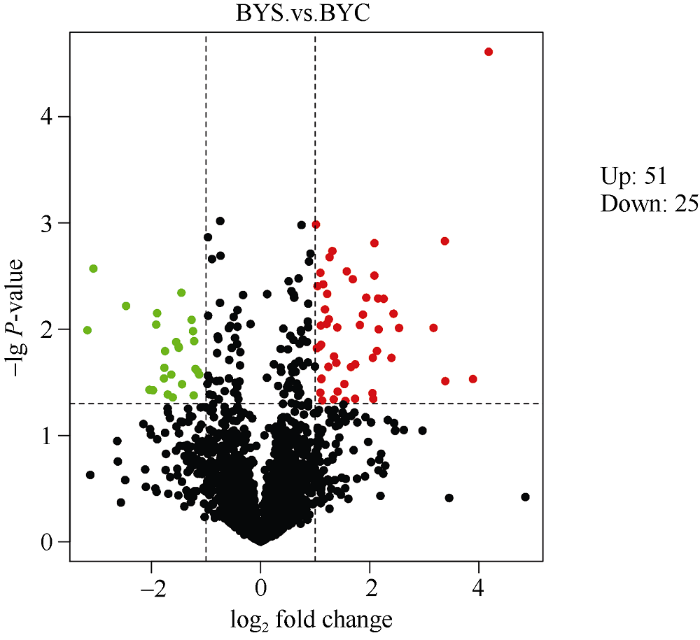 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2差异蛋白火山图
横坐标表示差异蛋白的差异倍数(log2值), 纵轴表示P-value (-lg值), 黑色代表差异不显著的蛋白, 红色代表上调蛋白, 绿色代表下调蛋白。
Fig. 2Differential protein volcano map
The abscissa indicates the difference fold (log2 value) of the differential protein, the ordinate indicates P-value (-lg value), black indicates the protein with insignificant difference, red indicates the up-regulated protein, and green indicates the down-regulated protein.
2.3 盐胁迫下差异表达蛋白质聚类分析
由图3可知, 不同蛋白在不同样品间的上调、下调情况, 且两组样品中3次重复样品间的相似性极高, 说明筛选差异蛋白的合理性; 76个差异蛋白通过COG数据库有62个差异蛋白可被注释并进行功能分类(表1)。图3
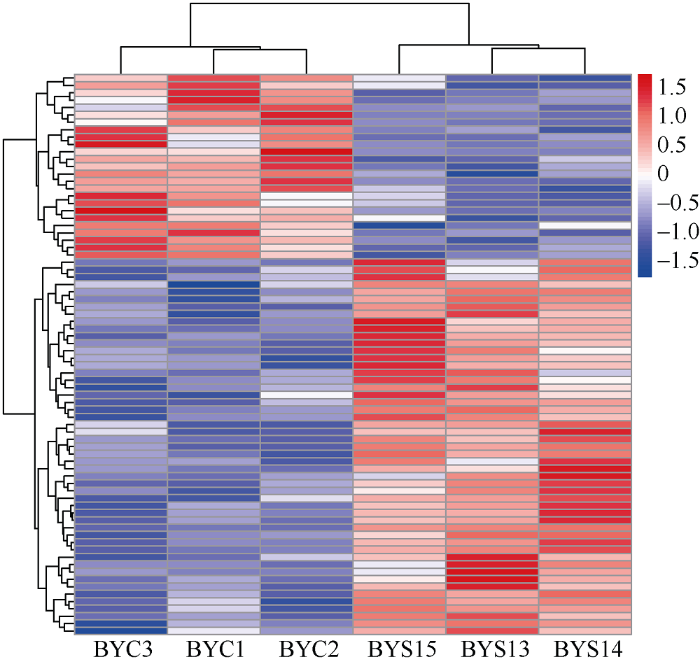 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3差异蛋白聚类热图
纵向是样品的聚类, 横向是蛋白的聚类。从纵向聚类可以看出样品间蛋白含量的模式聚类。
Fig. 3Differential protein clustering heat map
Vertical direction shows the clustering of samples, and horizontal direction shows the clustering of proteins. Pattern clustering of protein content between samples can be seen from longitudinal clustering.
Table 1
表1
表1BYC与BYS差异蛋白
Table 1
| 功能类 Functional class | 蛋白名称 Protein ID | 功能描述 Functional description | COG 编号 COG gene ID | 可信度 Identity | 上调/下调 Up(↑)/Down(↓) |
|---|---|---|---|---|---|
| 能量生产和转换 Energy production and conversion (9) | |||||
| C | A0A193CI13 | FoF1-type ATP synthase, beta subunit | YP_722959 | 0.91 | ↑ |
| C | A9LIN4 | Malic enzyme | YP_007218386 | 0.53 | ↑ |
| C | I1GZ41 | Acyl-CoA reductase or other NAD-dependent aldehyde dehydrogenase | YP_722268 | 0.61 | ↑ |
| C | A0A1V0EL65 | Hemoglobin-like flavoprotein | YP_007103351 | 0.30 | ↓ |
| C | A0A1D5S6L5 | Malic enzyme | YP_005607828 | 0.45 | ↑ |
| C | A0A1D6CUL3 | NADH dehydrogenase, FAD-containing subunit | YP_003322333 | 0.33 | ↑ |
| C | I1I051 | Coenzyme F420-reducing hydrogenase, beta subunit | YP_007131148 | 0.55 | ↓ |
| C | A0A1D6AN16 | Ferredoxin-NADP reductase | YP_007118319 | 0.52 | ↓ |
| C | W4ZQA0 | Hemoglobin-like flavoprotein | YP_003692354 | 0.33 | ↓ |
| 翻译后修饰、蛋白质周转和分子伴侣 Posttranslational modification, protein turnover, and chaperones (15) | |||||
| O | A0A165FYR3 | Glutathione S-transferase | YP_007096787 | 0.31 | ↑ |
| O | Q3I0N4 | Molecular chaperone IbpA, HSP20 family | YP_005887446 | 0.38 | ↑ |
| O | A0A1C6ZYA4 | Molecular chaperone, HSP90 family | YP_634186 | 0.45 | ↑ |
| O | I1H6R7 | Glutathione S-transferase | YP_007063563 | 0.28 | ↑ |
| O | F4Y589 | Molecular chaperone, HSP90 family | YP_634186 | 0.47 | ↑ |
| O | A0A1D5Y3B7 | ATP-dependent Clp protease ATP-binding subunit ClpA | YP_007121403 | 0.69 | ↑ |
| O | I1IF07 | Molecular chaperone IbpA, HSP20 family | YP_001357091 | 0.31 | ↑ |
| O | M8BCN0 | Molecular chaperone DnaK (HSP70) | YP_005440675 | 0.49 | ↑ |
| O | I1GZ93 | Molecular chaperone IbpA, HSP20 family | YP_522114 | 0.38 | ↑ |
| O | M0Y631 | Chaperonin GroEL (HSP60 family) | YP_006371119 | 0.62 | ↓ |
| O | F2E3N4 | FKBP-type peptidyl-prolyl cis-trans isomerase | YP_007057296 | 0.66 | ↑ |
| O | M8AN59 | ATP-dependent Zn proteases | YP_723906 | 0.37 | ↑ |
| O | F2DYT5 | Molecular chaperone DnaK (HSP70) | YP_005440675 | 0.49 | ↑ |
| O | A0A1D5SA32 | ATP-dependent Clp protease ATP-binding subunit ClpA | YP_007132111 | 0.52 | ↑ |
| O | W5EGU4 | Molecular chaperone DnaK (HSP70) | YP_423803 | 0.71 | ↑ |
| 氨基酸转运和代谢; 辅酶转运和代谢; 一般功能预测; 次生代谢产物的生物合成、转运和分解代谢 Amino acid transport and metabolism; Coenzyme transport and metabolism; General function prediction only; Secondary metabolites biosynthesis, transport, and catabolism (15) | |||||
| E | A0A1D5XXT6 | Monoamine oxidase | YP_005086783 | 0.30 | ↓ |
| E | A0A1D6CAG8 | Monoamine oxidase | YP_005086783 | 0.29 | ↓ |
| E | A0A1D5YV26 | 3-dehydroquinate dehydratase | NP_867287 | 0.36 | ↑ |
| E | I1GLP9 | 5,10-methylenetetrahydrofolate reductase | YP_112676 | 0.40 | ↑ |
| E | A0A1D6BBP7 | Aminopeptidase N | YP_006773912 | 0.36 | ↑ |
| E | I1HGT7 | Threonine synthase | YP_002463167 | 0.62 | ↑ |
| EH | I1GU32 | 3'-phosphoadenosine 5'-phosphosulfate sulfotransferase (PAPS reductase)/FAD synthetase or related enzyme | YP_007109372 | 0.63 | ↓ |
| H | I1HWV1 | Glutamine amidotransferase PdxT (pyridoxal biosynthesis) | YP_005441479 | 0.52 | ↑ |
| HR | F2CYS4 | Hydroxymethylpyrimidine pyrophosphatase and other HAD family phosphatases | YP_007159391 | 0.34 | ↑ |
| R | A0A1D6APK4 | Zn-dependent alcohol dehydrogenase | YP_001983794 | 0.67 | ↓ |
| 功能类 Functional class | 蛋白名称 Protein ID | 功能描述 Functional description | COG 编号 COG gene ID | 可信度 Identity | 上调/下调 Up(↑)/Down(↓) |
| R | T1MRH6 | Predicted oxidoreductase (related to aryl-alcohol dehydrogenase) | YP_007098133 | 0.45 | ↑ |
| R | W4ZPA5 | Uncharacterized metalloenzyme YdcJ, glyoxalase superfamily | YP_628605 | 0.26 | ↑ |
| QR | A0A1D5VID8 | NADPH-dependent curcumin reductase CurA | YP_003953479 | 0.52 | ↑ |
| Q | I1ICZ4 | Carotenoid cleavage dioxygenase or a related enzyme | YP_007064275 | 0.35 | ↓ |
| Q | A0A1D5UEP5 | Cu2+-containing amine oxidase | YP_005086886 | 0.34 | ↑ |
| 碳水化合物的运输和代谢 Carbohydrate transport and metabolism (7) | |||||
| G | Q38786 | Beta-glucosidase/6-phospho-beta-glucosidase/beta-galactosidase | YP_004449345 | 0.40 | ↓ |
| G | Q8S311 | Sucrose-6-phosphate hydrolase SacC, GH32 family | YP_002315570 | 0.29 | ↑ |
| G | M0WF67 | 6-phosphogluconate dehydrogenase | YP_007142557 | 0.47 | ↓ |
| G | I1IJ14 | Ribulose bisphosphate carboxylase small subunit | YP_007111326 | 0.63 | ↑ |
| G | I1I5R4 | Predicted arabinose efflux permease, MFS family | YP_003405887 | 0.40 | ↑ |
| G | A0A1D6RIU0 | 1,4-alpha-glucan branching enzyme | YP_677957 | 0.47 | ↑ |
| G | Q7X9A2 | Ribulose bisphosphate carboxylase small subunit | YP_007111326 | 0.63 | ↑ |
| 翻译、核糖体结构和生物发生 Translation, ribosomal structure, and biogenesis (5) | |||||
| J | M0WGV4 | Ribosome-associated translation inhibitor RaiA | YP_007127538 | 0.38 | ↓ |
| J | I1GM81 | Ribosomal protein L4 | NP_275148 | 0.41 | ↓ |
| J | F2DBP2 | Ribosomal protein L6P/L9E | YP_007126712 | 0.54 | ↑ |
| J | I1IU29 | Ribosomal protein L3 | YP_004004412 | 0.39 | ↓ |
| J | M8CXZ0 | RNA recognition motif (RRM) domain | YP_844222 | 0.44 | ↓ |
| 无机离子转运和代谢 Inorganic ion transport and metabolism (2) | |||||
| P | I1I9J4 | Cu/Zn superoxide dismutase | YP_003290884 | 0.49 | ↓ |
| P | R7WAY7 | Carbonic anhydrase | YP_004682419 | 0.38 | ↓ |
| 脂质运输和新陈代谢 Lipid transport and metabolism (3) | |||||
| I | M7ZQT4 | Isopentenyldiphosphate isomerase | YP_006428266 | 0.39 | ↑ |
| I | I1IT00 | NADPH-dependent2,4-dienoyl-CoA reductase, sulfur reductase, or a related oxidoreductase | YP_824941 | 0.32 | ↑ |
| I | O65195 | Myo-inositol-1-phosphate synthase | YP_005007067 | 0.35 | ↓ |
| 信号转导机制 Signal transduction mechanisms (2) | |||||
| T | I1J3C6 | Phosphohistidine swiveling domain of PEP-utilizing enzymes | YP_002508078 | 0.47 | ↓ |
| T | I1HYN7 | Predicted NTPase, NACHT family domain | YP_003890644 | 0.30 | ↑ |
| 细胞壁/膜/包膜生物发生 Cell wall/membrane/envelope biogenesis (2) | |||||
| M | W5BNP0 | Glycosyltransferase involved in cell wall bisynthesis | YP_004596549 | 0.26 | ↑ |
| M | I1I6H2 | Nucleoside-diphosphate-sugar epimerase | YP_007093680 | 0.72 | ↑ |
| 防御机制 Defense mechanisms (1) | |||||
| V | I1GTZ4 | Enamine deaminase RidA, house cleaning of reactive enamine intermediates, YjgF/YER057c/UK114 family | YP_003319835 | 0.55 | ↑ |
| 功能未知 Function unknown (1) | |||||
| S | I1IC12 | Uncharacterized protein YjbI, contains pentapeptide repeats | YP_007125802 | 0.50 | ↑ |
新窗口打开|下载CSV
2.4 GO注释与GO富集分析
如图4所示, 对76个差异蛋白通过GO注释到27个, 显著富集16个代谢路径, 主要表现在氧化还原过程(oxidation-reduction process) 33.9%; 氧化还原酶活性(oxidoreductase activity) 28.6%; 氧化还原酶活性, 作用于供体的CH-OH基团, NAD或NADP作为受体(oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor) 7.1%; 未折叠的蛋白质结合(unfolded protein binding) 5.4%。从细胞组分(cellular component)、分子功能(molecular function)以及生物过程(biological process)这三个方面分类, 在细胞组分没有成分发生变化; 分子功能有10个成分发生变化, 其中level 3统计富集的生物学过程有2个, 分别为氧气结合(oxygen binding)和氧化还原酶活性(oxidoreductase activity); 生物过程有6个成分发生变化。图4
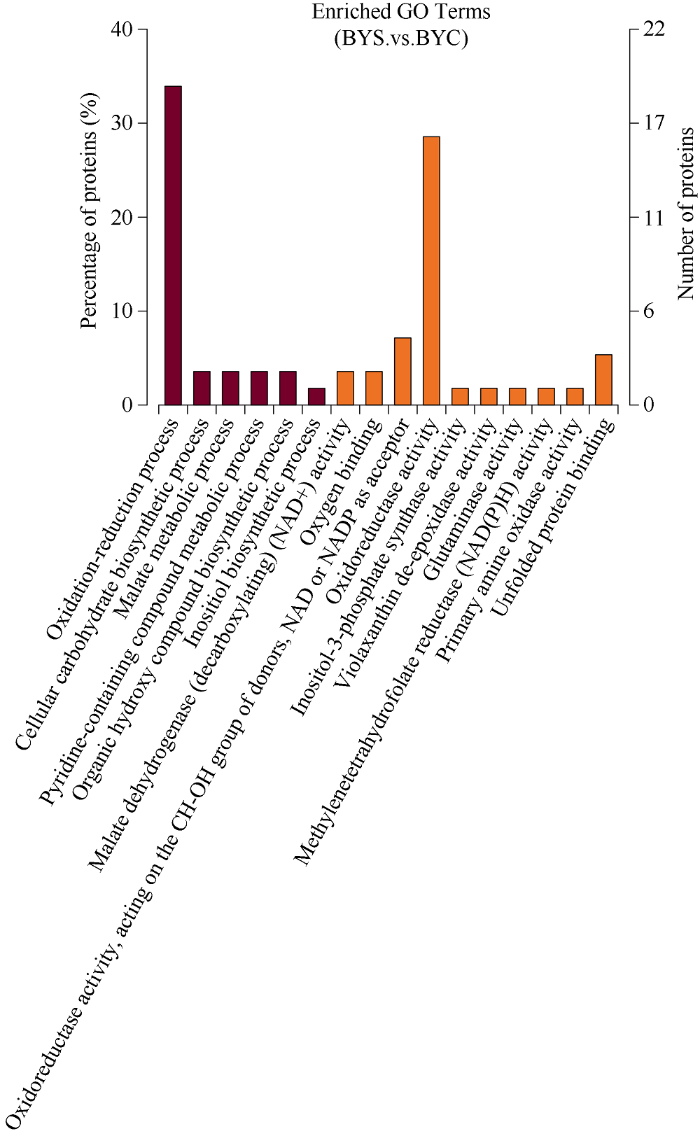 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4差异蛋白GO富集结果
图中展示了3个类别中的富集结果, 每种最多展示20种。
Fig. 4Differential protein GO enrichment results
The enrichment results in the three categories are shown in the figure, with up to 20 of each.
2.5 KEGG注释与KEGG富集分析
在盐胁迫过程中, 蛋白功能的行使是依靠多个蛋白的协同作用, 导致大量蛋白发生明显变化。而通路分析可以更全面、更系统地了解每个蛋白的生物学过程及应对盐胁迫的机制。通过KEGG富集, 76个差异蛋白中有22个差异蛋白显著富集10个生化代谢途径, 如图5所示, 分别为β-丙氨酸代谢(beta-Alanine metabolism)、长寿调节途径(Longevity regulating pathway-multiple species)、抗原处理和呈现(Antigen processing and presentation)、内质网中的蛋白质加工(Protein processing in endoplasmic reticulum)、雌激素信号通路(Estrogen signaling pathway)、维生素B6代谢(Vitamin B6 metabolism)、氯代烷烃和氯代烯烃降解(Chloroalkane and chloroalkene degradation)、精氨酸和脯氨酸代谢(Arginine and proline metabolism)、柠檬烯和蒎烯降解(Limonene and pinene degradation)、沙门氏菌感染(Salmonella infection), 且Protein processing in endoplasmic reticulum、Longevity regulating pathway-multiple species、Antigen processing and presentation和Estrogen signaling pathway这4个途径在盐胁迫条件下发生了非常显著变化。图5
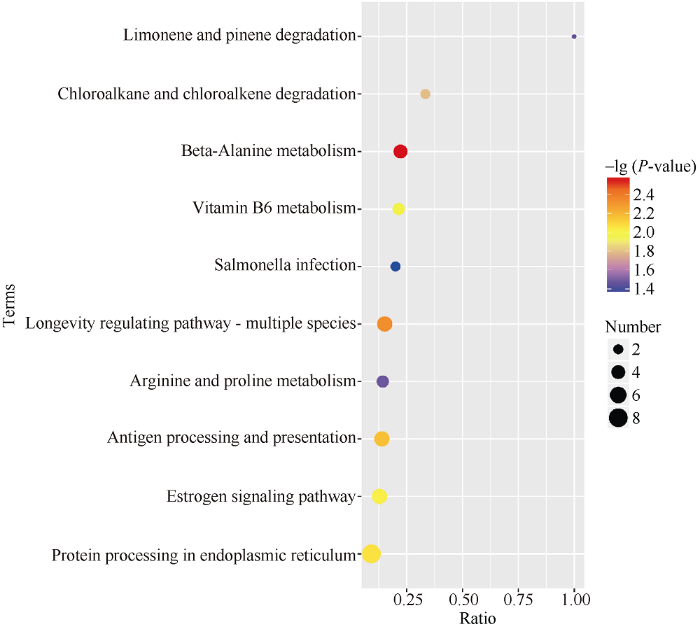 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5差异蛋白KEGG富集结果气泡图
横坐标为相应term中差异蛋白的数目与鉴定出的总蛋白数目的比值, 值越大, 说明在该term中差异蛋白富集程度越高。点的颜色代表超几何检验的P-value值, 值越小,说明检验的可靠性越大、越具统计学意义。点的大小代表相应term中差异蛋白的数目, 越大, 该term内差异蛋白就越多。
Fig. 5Bubble diagram of differential protein KEGG enrichment results
The abscissa is the ratio of the number of differential proteins in the corresponding term to the number of total proteins identified. The larger the value, the higher the degree of differential protein enrichment in this term. The color of the point represents the P-value of the hypergeometric test. The smaller the value, the greater the reliability and statistical significance of the test. The size of the dots represents the number of differential proteins in the corresponding term, the larger the size the more differential protein number in the term.
2.6 差异蛋白质互作调控网络
76个差异蛋白通过String在线软件(http://www.string- db.org/)构建蛋白质互相作用调控网络, 并隐藏没有相互作用的蛋白, 得到21个差异蛋白共建立50种互作关系, 如图6所示, 与蛋白质F2DYT5互作的蛋白数最多, 有6个; 有4个蛋白质与F4Y589和A0A1D5SA32互作; 均有3个蛋白质与I1GZ93、I1GU32、W5EGU4、I1IF07、M0Y631、A0A1D5Y3B7互作; 2个蛋白质与A9LIN4、I1IU29、A0A1D5S6L5、I1J3C6、Q3I0N4、F2E3N4互作; 仅有一个与之互作的蛋白质有I1GM81、A0A1D6AN16、O65195、Q8S311、G8CLP1和I1HU73。图6
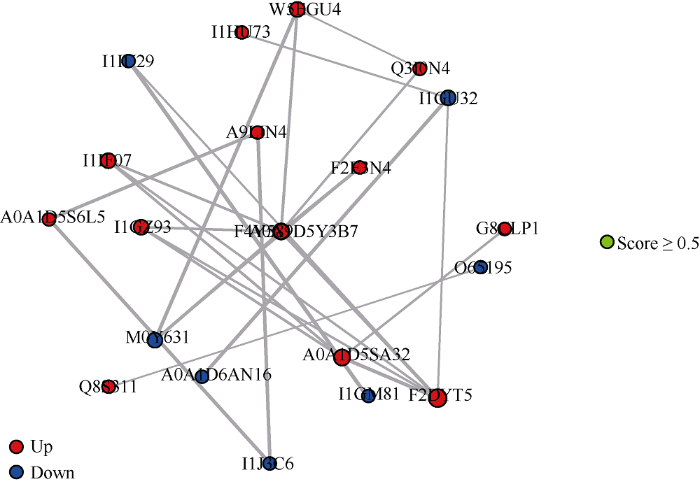 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6蛋白质互相作用网络图
红色节点代表上调蛋白, 蓝色节点代表下调蛋白, 节点越大, 代表与之互作的蛋白数越多, 节点间连线的粗细代表相互作用强度。
Fig. 6Protein interaction network diagram
The red node represents the up-regulated protein, the blue node represents the down-regulated protein, and the larger the node, the more the number of proteins interacting with it, and the thickness of the line between the nodes represents the interaction strength.
3 讨论
植物抗盐性指标是研究植物抗盐机理和能力的基础, MDA间接表示膜受损状况, 且有反馈作用[18]。高彩婷等[19]发现高盐胁迫24 h燕麦叶片MDA含量最高, 之后迅速下降。本研究盐胁迫6 d MDA含量下降, 可能是长时间胁迫造成的。SOD、POD在植物体内活性氧清除过程中起重要作用[20]。周莹等[21]研究表明, SOD活性随NaCl浓度升高呈先增后降趋势, POD活性呈逐渐下降趋势。本试验中, 150 mmol L-1为燕麦忍受盐胁迫的临界浓度[22], SOD、POD活性均降低, 与前人研究结果一致, 且SOD活性的变化规律也反映了与其相关蛋白I1I9J4的表达情况, 在盐胁迫下呈下调表达。前人研究表明草木樨幼苗在NaCl胁迫下, 产生更多的Pro维持细胞渗透平衡[23]。本试验与其结果一致, 盐胁迫下燕麦叶片Pro含量增加。通过比较CK与盐胁迫处理蛋白质变化, 共鉴定到76个差异蛋白, 通过GO显著富集16个代谢途径, KEGG显著富集10个代谢途径; COG数据库注释得到62个差异蛋白分属于不同蛋白质家族, 包含11个丰富的代谢途径, 这与ZHAO[24]研究结果相似。其中翻译后修饰、蛋白质周转、分子伴侣等功能包含最多的差异蛋白质, 为15个, 且除M0Y631外全部上调。蛋白质互相作用网络图表明大部分蛋白来自这一代谢途径, 表明盐胁迫下燕麦通过合成大量蛋白来抵御逆境[25]。本研究鉴定的差异蛋白质中, 有9个与分子伴侣相关, 4个与蛋白质周转相关, 2个与蛋白质翻译后修饰相关。分子伴侣蛋白不仅对蛋白质前体的转运起重要作用[26], 还能调控蛋白质折叠、积累及其定位和降解[27,28,29,30]。本研究鉴定的9个分子伴侣相关蛋白均属热激蛋白, 在盐胁迫条件下8个上调, 且与F2DYT5、F4Y589互作的蛋白数最多。Hamilton等[31]研究发现玉米就是通过合成热激蛋白应对高盐胁迫对其造成的伤害。Ribosomal是蛋白质合成的主要“工厂”, 在mRNA翻译成多肽的过程中起重要作用[12]。本试验中F2DBP2上调, 推测其与热激蛋白的合成有关。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
