 ,*, 赵丹阳*西北农林科技大学农学院, 陕西杨凌 712100
,*, 赵丹阳*西北农林科技大学农学院, 陕西杨凌 712100Efficient Separation and Identification of High Molecular Weight Glutenin Subunits by HPCE
WANG Wei-Dong, GAO Xiang ,*, ZHAO Dan-Yang*College of Agronomy, Northwest A&F University, Yangling 712100, Shaanxi, China
,*, ZHAO Dan-Yang*College of Agronomy, Northwest A&F University, Yangling 712100, Shaanxi, China通讯作者:
第一联系人:
收稿日期:2017-11-24接受日期:2018-03-26网络出版日期:2018-04-16
| 基金资助: |
Received:2017-11-24Accepted:2018-03-26Online:2018-04-16
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (0KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王卫东, 高翔, 赵丹阳. 高分子量麦谷蛋白亚基HPCE高效分离及图谱鉴定[J]. 作物学报, 2018, 44(7): 966-976. doi:10.3724/SP.J.1006.2018.00966
WANG Wei-Dong, GAO Xiang, ZHAO Dan-Yang.
胚乳贮藏蛋白是决定小麦加工品质的重要因素, 它主要包括麦谷蛋白和醇溶蛋白[1]两大类。依据电泳迁移率, 可将麦谷蛋白进一步分为高分子量麦谷蛋白亚基(high molecular weight glutenin subunit, HMW-GS)和低分子量麦谷蛋白亚基(low molecular weight glutenin subunit, LMW-GS)。研究表明, 小麦品质变异的30%~79%归因于编码HMW-GS等位基因的变化[2]。普通小麦一般有3~5个HMW-GS, 由1A、1B、1D染色体长臂上的Glu-1位点编码, 每个位点含有两个紧密连锁的基因, 分别编码分子量较大的x-型亚基和分子量较小的y-型亚基[3]。HMW-GS的组成与面包烘烤品质密切相关, 其快速、准确的分离与鉴定已成为小麦品质研究的重要环节。
用于HMW-GS分离鉴定的常规方法主要包括SDS-PAGE、分子标记、RP-HPLC等[4,5,6,7]。SDS-PAGE是最常用的鉴定方法, 其操作简单、成本较低, 但是对于迁移率比较接近的亚基难以准确鉴别, 且分离所需时间较长。分子标记技术鉴定准确性高, 但受限于标记种类且往往需要与SDS-PAGE相结合检验基因是否表达。RP-HPLC技术选择性好, 检测灵敏度高, 但同样存在用时较长等缺点, 不适宜大量样品分析。近年来高效毛细管电泳技术(HPCE)由于其用样少、速度快、分辨率高等特点, 显示了在HMW-GS的分离与鉴定中良好的应用前景。
对HPCE的分离效率, 已从磷酸盐缓冲液系统、硼酸盐缓冲液系统、乳酸铝缓冲液系统及Prosot SDS等方面进行了研究[8,9,10,11,12], 验证了其在蛋白定性分析中的可行性, 但是用HPCE分离小麦HMW-GS的研究尚处起步阶段, 有关标准鉴定图谱的研究甚少, 且已报道的分离体系在分离速度和分辨率上还有待提高。本研究以此为切入点, 引入IDA缓冲液系统, 通过分析不同组分浓度、pH对分离效果的影响并结合电泳参数的优化, 建立HPCE高效分离体系, 并以此为基础研究不同亚基的迁移图谱, 为小麦HMW-GS的定性分析及种质筛选提供了技术支持。
1 材料与方法
1.1 试验品种
选用12个小麦品种, 包括中国春、西农979、济南13、晋麦47、济麦4号、豫麦41、烟农19、莱州137、鲁麦23、矮抗58、豫麦50和郑麦366。中国春、西农979、济南13、矮抗58、豫麦50由国家小麦改良中心杨凌分中心品质实验室提供, 其他品种由山西省农业科学院小麦研究所提供。本课题组前期研究证实这些品种在HMW-GS组成上具有多样性。1.2 HMW-GS的提取和鉴定
参考Jang等[13]描述的方法, 并稍作修改。取单粒小麦种子, 研磨成粉, 加入55%异丙醇1 mL, 65°C振荡冲洗3次, 每次30 min, 充分去除水溶性蛋白及单体醇溶蛋白。向残余沉淀中加入100 μL提取液(含50%异丙醇, 80 mmol L-1 Tris-HCl, pH 8.8, 1% DTT), 充分混匀, 65°C水浴30 min, 加入1.4% 4-乙烯基吡啶(v/v), 混匀后, 保持同一水浴环境烷基化反应30 min。常温15 000× g离心15 min, 取上清液加入丙酮, 使其在混合液中的终浓度为40%, 静置2 h, 15 000× g离心, 弃上清液, 风干后沉淀于-20°C保存备用。采用Lee等[14]改良的SDS-PAGE方法, 利用MV-10DSYS电泳仪对小麦各品种进行HMW-GS的初步鉴定。样品中加入20 μL提取缓冲液(含2% SDS, 0.02%溴酚蓝, 0.08 mol L-1 Tris-HCl, pH 8.0, 40%甘油), 振荡混匀, 沸水浴中煮沸5 min, 取8 μL上样。采用12%分离胶与5%浓缩胶, 12 mA恒流电泳, 指示条带出胶后继续电泳2 h。使用1%考马斯亮蓝R-250染色过夜, 脱色至背景清晰, 照相记录结果。
用分子标记法验证HMW-GS的初步鉴定结果。取光照培养1周的幼苗嫩叶, 用CTAB法[15]提取基因组DNA。各亚基基因的分子标记信息见表1。
1.3 HPCE高效分离体系的建立
1.3.1 缓冲液系统 将单粒种子所得HMW-GS样品, 用200 μL溶解液A (丙三醇15 mL, 尿素18 g, 乙酸75 μL, 加ddH2O定容至50 mL)溶解, 然后25 °C超声(50W)处理30 min, 充分去除气泡。使用P/ACE MDQ plus毛细管电泳仪(BECKMAN, USA)进行HPCE分析, 参考Robertson等[26]描述的方法, 并做适当改进。新管平衡程序为0.1 mol L-1 NaOH冲洗1 min, ddH2O冲洗2 min, 1 mol L-1 H3PO4冲洗1 min, 缓冲液冲洗 5 min。进样前, 用1 mol L-1 H3PO4 冲洗1 min, 0.1 mol L-1 NaOH冲洗2 min, dd H2O冲洗1 min。两次样品间用1 mol L-1 H3PO4 冲洗30 s, ddH2O冲洗1 min, 缓冲液冲洗1 min。分析缓冲液不同组分浓度及pH对HMW-GS分离效果的影响。依据蛋白分析中IDA缓冲液系统条件范围[27], 设置2.2、2.5和2.8的pH梯度。IDA浓度为50、75和100 mmol L-1。HPMC浓度为0.05%、0.50%和不添加。ACN浓度为10%、15%和20%。Table 1
表1
表1HMW-GS分子标记
Table 1
| 亚基 Subunit | 标记序列 Sequence (5′→3′) | 片段大小 Size of fragment (bp) | 参考文献 Reference | |
|---|---|---|---|---|
| 1Ax1 | F: TCACCGACAGTCCACCGA; R: ACCAAGCGAGCTGCAGAG | 2532 | Bustos et al. [16] | |
| Null | F: ACGTTCCCCTACAGGTACTA; R: TATCACTGGCTAGCCGACAA | 920 | Lafiandra et al. [17] | |
| 1Ax2* | F: ATGACTAAGCGGTTGGTTCTT; R: ACCTTGCTCCCCTTGTCTTT | 1319 | Ma et al. [18] | |
| 1Bx6 | F: CACTGAGATGGCTAAGCGCC; R: GCCTTGGACGGCACCACAGG | 321 | Schwarz et al. [19] | |
| 1Bx7/1Bx17 | F: CGCAACAGCGAGGACAATT; R: TGGTCCGTCACTATCTTGAGA | 766/669 | Ma et al. [18] | |
| 1Bx13 | F: ATGAGCTAAGCGCGCTGGTCCTCTTTG; R: CTATCACTGCCTGGTCCGACAATGCG | 900 | Pang and Zhang [20] | |
| 1Bx20 | F: CCTCAGCATGCAAACATGCAGC; R: CTGAAACCTTTGGCCAGTCATGTC | 800 | Butow et al. [21] | |
| 1By8 | F: TTAGCGCTAAGTGCCGTCT; R: TTGTCCTATTTGCTGCCCTT | 527 | Lei et al. [22] | |
| 1By9 | F: TTCTCTGCATCAGTCAGGA; R: AGAGAAGCTGTGTAATGCC | 707 | Lei et al. [22] | |
| 1By16 | F: GCAGTACCCAGCTTCTCAA; R: CCTTGTCTTGTTTGTTGCC | 3 fragments | Lei et al. [22] | |
| 1By18 | F: CAACAAAACGGGCGTTGT; R: CAACAAAACGGGCGTTGT | 365 | Liang et al. [23] | |
| 1Dx2/1Dx5 | F: GGGACAATACGAGCAGCAAA; R: CTTGTTCCGGTTGTTGCCA | 299/281 | Liu et al. [24] | |
| 1Dy10/1Dy12 | F: GTTGGCCGGTCGGCTGCCATG; R: TGGAGAAGTTGGATAGTACC | 576/612 | Ahmad [25] | |
新窗口打开|下载CSV
1.3.2 电泳参数 在相同缓冲液条件下, 比较电泳参数对分离效果的影响。常规电泳参数为毛细管内径50 μm, PDA检测波长214 nm, 分离电压15 kV, 运行温度25°C [28]。优化后的电泳参数为毛细管内径25 μm, PDA检测波长200 nm, 分离电压20 kV, 运行温度30°C。
1.4 HPCE高效分离体系的验证
1.4.1 连续重复电泳 结合Werner等[9]和余建中[29]的研究结果初步分析分离图谱的特点。连续30次重复试验, 对HPCE高效分离体系下图谱峰形、峰高稳定性进行检验。1.4.2 RP-HPLC分析 与RP-HPLC比对分析, 验证分离速度, 记录出峰时间并计算RSD值, 检验分离重现性。仪器为Agilent 1100 (USA)液相色谱仪及反向高效液相色谱柱(ZORBAX 300SB-C18 Stable Band Analytical 4.6 × 250 mm, 5-Micron)。参照Dong等[30]描述的方法, 用100 μL溶解液B (21% ACN 0.2 mL, 0.1% TFA)溶解HMW-GS样品。进样量25 μL, 设置柱温60 °C, 流速1 mol L-1, 洗脱梯度50 min内流动相(0.06% TFA-ACN溶液)的体积比由21%增加到41%; 相邻样品间柱洗涤时间15 min; 紫外光检测波长210 nm。
1.5 HMW-GS图谱鉴定
以上述HPCE高效分离体系为基础, 比较不同类型HMW-GS的迁移顺序及标准出峰时间, 为减小误差, 对亚基组成相似的品种采用混合进样方式[31]。2 结果与分析
2.1 供试品种的HMW-GS组成鉴定
SDS-PAGE结合“Payne标准图谱”初步鉴定结果表明, 供试品种在Glu-1A位点的类型有null、1Ax1和1Ax2*; 在Glu-1B位点的亚基组合类型有1Bx20、1Bx7+1By8、1Bx7+1By9、1Bx6+1By8、1Bx13+1By16、1Bx17+1By18和1Bx20+1By20, 除鲁麦23外均成对亚基; 在Glu-1D位点的亚基组合类型有1Dx2+1Dy12、1Dx3+1Dy12、1Dx4+1Dy12和1Dx5+1Dy10 (图1-A)。图1
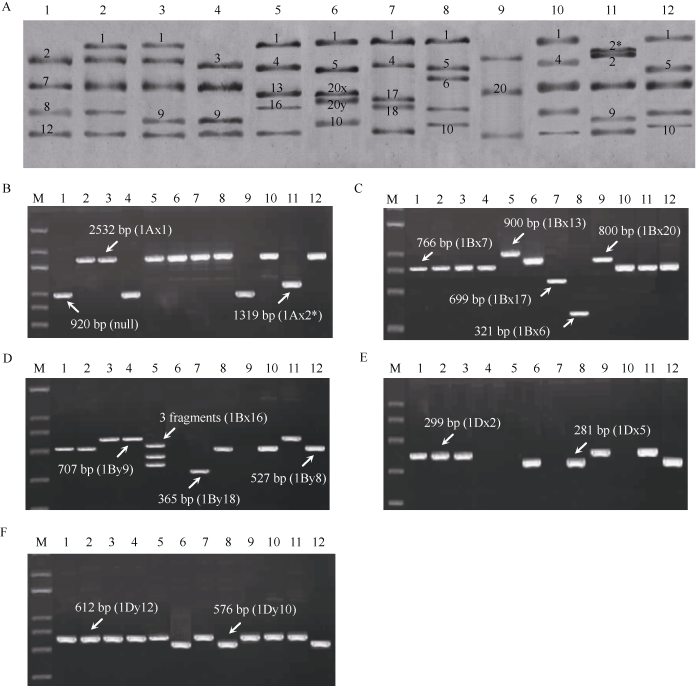 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1小麦Glu-1位点亚基组成和编码基因的鉴定
A: SDS-PAGE分析; B~F: HMW-GS分子标记鉴定。M: Trans 2k plus; 1: 中国春; 2: 西农979; 3: 济南13; 4: 晋麦47; 5: 济麦4号; 6: 豫麦41; 7: 烟农19; 8: 州137; 9: 鲁麦23; 10: 矮抗58; 11: 豫麦50; 12: 郑麦366。
Fig. 1Identification of the subunit composition and coding genes of Glu-1 loci in wheat
A: analysis of SDS-PAGE; B-F: identification of HMW-GS by molecular markers. M: Trans 2k plus DNA marker; 1: Chinese Spring; 2: Xinong 979; 3: Jinan 13; 4: Jinmai 47; 5: Jimai 4; 6: Yumai 41; 7: Yannong 19; 8: Laizhou 137; 9: Lumai 23; 10: Aikang 58; 11: Yumai 50; 12: Zhengmai 366.
分子标记鉴定与SDS-PAGE分析结果一致, 由此确定了供试12个品种的HMW-GS的组成, 共包含18个亚基(图1-B~F, 表2)。Glu-1A位点, 仅豫麦50含1Ax2*亚基, 中国春、晋麦47和鲁麦23为null, 其余品种均含1Ax1亚基; Glu-1B位点, 中国春等7个品种含1Bx7亚基, 豫麦41和鲁麦23含1Bx20, 烟农19含1Bx17, 莱州137含1Bx6, 中国春等5个品种含1By8, 济南13、晋麦47和豫麦50含1By9, 济麦4号含1By16, 烟农19含1By18; Glu-1D位点, 中国春等5个品种含1Dx2亚基, 豫麦41、莱州137和郑麦366同时含1Dx5和1Dy10, 其余品种y型亚基均为1Dy12。
Table 2
表2
表2材料品种HMW-GS组成
Table 2
| 品种 Variety | Glu-1A | Glu-1B | Glu-1D |
|---|---|---|---|
| 中国春 Chinese Spring | null | 7+8 | 2+12 |
| 西农979 Xinong 979 | 1 | 7+8 | 2+12 |
| 济南13 Jinan 13 | 1 | 7+9 | 2+12 |
| 晋麦47 Jinmai 47 | null | 7+9 | 3+12 |
| 济麦4号 Jimai 4 | 1 | 13+16 | 4+12 |
| 豫麦41 Yumai 41 | 1 | 20x+20y | 5+10 |
| 烟农19 Yannong 19 | 1 | 17+18 | 4+12 |
| 莱州137 Laizhou 137 | 1 | 6+8 | 5+10 |
| 鲁麦23 Lumai 23 | null | 20 | 2+12 |
| 矮抗58 Aikang 58 | 1 | 7+8 | 4+12 |
| 豫麦50 Yumai 50 | 2* | 7+9 | 2+12 |
| 郑麦366 Zhengmai 366 | 1 | 7+8 | 5+10 |
新窗口打开|下载CSV
2.2 HPCE高效分离体系及其影响因子分析
2.2.1 缓冲液pH的影响 中国春有4个HMW-GS特征峰, 按出峰时间由前向后依次为1Dy12、1By8、1Bx7和1Dx2 [32,33]。本研究以中国春为标准样, 分析缓冲液不同组分浓度及pH值对分离效果的影响。缓冲液系统不同pH值对HMW-GS峰高、峰宽及基线均有显著影响。随着pH值的降低, 亚基峰宽逐渐增大, y型亚基峰高有增大的趋势, 而x型亚基则相反。pH为2.8和2.2时均出现不同程度的基线波动, 尤其是pH 2.8时, 电泳分离后期有基线上升的趋势, 1By8亚基有副峰的影响。调整pH为2.5时基线状况最佳, 且亚基分离度较高, 整体迁移速率介于前两者之间, 4个特征峰的出峰时间分别为10.24、11.94、13.40和15.00 min (图2-A)。可见, 缓冲液pH 2.5为最佳条件。
2.2.2 IDA浓度的影响 提高IDA浓度, 对亚基迁移速率有促进的作用, 对亚基峰宽、峰高、分离度及基线影响不大。通过连续两针电泳比较发现, 不同IDA浓度对图谱重现性有显著影响。IDA浓度
为100 mmol L-1和50 mmol L-1时, 连续两针电泳亚基出峰时间均出现了较大的偏差。调整IDA浓度为75 mmol L-1时, 两针电泳下亚基主峰几乎重合, 亚基峰形、峰高均保持较高的一致性, 该浓度下亚基迁移速率介于50 mmol L-1和100 mmol L-1之间(图2-B)。因此, IDA适宜浓度为75 mmol L-1。
2.2.3 HPMC浓度的影响 HPMC是HPCE分离体系的常用缓冲液添加剂, 不添加HPMC时, 电泳整体迁移速率最大, 但亚基之间未完全分离, 且峰形不规则, 峰宽过大, 显著影响了图谱分辨率; 添加HPMC后, 亚基分离度明显提高, 浓度为0.5%时, 亚基峰宽较小, 但基线噪音很大, 当调整浓度为0.05%时, 峰宽增大, 基线噪音显著减小, 且主峰峰形规则, 因此图谱分辨率更高(图2-C)。可见, HPMC的加入对分离效率有促进作用, 且适宜浓度为0.05%。
2.2.4 ACN浓度的影响 3种ACN浓度下均获得清晰对称的峰形。随着ACN浓度的减小, 亚基分离度增大, 当ACN浓度为10%时, 分离度最高, 但是亚基峰高有明显降低, 同时由于该浓度下峰宽较大, 导致亚基纵向扩散与峰展宽的比例过小, 影响了图谱分辨率(图2-D)。ACN浓度为15%和20%时这一比例差异不大, 但前者分离度更高, 这将有利于其他类型亚基的加入和识别。因此, 推荐ACN适用浓度为15%。
图2
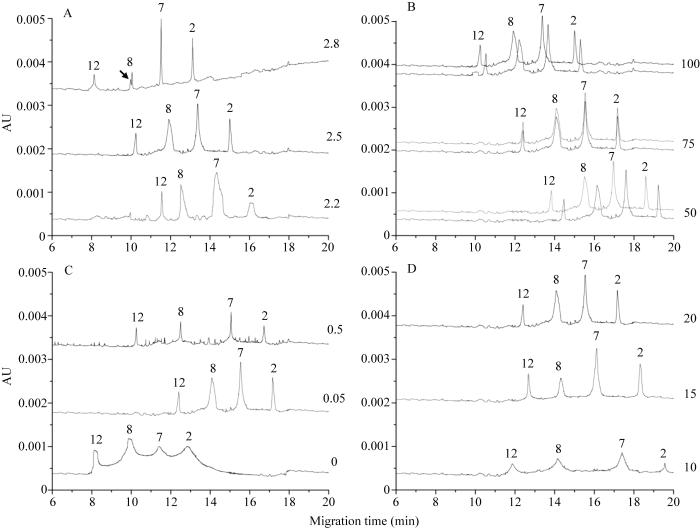 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2缓冲液组分浓度、pH对HMW-GS电泳分离的影响
A: 不同pH对分离效果的影响, 缓冲液组分为100 mmol L-1 IDA+0.05% HPMC+20% ACN。B: 缓冲液不同IDA浓度下连续两针电泳分离结果, 其他组分为0.05% HPMC+20% ACN, pH 2.5。C: 缓冲液不同HPMC浓度对分离效果的影响, 其他组分为75 mmol L-1 IDA+20% ACN, pH 2.5。D: 缓冲液不同ACN浓度对分离效果的影响, 其他组分为75 mmol L-1 IDA+0.05% HPMC, pH 2.5。A~D电泳参数均为毛细管内径50 μm, PDA检测波长214 nm, 分离电压15 kV, 运行温度25 °C。箭头所示为副峰。
Fig. 2Effect of constituent concentration and pH values of buffer on electrophoresis separation of HMW-GS
A: effect of different pH on the electrophoresis separation, the constituents of the buffer were 100 mmol L-1 IDA + 0.05% HPMC + 20% ACN. B: results of two successive electrophoretic separation under different concentrations of IDA, the other constituents of the buffer were 0.05% HPMC + 20% ACN, the pH was 2.5. C: effect of different concentrations of HPMC on the electrophoresis separation, the other constituents of the buffer were 75 mmol L-1 IDA + 20% ACN, the pH was 2.5. D: effect of different concentrations of ACN on the electrophoresis separation, the other constituents of the buffer were 75 mmol L-1 IDA + 0.05% HPMC, the pH was 2.5. Electrophoresis parameters of A-D: the inner diameter of the capillary was 50 μm; the detection wavelength was 214 nm; the separation voltage was 15 kV; the operating temperature was 25 °C. The arrow indicates the position of the secondary peak.
2.2.5 电泳参数的影响 参数优化后HPCE图谱整体迁移速率提高, 分离所需时间变短, 同时由于亚基峰高增大、峰宽减小, 亚基纵向扩散与峰展宽的比例扩大, 图谱分辨率得到进一步提升(图3), 在后期分离鉴定迁移率比较接近的亚基时将更加有利。
图3
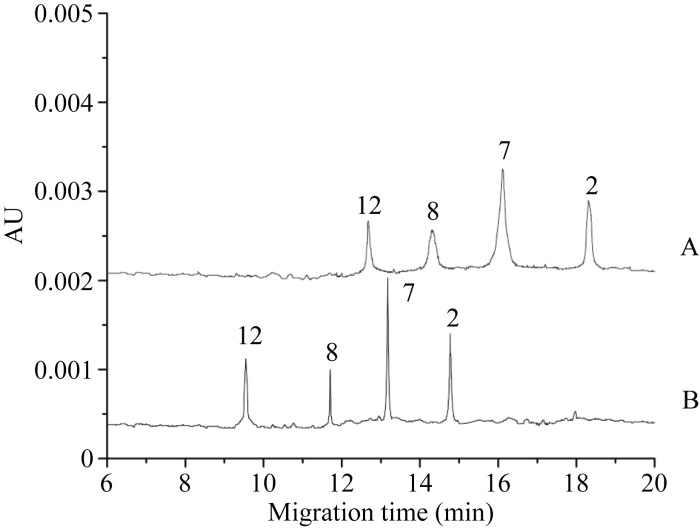 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3电泳参数优化前(A)和优化后(B)的HPCE图谱
缓冲液成分: 75 mmol L-1 IDA + 0.05% HPMC + 15% ACN, pH 2.5。优化前电泳参数: 毛细管内径50 μm, PDA检测波长214 nm, 分离电压15 kV, 运行温度25°C; 优化后电泳参数: 毛细管内径25 μm, PDA检测波长200 nm, 分离电压20 kV, 运行温度30°C。
Fig. 3HPCE profile before (A) and after (B) optimization of electrophoresis parameters
Buffer constituents: 75 mmol L-1 IDA + 0.05% HPMC + 15% ACN, pH 2.5. Electrophoresis parameters were 50 μm of the inner diameter of capillary, 214 nm of detection wavelength, 15 kV of separation voltage, and 25°C of operating temperature before optimization and 25 μm of the inner diameter of capillary, 200 nm of the detection wavelength, 20 kV of separation voltage, and 30°C of operating temperature after optimization.
综合考虑各种因子对分离效果的影响, 确定了最佳缓冲液体系为75 mmol L-1 IDA + 0.05% HPMC + 15% ACN, pH 2.5, 电泳参数为毛细管内径25 μm, PDA检测波长200 nm, 分离电压20 kV, 运行温度30°C。据此建立了小麦HMW-GS的HPCE高效分离体系。
2.3 HPCE高效分离体系验证
与前人报道一致[9,29], HPCE图谱中1~2 min为溶剂峰, 3~7 min为少量LMW-GS区域, HMW-GS区域始于9 min (图4)。1Dy12、1By8、1Bx7和1Dx2亚基在16 min内全部分离完成。连续30次电泳, 图谱峰形及峰高均保持稳定, 且基线水平、噪音低, 图谱分辨率良好。图4
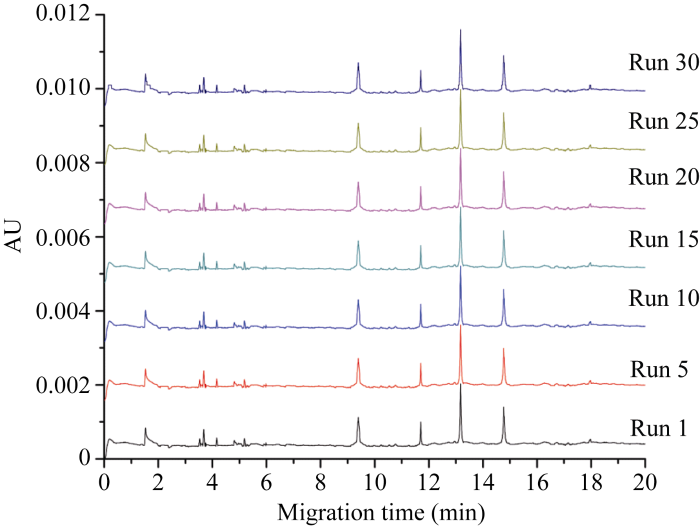 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4中国春HMW-GS连续30次HPCE分离
图中曲线分别对应第1、5、10、15、20、25和30次电泳分离。
Fig. 4Thirty separations of HMW-GS of Chinese Spring by HPCE
The curves correspond to the 1st, 5th, 10th, 15th, 12th, 25th, and 30th electrophoresis separation, respectively.
通过比较HMW-GS分离度及迁移速度, 发现本研究建立的HPCE高效分离体系优于RP-HPLC (图5), 更利于鉴别迁移率相近亚基。比较亚基出峰时间(表3), HPCE高效分离体系相对标准偏差在0.2%以下, 远低于RP-HPLC的1.0%以下, 因此分离重现性更高。良好的稳定性、分离速度和重现性保证了HMW-GS图谱鉴定的准确性。
图5
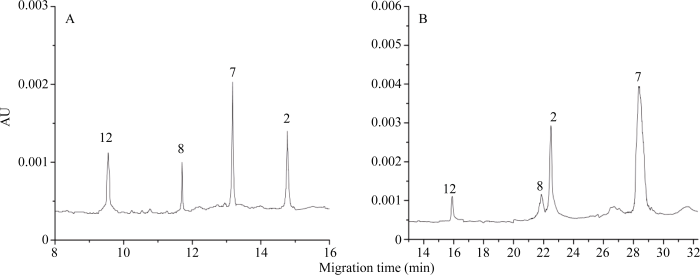 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5HPCE (A)与RP-HPLC (B)的分离图谱
以中国春HMW-GS为标准样品。参照Yan等[34]的方法命名RP-HPLC中的特征峰。
Fig. 5Separation profile of HPCE (A) and RP-HPLC (B)
Chinese Spring HMW-GS was the standard. Nomenclature of characteristic peaks in the RP-HPLC profile follows the descriptions of Yan et al. [34]
Table 3
表3
表3HMW-GS出峰时间相对标准偏差
Table 3
| 亚基 Subunit | HPCE | RP-HPLC | ||
|---|---|---|---|---|
| 出峰时间 Peak time (min) | 相对标准偏差 Relative standard deviation (%) | 出峰时间 Peak time (min) | 相对标准偏差 Relative standard deviation (%) | |
| 1Dx2 | 14.73±0.02 | 0.12 | 22.31±0.11 | 0.40 |
| 1Bx7 | 13.18±0.01 | 0.07 | 28.42±0.22 | 0.63 |
| 1By8 | 11.70±0.01 | 0.14 | 21.86±0.21 | 0.78 |
| 1Dy12 | 9.39±0.02 | 0.18 | 15.91±0.18 | 0.92 |
Peak time is the average ± standard derivation of 30 consecutive tests.
新窗口打开|下载CSV
2.4 HMW-GS图谱鉴定
中国春1Dy12、1By8、1Bx7、1Dx2亚基出峰时间依次为9.39、11.70、13.18和14.73 min (图6-A)。在中国春HMW-GS的基础上, 添加西农979 (1, 7+8, 2+12)混合进样, 在13.73 min处获得1Ax1亚基主峰, 其迁移速率介于1Bx7和1Dx2亚基之间(图6-B)。继续添加济南13 (1, 7+9, 2+12)混合进样, 于10.30 min处获得1By9亚基特征峰, 其迁移时间晚于1Dy12亚基(图6-C)。图6
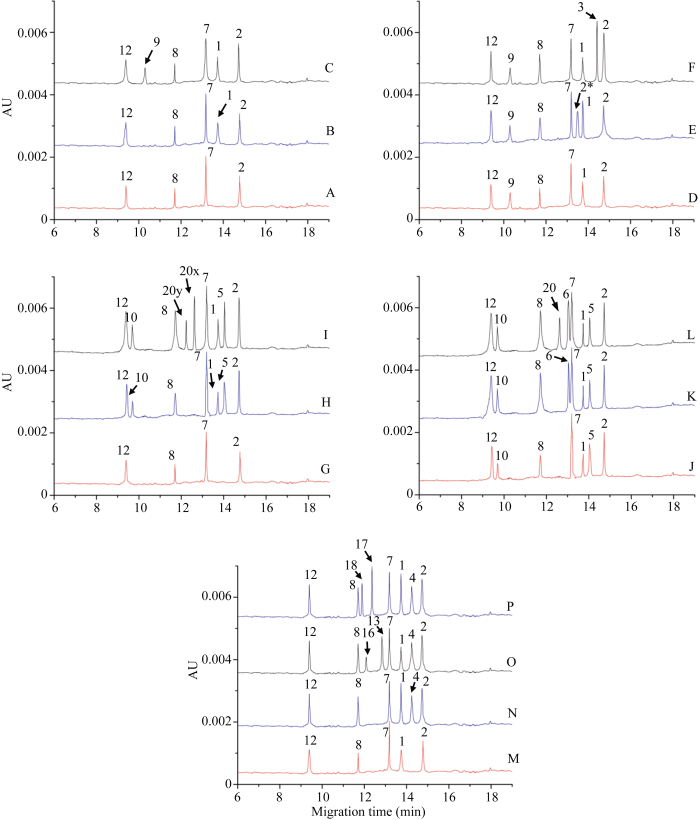 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6不同HMW-GS的HPCE图谱(混合进样)
A: 中国春(null, 7+8, 2+12); B: 中国春+西农979 (1, 7+8, 2+12); C: 中国春+西农979+济南13 (1, 7+9, 2+12); D: 中国春+西农979+济南13; E: 中国春+西农979+济南13+豫麦50 (2*, 7+9, 2+12); F: 中国春+西农979+济南13+晋麦47 (null, 7+9, 3+12); G: 中国春; H: 中国春+郑麦366 (1, 7+8, 5+10); I: 中国春+郑麦366+豫麦41 (1, 20x+20y, 5+10); J: 中国春+郑麦366; K: 中国春+郑麦366+莱州137 (1, 6+8, 5+10); L: 中国春+郑麦366+莱州137+鲁麦23 (null, 20, 2+12)。M: 中国春+西农979; N: 中国春+西农979+矮抗58 (1, 7+8, 4+12); O: 中国春+西农979+矮抗58+济麦4号 (1, 13+16, 4+12); P: 中国春(null, 7+8, 2+12) +西农979+矮抗58+烟农19 (1, 17+18, 4+12)。
Fig. 6HPCE spectrum of different HMW-GS (mixed injection)
A: Chinese Spring (null, 7+8, 2+12); B: Chinese Spring + Xinong 979 (1, 7+8, 2+12); C: Chinese Spring + Xinong 979 + Jinan 13 (1, 7+9, 2+12); D: Chinese Spring + Xinong 979 + Jinan 13; E: Chinese Spring + Xinong 979 + Jinan 13 + Yumai 50 (2*, 7+9, 2+12); F: Chinese Spring + Xinong 979 + Jinan 13 + Jinmai 47 (null, 7+9, 3+12); G: Chinese Spring; H: Chinese Spring + Zhengmai 366 (1, 7+8, 5+10); I: Chinese Spring + Zhengmai 366 + Yumai 41 (1, 20x+20y, 5+10); J: Chinese Spring + Zhengmai 366; K: Chinese Spring + Zhengmai 366 + Laizhou 137 (1, 6+8, 5+10); L: Chinese Spring + Zhengmai 366+Laizhou 137+Lumai 23 (Null, 20, 2+12). M: Chinese Spring + Xinong 979; N: Chinese Spring + Xinong 979+Aikang 58 (1, 7+8, 4+12); O: Chinese Spring + Xinong 979+Aikang 58+Jimai 4 (1, 13+16, 4+12); P: Chinese Spring + Xinong 979 + Aikang 58 + Yannong 19 (1, 17+18, 4+12).
在中国春(null, 7+8, 2+12)、西农979 (1, 7+8, 2+12)和济南13 (1, 7+9, 2+12)的基础上(图6-D), 添加豫麦50 (2*, 7+9, 2+12)获得1Ax2*亚基特征峰(图6-E), 添加晋麦47 (null, 7+9, 3+12)获得1Dx3亚基特征峰(图6-F)。1Ax2*亚基出峰时间为13.50 min, 该亚基的迁移时间介于1Bx7和1Ax1之间, 且与1Ax1亚基峰比较接近。1Dx3亚基迁移速率略快于1Dx2亚基, 出峰时间为14.46 min。
将中国春(null, 7+8, 2+12)与郑麦366 (1, 7+8, 5+10)混合进样, 在9.69、13.73和14.04 min处获得3个特征峰(图6-G, H)。已知13.73 min为1Ax1亚基, 14.04 min主峰面积大于9.69 min, 并且同一位点上x型亚基的含量大于y型亚基[3], HPCE图谱中峰面积大小代表了亚基含量的高低, 因此可知, 9.69 min为1Dy10亚基, 14.04 min为1Dx5亚基。同理, 继续添加豫麦41 (1, 20x+20y, 5+10)进样, 在12.22 min和12.62 min获得两个新的特征峰(图6-I), 前者峰面积较小, 为1By20亚基, 后者为1Bx20亚基。
向中国春、郑麦366混合样中添加莱州137, 获得1Bx6亚基峰, 出峰时间为13.08 min, 迁移速率与1Bx7亚基很接近(图6-J, K)。继续添加鲁麦23同样获得了1Bx20亚基峰(图6-L), 与图6-I鉴定结果一致。
向中国春与西农979混合样中添加矮抗58, 确定了1Dx4亚基峰, 其迁移速率介于1Ax1与1Dx2之间, 出峰时间为14.24 min (图6-M, N)。向中国春、西农979、矮抗58混合样分别添加济麦4号和烟农19, 并比较新加入峰面积, 获得1By16、1Bx13、1By18和1Bx17亚基特征峰(图6-O, P), 出峰时间分别为12.09、12.83、11.89和12.36 min。
获得的18个亚基的标准图谱, 亚基迁移顺序为1Dy12→1Dy10→1By9→1By8 → 1By18 → 1By16→ 1By20 → 1Bx17 → 1Bx20 →1Bx13→1Bx6→1Bx7→ 1Ax2*→1Ax1→1Dx5→1Dx4→1Dx3→1Dx2, 标准出峰时间如表4所示, 相对标准偏差在0.2%以下。以1Bx17为分界线, 9.39 min到12.36 min为y型亚基区域, 12.36 min到14.76 min为x型亚基区域。
Table 4
表4
表418个HMW-GS的出峰时间
Table 4
| Glu-1位点 Glu-1 locus | 亚基类型 Type of subunit | 亚基 Subunit | 出峰时间 Peak time (min) |
|---|---|---|---|
| Glu-1A | x-type | 1Ax1 | 13.73 |
| 1Ax2* | 13.50 | ||
| Glu-1B | x-type | 1Bx6 | 13.08 |
| 1Bx7 | 13.18 | ||
| 1Bx13 | 12.83 | ||
| 1Bx20 | 12.62 | ||
| 1Bx17 | 12.36 | ||
| y-type | 1By8 | 11.70 | |
| 1By9 | 10.30 | ||
| 1By20 | 12.22 | ||
| 1By16 | 12.09 | ||
| 1By18 | 11.89 | ||
| Glu-1D | x-type | 1Dx2 | 14.73 |
| 1Dx3 | 14.46 | ||
| 1Dx4 | 14.24 | ||
| 1Dx5 | 14.04 | ||
| y-type | 1Dy10 | 9.69 | |
| 1Dy12 | 9.39 |
新窗口打开|下载CSV
3 讨论
准确有效地鉴定HMW-GS将有助于加快品质改良步伐。HPCE技术在分离速度、分辨率等方面很大程度克服了传统方法的不足。HPCE中分离体系的构建是研究亚基标准图谱的前提。体系中缓冲液系统是影响分离效果的主要因素, Bean和Lookhart[12]最早将IDA缓冲系统引入HPCE, 主要对醇溶蛋白的分离进行分析。本研究将IDA引入HMW-GS的HPCE分析, 通过优化条件, 获得了良好的分离效果, 与常规的磷酸盐、硼酸盐、乳酸铝及Prosot SDS缓冲液系统[8,9,10,11,12]相比, 在分离重现性上更具有优势, 结合电泳参数的优化, 亚基出峰时间的相对标准偏差控制在0.2%以下, 误差范围在±0.02 min内, 从而保障了标准图谱分析的精确性。
在优化分离体系时, 缓冲液系统各组分浓度及pH值主要通过改变电渗流而影响分离[35]。小麦HMW-GS属于大分子蛋白[36], 极易吸附于毛细管内壁, 极端pH可以产生抑制作用[37], 稳定溶液电渗流, 实验中pH 2.8时电泳后期有基线上升的现象, 推测可能是该条件下抑制作用急剧下降, 使毛细管内产生了较多的焦耳热。IDA同样具有稳定电渗流的作用, 因此与分离重现性密切相关。Machiste等[38]认为加入HPMC明显提高缓冲液的分离效率, 因此可将HPMC作为添加剂使用。本研究印证了这一观点。在本研究中, ACN浓度升高将会使分离度下降, 其原因可能是ACN对电渗流的影响加快了样品堆积[39]。
在亚基标准图谱研究中, 本研究采用控制变量混合进样分离[31]的方式, 有助于减小试验误差。Werner等[9]、Sutton和Bietz[11]利用ProSort SDS商用试剂进行小麦HMW-GS的HPCE分析, 获得了1Ax1、1Dx2、1Bx7、1By8、1By9、1Dy10、1Dy12、1Bx17、1By18等亚基的迁移图谱, 出峰时间RSD在3%左右。亚基迁移顺序表现为1Dy12→1Dy10→ 1By9→1By8→1By18→1Bx17→1Bx7→1Ax1→1Dx2, 但对1Dy12与1Dy10、1By9与1By8等迁移率比较接近的亚基分离度不高。本研究增加了图谱中能够表征的亚基类型, 上述亚基出峰顺序与前人结果[9,11]一致, 但是迁移速率上略有差异, 在出峰时间相对标准偏差降低的同时, 亚基分离度获得了提升。
与SDS-PAGE类似, HPCE图谱中具有明显的x型、y型亚基分界, 整体上表现为y型亚基先洗脱, 然后是x型亚基。Salmanowicz等[33]研究表明, HMW-GS中y型亚基的pI为6.70~6.98, 近中性, 而x型亚基pI为4.72~5.23, 呈弱酸性, 在毛细管高压电场下, 碱性或近中性亚基的迁移时间比弱酸性亚基短。本研究发现, 1Ax1和1Ax2*的迁移速率均大于1Dx5, 与在SDS-PAGE中截然不同[40]。这可能与该类型亚基在两种分离环境中所处的分子形态有关, Werner等[9]也认为, Glu-1A位点亚基在CE动态筛分环境和SDS-PAGE固定孔筛分装置中分子形态的不同是导致分离速率差异的主要因素。
结合亚基迁移顺序、标准出峰时间及电泳图谱等, 可对小麦相关HMW-GS进行HPCE快速鉴定, 为大规模种质资源筛选提供技术条件, 同时由于亚基在HPCE图谱中是以峰的形式出现, 且对应具体的迁移时间, 因此亦可对新HMW-GS进行初步鉴别。
4 结论
通过对缓冲液系统组分浓度、pH及电泳参数的优化, 成功构建了小麦HMW-GS的HPCE高效分离体系, 该体系具有良好的分离效率及分离重现性。以此为基础, 获得了1Dy12、1Dy10、1By9、1By8、1By18、1By16、1By20、1Bx17、1Bx20、1Bx13、1Bx6、1Bx7、1Ax2*、1Ax1、1Dx5、1Dx4、1Dx3和1Dx2共18个亚基的标准图谱, 依据亚基迁移顺序、标准出峰时间及图谱特点可以进行HMW-GS的HPCE快速鉴定。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1146/annurev.pp.38.060187.001041URL [本文引用: 1]

; Plant breeding inst., Cambridge CB2 2LQ, ROYAUME-UNI02 0202 02 0202ARPPA302 1987,02vol.0238,02pp.02141-15302(54 ref.)AnglaisRevue : MultilingueAnnual Reviews, Palo Alto, CA, ETATS-UNIS02 (1950-1987) (Revue); INIST-CNRS, Cote INIST : 5650
DOI:10.1016/S0733-5210(09)80062-3URL [本文引用: 1]

The high molecular weight (HMW) subunits of glutenin are of considerable interest because of their relationship to breadmaking quality. We review recent studies of their genetics, amino acid sequences and conformations, and discuss how they may be assembled to form disulphide-bonded polymers that confer elasticity on dough. We also speculate on how their structure and functionality may be explored using engineering and expression in microorganisms or in developing seeds of transgenic .
URL [本文引用: 2]

The alleles for the gene loci, Glu-A1, Glu-B1 and Glu-D1 are formally defined and cross referenced to the lettering and numbering systems used by the authors and by other research groups to describe the gene products, the high-molecular-weight subunits of glutenin. It is recommended that these alleles be referred to in future by all research groups, in addition to using their own subunit nomenclature, so enabling the work of different laboratories to be compared without difficulty.
DOI:10.1007/s12161-015-0218-3URL [本文引用: 1]

Durum wheat ( Triticum turgidum L.) flour is instrumental for the production of pasta worldwide. The quality of this food rests on flour processing and on its protein content and composition. Gluten proteins as high and low-molecular weight glutenins (GS) are important to predict the flour technological property in pasta making. Different methods were compared to separate, identify and quantify GS in flours from two wheat cultivars. Sodium dodecyl sulphate-polyacrilamide gel electrophoresis (SDS-PAGE) gave in a fast way information about the GS assets. Two-dimensional gel electrophoresis (2D-GE) allowed for the highest resolution in detecting and quantifying single GS, subsequently identified by liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS/MS). Reversed-phase high-performance liquid chromatography (RP-HPLC) is a non-gel alternative system for separation and quantification of single GS that when combined with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) gave information about their exact masses. This method gives also quantitative indications of each individual GS. Different GS patterns and contents were detected in the flour of the two cultivars, underlining the importance of these analytical methods before determining the best flour processing procedure in pasta making. The different methods were evaluated with a modular approach consisting of a grid of different parameters and a non-linear score within each module.
DOI:10.1111/pbr.12506URL [本文引用: 1]

Wheat, among all cereal grains, possesses unique characteristics conferred by gluten; in particular, high molecular weight glutenin subunits (HMW‐GS) are of considerable interest as they strictly relate to bread‐making quality and contribute to strengthening and stabilizing dough. Thus, the identification of allelic composition, in particular at the Glu‐B1 locus, is very important to wheat quality improvement. Several PCR‐based molecular markers to tag‐specific HMW glutenin genes encoding Bx and By subunits have been developed in recent years. This study provides a survey of the molecular markers developed for the HMW‐GS at the Glu‐B1 locus. In addition, a selection of molecular markers was tested on 31 durum and bread wheat cultivars containing the By8, By16, By9, Bx17, Bx6, Bx14 and Bx17 Glu‐B1 alleles, and a new assignation was defined for the ZSBy9_aF1/R3 molecular marker that was specific for the By20 allele. We believe the results constitute a practical guide for results that might be achieved by these molecular markers on populations and cultivars with high variability at the Glu‐B1 locus.
DOI:10.1371/journal.pone.0172819URLPMID:5325591 [本文引用: 1]

Abstract Gluten proteins from wheat, rye, barley and, in rare cases, oats, are responsible for triggering hypersensitivity reactions such as celiac disease, non-celiac gluten sensitivity and wheat allergy. Well-defined reference materials (RM) are essential for clinical studies, diagnostics, elucidation of disease mechanisms and food analyses to ensure the safety of gluten-free foods. Various RM are currently used, but a thorough characterization of the gluten source, content and composition is often missing. However, this characterization is essential due to the complexity and heterogeneity of gluten to avoid ambiguous results caused by differences in the RM used. A comprehensive strategy to isolate gluten protein fractions and gluten protein types (GPT) from wheat, rye, barley and oat flours was developed to obtain well-defined RM for clinical assays and gluten-free compliance testing. All isolated GPT (ω5-gliadins, ω1,2-gliadins, α-gliadins, γ-gliadins and high- and low-molecular-weight glutenin subunits from wheat, ω-secalins, γ-75k-secalins, γ-40k-secalins and high-molecular-weight secalins from rye, C-hordeins, γ-hordeins, B-hordeins and D-hordeins from barley and avenins from oats) were fully characterized using analytical reversed-phase high-performance liquid chromatography (RP-HPLC), sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), N-terminal sequencing, electrospray-ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS) and untargeted LC-MS/MS of chymotryptic hydrolyzates of the single GPT. Taken together, the analytical methods confirmed that all GPT were reproducibly isolated in high purity from the flours and were suitable to be used as RM, e.g., for calibration of LC-MS/MS methods or enzyme-linked immunosorbent assays (ELISAs).
DOI:10.3390/molecules22071055URLPMID:28672820 [本文引用: 1]

Abstract The accurate identification of alleles for high-molecular weight glutenins (HMW-GS) is critical for wheat breeding programs targeting end-use quality. RP-HPLC methods were optimized for separation of HMW-GS, resulting in enhanced resolution of 1By and 1Dx subunits. Statistically significant differences in retention times (RTs) for subunits corresponding to HMW-GS alleles were determined using 16 standard wheat cultivars with known HMW-GS compositions. Subunits that were not identified unambiguously by RP-HPLC were distinguished by SDS-PAGE or inferred from association with linked subunits. The method was used to verify the allelic compositions of 32 Korean wheat cultivars previously determined using SDS-PAGE and to assess the compositions of six new Korean cultivars. Three cultivars contained subunits that were identified incorrectly in the earlier analysis. The improved RP-HPLC method combined with conventional SDS-PAGE provides for accurate, efficient and reliable identification of HMW-GS and will contribute to efforts to improve wheat end-use quality.
DOI:10.1016/S0023-6438(95)91346-7URL [本文引用: 2]

Wheat gluten proteins have important functional properties, and are reliable indicators of genotype. They are also very heterogeneous, demanding use of powerful analytical techniques. Gel electrophoresis methods are widely used for gluten analysis, but they are labor-intensive, and their data are not easily quantified. Methods of capillary electrophoresis (CE) are now available that promise to overcome these disadvantages. We here report successful application and optimization of two CE procedures for gliadin analysis. The first procedure used 0.06 mol/L sodium borate buffer pH 9.0, containing 200 mL/L acetonitrile + 10 g/L sodium dodecyl sulfate ; separations were excellent, but reproducibility was difficult to maintain. The second buffer 0.1 mol/L phosphate, pH 2.5, containing a linear hydrophilic polymer overcame this problem, providing separations of gliadins that are rapid, high-resolution, automatic, and easily quantified. CE analyses of gliadin also reliably differentiate varieties ofall US. wheat classes. CE is thus a valuable technique for gliadin analysis, complementary to other methods of electrophoresis and chromatography. CE has much potential to become a routine tool for wheat varietal identification and classification, and for prediction of quality.
DOI:10.1021/bp00029a017URL [本文引用: 7]

The two main polyacrylamide gel electrophoresis (PAGE) methods used for wheat varietal fingerprinting have been low pH analysis (acid-PAGE) of the alcohol-soluble proteins, and sodium dodecyl sulfate (SDS-PAGE) of total gluten proteins denatured and extracted by the detergent SDS in the presence of reducing agents. Equivalent separations have now been achieved in the capillary electrophoresis format. The acid-PAGE separation was accomplished by free-solution capillary electrophoresis with a charge-reversed capillary. A separation analogous to SDS-PAGE for the analysis of reduced and denatured gluten was accomplished in the capillary format by including a soluble polyacrylamide sieving matrix in the buffer. This replaceable sieving matrix permitted analysis of multiple samples without cross-contamination between samples. The further addition of a small amount of organic solvent to the sieving matrix allowed for excellent resolution of the high molecular weight glutenin subunits that correlate with breadmaking quality. These techniques also possessed the inherent advantages of capillary electrophoresis: low mass requirements, fast separations, and quantitative analysis through on-capillary UV-detection.
DOI:10.1021/bp00033a016URL [本文引用: 2]

The high molecular weight glutenin subunits of endosperm migrate anomalously in (SDS) gel electrophoresis. This anomalous migration was studied by capillary electrophoresis employing an entangled polymer solution that contained SDS. The nonrigid solution nature of the sieving matrix allowed for the easy preparation of the different matrix concentrations required for the construction of a Ferguson plot. A Ferguson plot analysis of these suggested that the high molecular weight glutenin subunits possessed frictional coefficients similar to those determined for standard of the same size, indicating that anomalous migration in gels was due to decreased of SDS and not to a unique structural conformation in the presence of SDS.
DOI:10.1006/jcrs.1996.9999URL [本文引用: 4]
DOI:10.1002/elps.1150191823URLPMID:9932814 [本文引用: 3]

Abstract Studies were conducted to produce faster, simpler, more rugged protocols for separating wheat proteins by high performance capillary electrophoresis (HPCE). Three areas were targeted for improvement: initial capillary equilibration procedures, buffer composition, and post-separation rinsing procedures. For the initial equilibration of capillaries, a brief rinse with a hydroxypropylmethylcellulose (HPMC) solution was the most critical factor for successful separation of wheat proteins. To reduce separation time and maintain resolution, -alanine and glycine were each used in place of sodium phosphate as buffer ions. Two isoelectric buffers, aspartic acid and iminodiacetic acid (IDA) were also tested. Each of these four buffer systems generated substantially lower currents, and provided faster separations, than sodium phosphate-based buffers. Finally, post-separation rinsing procedures were re-examined with the goal of reducing the time necessary to rinse the capillary after each separation. A critical factor in achieving this goal was removal of albumins and globulins prior to separation. These proteins bind to the capillary wall and cause rising baselines and excessive peak tailing. Once these proteins were removed, capillaries could be rinsed with buffer for only 2 min between separations. Capillary equilibration procedures were shortened from 90 min to 30 min. Likewise, separation times were reduced by 40% (25 min to 15 min) by using glycine in place of sodium phosphate in the separation buffer. Finally, post-separation times were reduced by 80% (10 min to 2 min). Overall, these factors resulted in a reduction in total separation time of 50% (35 to 17 min) and maintained high resolution separations and good run-to-run repeatability.
DOI:10.3390/molecules22071055URLPMID:28672820 [本文引用: 1]

Abstract The accurate identification of alleles for high-molecular weight glutenins (HMW-GS) is critical for wheat breeding programs targeting end-use quality. RP-HPLC methods were optimized for separation of HMW-GS, resulting in enhanced resolution of 1By and 1Dx subunits. Statistically significant differences in retention times (RTs) for subunits corresponding to HMW-GS alleles were determined using 16 standard wheat cultivars with known HMW-GS compositions. Subunits that were not identified unambiguously by RP-HPLC were distinguished by SDS-PAGE or inferred from association with linked subunits. The method was used to verify the allelic compositions of 32 Korean wheat cultivars previously determined using SDS-PAGE and to assess the compositions of six new Korean cultivars. Three cultivars contained subunits that were identified incorrectly in the earlier analysis. The improved RP-HPLC method combined with conventional SDS-PAGE provides for accurate, efficient and reliable identification of HMW-GS and will contribute to efforts to improve wheat end-use quality.
DOI:10.1116/1.585038URL [本文引用: 1]

The high-molecular-weight glutenin subunit (HMW-GS) 1Dx2.2 from the Korean wheat cultivar Uri was identified and characterized by one and two dimensional gel electrophoresis, and by liquid chromatography electrospray ionization-tandem mass spectrometry (LC-ESI MS/MS). The amplified coding region of this gene was also cloned and sequenced. Molecular characterization of the 1Dx2.2 gene revealed a...
DOI:10.3732/apps.1600109URL [本文引用: 1]

An efficient, effective DNA extraction method is necessary for comprehensive analysis of plant genomes. This study analyzed the quality of DNA obtained using paper FTA cards prepared directly in the field when compared to the more traditional cetyltrimethylammonium bromide (CTAB) ased extraction methods from silica-dried samples. DNA was extracted using FTA cards according to the manufacturer protocol. In parallel, CTAB-based extractions were done using the automated AutoGen DNA isolation system. DNA quality for both methods was determined for 15 non-agricultural species collected in situ, by gel separation, spectrophotometry, fluorometry, and successful amplification and sequencing of nuclear and chloroplast gene markers. The FTA card extraction method yielded less concentrated, but also less fragmented samples than the CTAB-based technique. The card-extracted samples provided DNA that could be successfully amplified and sequenced. The FTA cards are also useful because the collected samples do not require refrigeration, extensive laboratory expertise, or as many hazardous chemicals as extractions using the CTAB-based technique. The relative success of the FTA card method in our study suggested that this method could be a valuable tool for studies in plant population genetics and conservation biology that may involve screening of hundreds of individual plants. The FTA cards, like the silica gel samples, do not contain plant material capable of propagation, and therefore do not require permits from the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) for transportation.
DOI:10.1007/s001220051390URL [本文引用: 1]

The present work reports new PCR markers that amplify the complete coding sequence of the specific alleles of the high molecular weight (HMW) glutenin genes. A set of AS-PCR molecular markers was designed which use primers from nucleotide sequences of the Glu-A1 and Glu-D1 genes, making use of the minor diffeences between the sequences of the x1 , x2 * of Glu-A1 , and the x5 and y10 of Glu-D1 . These primers were able to distinguish between x2 * and the x1 or xNull of Glu-A1. Also x5 was distinguishable from x2, and y10 from y12. The primers amplified the complete coding regions and corresponded to the upstream and downstream flanking positions of Glu-A1 and Glu-D1. Primers designed to amplify the Glu-A1 gene amplified a single product when used with genomic DNA of common wheats and the xNull allele of this gene. This work also describes the cloning and characterisation of the nucleotide sequence of this allele. It possesses the same general structure as x2 * and x1 (previously determined) and differs from these alleles in the extension of the coding sequence for a presumptive mature protein with only 384 residues. This is due to the presence of a stop codon (TAA) 1215-bp downstream from the start codon. A further stop codon (TAG), 2280-bp downstream from the starting codon is also found. The open reading frame of xNull and x1 alleles has the same size in bp. Both are larger than x2 * which shows two small deletions. The reduced size of the presumptive mature protein encoded by xNull could explain the negative effect of this allele on grain quality.
DOI:10.1007/s001220050405URL [本文引用: 1]

Genes (x-type) corresponding to different high-molecular-weight glutenin subunits encoded at the Glu-A1 locus present in bread- and durum-wheat cultivars have been selectively amplified by the polymerase chain reaction (PCR). DNA fragments corresponding to an unexpressed x-type gene were also amplified. As unexpressed y-type genes may or may not contain an 8-kb transposon-like insertion, two different sets of primers were designed to obtain amplification of DNA fragments corresponding to these genes. Amplified DNA fragments were also digested with restriction enzymes. The digestion patterns of amplified fragments corresponding to unusual x-type subunits showed similarities with genes encoding the most common subunits 2 * and 1. The unexpressed amplified x-type gene showed a restriction pattern similar to the one obtained with the allelic gene encoding high-molecular-weight glutenin subunit 1; homologies were also found within the repetitive region of the linked y-type genes. On the basis of these observations it is postulated that an ancestral active x-type gene, most likely corresponding to subunit 1, was silenced following the insertion of the 8-kb transposon-like fragment into the linked y-type gene.
DOI:10.1023/A:1026191918704URL [本文引用: 2]

In Australian commercial cultivars, each high molecular weight glutenin ( Glu-1 ) homoeologous locus consists of one of two predominant alleles: Glu-A1a (subunit Ax1) or Glu-A1b (subunit Ax2*) at the GluA1 locus, Glu-B1b (Bx7 and By8 subunits) or Glu-B1i (Bx17 and By18 subunits) at the Glu-B1 locus, and Glu-D1d (Dx5 and Dy10 subunits) or Glu-D1a (Dx2 and Dy12 subunits) at the Glu-D1 locus. PCR-based assays have been developed in this study to discriminate between these common alleles at each locus. Primers specific for the Glu-A1 Ax2* gene give a single fragment of 1319 bp only in the presence of this gene. Primers targeting the Glu-B1 locus resulted in a co-dominant marker for which the Bx7 genotype produced two fragments (630 bp and 766 bp) and the Bx17 genotype a single fragment (669 bp). The third pair of primers was specific for the Dx5 gene and resulted in a single band of 478 bp. A multiplexed PCR assay was established which permitted the discrimination of the major HMW glutenins in a single PCR reaction and agarose gel assay. As the HMW glutenin composition of a wheat line is extremely important in determining the functional properties of wheat gluten, these markers are useful for the purposes of marker-assisted breeding. These markers may also be useful for the purpose of DNA-based identification of wheat varieties.
DOI:10.1007/s00122-004-1718-5URLPMID:15175854 [本文引用: 1]

Polymorphisms between the coding sequences of high-molecular-weight (HMW) glutenin x-type genes at the Glu-1 locus were used to amplify Glu-1B x-type-specific PCR fragments. PCR analysis in a wheat cultivar subset carrying different Glu-1B x-type alleles resulted in PCR fragments that differed in size for Glu-B1-1d ( B-x6 ) and non -Glu-B1-1d ( B-x6 ) genotypes. Subsequent sequencing analysis revealed a 15-bp in-frame insertion in the coding regions of all Glu-B1-1d ( B-x6 ) genotypes which allowed the development of a B-x6 -specific PCR assay for high-throughput allele sizing by ion-pair reversed-phase high-performance liquid chromatography. The assay was validated in a set of 86 German wheat cultivars, and genotyping data unequivocally verified the presence of HMW glutenin subunits GLU-B1-1D ( Bx-6 )02+02GLU-B1-2A ( By-8 ) by means of sodium dodecyl sulphate-polyacrylamide gel electrophoresis. These results demonstrate that the PCR assay can be applied for the detection and negative selection of the ‘poor breadmaking quality’ Glu-B1-1d ( B-x6 ) alleles in wheat breeding programs.
DOI:10.1111/j.1744-7909.2007.00573.xURLPMID:18713365 [本文引用: 1]

The high molecular weight glutenin subunit (HMW-GS) pair 1Bx13+1Byt6 are recognized to positively correlate with bread-making quality; however, their molecular data remain unknown. In order to reveal the mechanism by which 1By16 and 1Bx13 creates high quality, their open reading frames (ORFs) were amplified from common wheat Atlas66 and Jimai 20 using primers that were designed based on published sequences of HMW glutenin genes. The ORF of 1By16 was 2220bp, deduced into 738 amino acid residues with seven cysteines including 59 hexapeptides and 22 nanopeptides motifs. The ORF of 1Bx13 was 2385bp, deduced into 795 amino acid residues with four cysteines including 68 hexapeptides, 25 nanopeptides and six tripeptides motifs. We found that 1By16 was the largest y-type HMW glutenin gene described to date in common wheat. The 1By16 had 36 amino acid residues inserted in the central repetitive domain compared with 1By15. Expression in bacteria and western-blot tests confirmed that the sequence cloned was the ORF of HMW-GS 1By16, and that 1Bx13 was one of the largest 1Bx genes that have been described so far in common wheat, exhibiting a hexapeptide (PGQGQQ) insertion in the end of central repetitive domain compared with 1Bx7. A phylogenetic tree based on the deduced full-length amino acid sequence alignment of the published HMW-GS genes showed that the 1By16 was clustered with Glu-IB-2, and that the 1Bx13 was clustered with Glu-1B-1 alleles.
DOI:10.1007/s00122-004-1776-8URLPMID:15340686 [本文引用: 1]

Increased expression of the high molecular weight glutenin subunit (HMW-GS) Bx7 is associated with improved dough strength of wheat ( Triticum aestivum L.) flour. Several cultivars and landraces of widely different genetic backgrounds from around the world have now been found to contain this so-called ‘over-expressing’ allelic form of the Bx7 subunit encoded by Glu-B1al . Using three methods of identification, SDS-PAGE, RP-HPLC and PCR marker analysis, as well as pedigree information, we have traced the distribution and source of this allele from a Uruguayan landrace, Americano 44D, in the mid-nineteenth century. Results are supported by knowledge of the movement of wheat lines with migrants. All cultivars possessing the Glu-B1al allele can be identified by the following attributes: (1) the elution of the By sub-unit peak before the Dx sub-unit peak by RP-HPLC, (2) high expression levels of Bx7 (>39% Mol% Bx), (3) a 4302bp insertion in the matrix-attachment region (MAR) upstream of the gene promoter relative to Bx7 and an 1802bp nucleotide duplication in the coding region of the gene. Evidence is presented indicating that these 18 and 4302bp sequence insertions are not causal for the high expression levels of Bx7 as they were also found to be present in a small number of hexaploid species, including Chinese Spring, and species expressing Glu-B1ak and Glu-B1a alleles. In addition, these sequence inserts were found in different isolates of the tetraploid wheat, T. turgidum , indicating that these insertion/deletion events occurred prior to hexaploidization.
DOI:10.1016/j.jcs.2005.08.003URL [本文引用: 3]

A number of primers were designed which target DNA sequence variation of the coding and /or promoter regions of wheat HMW glutenin y-type genes located at the Glu-B1 locus. This allowed the development of a set of PCR-based markers for specific HMW glutenin genes encoding By-subunits for which no markers were previously available. Markers were validated using test cultivars containing specific Glu-B1 alleles confirmed by SDS-PAGE and RP-HPLC analysis. Among the specific markers developed, primer pair ZSBy8F5/R5 was specific for the By8 gene, which exists in Glu-B1b (Bx7+By8) and Glu-B1u (Bx7*+By8) alleles. This marker allows discrimination of alleles containing By8 and By8* that are usually difficult to distinguish using SDS-PAGE. Since the over-expressed Glu-B1 allele ( Glu-Bl al .) contains the By8* subunit, it is possible to use this marker in breeding programs for selecting for the over-expression of subunit Bx7 in crosses that segregate between normal Bx7 and over-expressed Bx7 subunits. This marker also represents an alternative for distinguishing two common Glu-B1 alleles: Glu-B1i (Bx17+By18) and Glu-B1b (Bx7+By8). Two primer pairs ZSBy9aF1/R3 and ZSBy9F7/R6 both gave characteristic banding patterns for Glu-B1c (Bx7+By9) and can therefore be used to discriminate By9 - containing alleles from non - By9 alleles. Primer pair ZSBy9F2/R2 produced amplicons with a diagnostic banding pattern for allele Glu-B1f (Bx13+By16) and also permitted the discrimination of Glu-B1h (Bx14+By15) and Glu-B1e (Bx20) that have opposing genetic effects on wheat quality and are difficult to discriminate by SDS-PAGE.
DOI:10.1007/s11032-015-0406-2URL [本文引用: 1]

Wheat grain high molecular weight glutenin subunits (HMW-GS) are the major determinants of dough elasticity and viscosity, and thus of breadmaking quality. Most known HMW-GS genes in bread wheat have...
DOI:10.1007/s00122-008-0886-0URLPMID:18797838 [本文引用: 1]

End-use quality is one of the priorities of modern wheat ( Triticum aestivum L.) breeding. Even though quality is a complex trait, high molecular weight (HMW) glutenins play a major role in determining the bread making quality of wheat. DNA markers developed from the sequences of HMW glutenin genes were reported in several previous studies to facilitate marker-assisted selection (MAS). However, most of the previously available markers are dominant and amplify large DNA fragments, and thus are not ideal for high throughput genotyping using modern equipment. The objective of this study was to develop and validate co-dominant markers suitable for high throughput MAS for HMW glutenin subunits encoded at the Glu-A1 and Glu-D1 loci. Indels were identified by sequence alignment of allelic HMW glutenin genes, and were targeted to develop locus-specific co-dominant markers. Marker UMN19 was developed by targeting an 18-bp deletion in the coding sequence of subunit Ax2* of Glu-A1 . A single DNA fragment was amplified by marker UMN19, and was placed onto chromosome 1AL. Sixteen wheat cultivars with known HMW glutenin subunits were used to validate marker UMN19. The cultivars with subunit Ax2* amplified the 362-bp fragment as expected, and a 344-bp fragment was observed for cultivars with subunit Ax1 or the Ax-null allele. Two co-dominant markers, UMN25 and UMN26, were developed for Glu-D1 by targeting the fragment size polymorphic sites between subunits Dx2 and Dx5, and between Dy10 and Dy12, respectively. The 16 wheat cultivars with known HMW glutenin subunit composition were genotyped with markers UMN25 and UMN26, and the genotypes perfectly matched their subunit types. Using an Applied Biosystems 3130xl Genetic Analyzer, four F 2 populations segregating for the Glu-A1 or Glu-D1 locus were successfully genotyped with primers UMN19, UMN25 and UMN26 labeled with fluorescent dyes.
DOI:10.1007/s001220051558URL [本文引用: 1]

While quality in hexaploid wheat ( Triticum aestivum L. em Thell.) is a very complex trait, it is known that the water-insoluble gluten proteins are responsible for the elasticity and chohesiveness (strength) of dough and are therefore important determinants of breadmaking quality. High-molecular-weight (HMW) glutenin subunits encoded by genes on the long arm of group 1 chromosomes have been associated with gluten strength, and a portion of the variability between cultivars can be attributed to glutenin subunit composition. Good or poor wheat breadmaking quality is associated with two allelic pairs at the Glu-D1 complex locus, designated 1Dx5-1Dy10 and 1Dx2-1Dy12, respectively. Among the HMW glutenin subunits encoded at Glu-B1 , Bx7 is quite common, being associated with either of two subunits, By8 or By9. Both allelic pairs contribute moderately well to good breadmaking quality by increasing dough elasticity. Glutenin subunit screening is accomplished using electrophoresis (SDS-PAGE). In this paper, I report the development of an alternative screening method based on glutenin genes themselves using the polymerase chain reaction (PCR). This easy, quick and non-destructive PCR-based approach is an efficient alternative to standard procedures for selecting bread-wheat genotypes with good breadmaking characteristics.
DOI:10.1094/CCHEM-10-11-0123URL [本文引用: 1]
DOI:10.1021/jf990962tURLPMID:10691639 [本文引用: 1]

Free zone capillary electrophoresis conditions have been improved to allow rapid (2-8 min) separations of grain proteins from several cereals (wheat, oats, rice, barley, and rye) with high resolution and reproducibility. This new method utilized the isoelectric compound iminodiacetic acid (IDA) in conjunction with 20% acetonitrile and 0.05% hydroxypropylmethylcellulose. Cultivars of all cereals tested could be differentiated in 3 min, including wheat, using either prolamin or glutelin protein patterns. Resolution was similar to or higher than that of separations in other acidic buffers. Migration time repeatability was excellent with run-to-run variability <1% RSD, day-to-day <1.4% RSD, and capillary-to-capillary <3.3% RSD. Because larger inner diameter capillaries (50 microm) could be used with this buffer, sensitivity was improved and capillary rinse times could be reduced when compared to smaller capillaries (25 microm i.d.). This also served to reduce total separation time so that the majority of cereal storage protein from several types of cereals could be analyzed with total analysis times of 2-8 min with extremely high resolution and repeatability. This method would allow unattended, high-throughput ( approximately 180-400 samples/24 h) analysis of cereal proteins without the generation of much organic solvent waste as well as automated data analysis and storage.
DOI:10.1002/elps.200390184URLPMID:12731030 [本文引用: 1]

This study focused on optimizing phosphate-based buffers and other capillary electrophoresis (CE) parameters for separating and characterizing high molecular weight glutenin subunits (HMW-GS) in bread wheat ( Triticum aestivum L., AABBDD, 2 n = 6 x = 42), emmer ( Triticum dicoccum , AABB, 2 n = 4 x = 28) and Aegilops tauschii (DD, 2 n = 2 x = 14). The fast and high-resolution separation of HMW-GS was achieved using 0.1 M phosphate-glycine buffer (pH 2.5, containing 20% acetonitrile and 0.05% hydroxypropylmethylcellulose) at 12.5 kV and 40C with 25 m inside diameter (ID)27 cm uncoated fused-silica capillary. In general, one sample separation can be analyzed in 15 min. The good run-to-run repeatable separation of HMW-GS could be obtained with a relative standard deviation of less than 1% when capillaries were rinsed with 1 M phosphoric acid for 2 min, followed by separation buffer for 2 min after each separation. The HMW-GS from some bread wheat cultivars as well as tetraploid and diploid accessions was separated by the CE method described above, and all subunits detected were well characterized and readily identified. Some HMW-GS showed reversed mobilities and elution order compared to the methods of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and SDS-CE. Particularly, most of the HMW-GS analyzed with the CE buffer used were separated into multiple peaks, generally a high peak plus a minor peak. CE appears to be capable of separating and characterizing HMW-GS with fast and high-resolution features, therefore it is expected to be useful for specific germplasm screening and desirable HMW-GS identification in wheat quality improvement.
URL [本文引用: 2]

小麦胚乳贮藏蛋白(包括醇溶蛋白和谷蛋白)是面包品质的主要决定 因素,其组成具有很高的异质性,是小麦品种鉴定和品质改良的重要标记.贮藏蛋白的高效分离与表征方法研究一直是国内外的重要课题,目前常用的方法是传统的 酸性聚丙烯酰胺凝胶电泳(A-PAGE)和十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)以及高效液相色谱(HPLC).由于上述方法的局限性, 看守年来高效毛细管电泳(HPCE)新技术逐步发展起来,已在蛋白质、氨基酸、核酸等生物大分子的分离分析中发挥重要作用,并显示出极大的优越性.该文结 合常规A-PAGE和SDS-PAGE技术,对小麦醇溶蛋白和高分子量谷...
URL [本文引用: 2]

小麦胚乳贮藏蛋白(包括醇溶蛋白和谷蛋白)是面包品质的主要决定 因素,其组成具有很高的异质性,是小麦品种鉴定和品质改良的重要标记.贮藏蛋白的高效分离与表征方法研究一直是国内外的重要课题,目前常用的方法是传统的 酸性聚丙烯酰胺凝胶电泳(A-PAGE)和十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)以及高效液相色谱(HPLC).由于上述方法的局限性, 看守年来高效毛细管电泳(HPCE)新技术逐步发展起来,已在蛋白质、氨基酸、核酸等生物大分子的分离分析中发挥重要作用,并显示出极大的优越性.该文结 合常规A-PAGE和SDS-PAGE技术,对小麦醇溶蛋白和高分子量谷...
DOI:10.1556/CRC.37.2009.1.8URL [本文引用: 1]

The wheat storage proteins, especially the high molecular weight glutenin subunits (HMW-GS), play important roles in the determination of flour processing and bread-making quality. Compared with the traditional SDS-PAGE method, reversed-phase high-performance liquid chromatography (RP-HPLC) was shown to have many advantages for the separation and characterization of HMW-GS because of its high resolving power, repeatability and automation. In this work, HMW-GS from bread and tetraploid wheats were separated and characterized by RP-HPLC. The elution time ranking of different HMW-GS was: 1Ax > 1Bx > 1Dx > 1By > 1Dy. Several subunit pairs associated with good quality properties and those with similar mobilities on SDS-PAGE, such as 1Bx7 and 1Bx7*, 1By8 and 1By8*, 1Dx2 and 1Ax2*, 1Bx6 and 1Bx6.1, were well separated and readily identified through RP-HPLC. However, other subunit pairs, such as 1Dy10 – 1Dy12, 1Dx5 – 1By18 and 1Dx2 – 1By16, could not be adequately separated and identified by RP-HPLC, whereas they displayed different mobilities on SDS-PAGE gels. Because 1Dx5 and 1Dx2 showed different hydrophobicities, RP-HPLC could distinguish 1Dx5 + 1Dy10 and 1Dx2 + 1Dy12. A comparative analysis between RP-HPLC and SDS-PAGE showed that a combination of both methods provided more effective identification of HMW-GS in wheat quality improvement and germplasm screening.
DOI:10.1094/CCHEM.2001.78.6.737URL [本文引用: 2]

Abstract Cereal Chem. 78(6):737-742 The use of capillary electrophoresis in SDS (SDS-CE) for separation and quantification of HMW glutenin subunits (HMW-GS) was investi- gated. HMW-GS were precipitated with 40% acetone from 50% 1-pro- panol extract of flour under reducing conditions after removal of mono- meric proteins with 50% 1-propanol. Poly (ethylene oxide) was used in the running buffer (3% w/v) for SDS-CE. The results indicated that HMW-GS could be well separated by SDS-CE, including subunits 7+8, 7+9, 2+12, 5+10, and 17+18. However, HMW-GS showed delayed migration times compared with molecular weight protein standards. Some HMW-GS were reversed in their mobilities in SDS-CE compared with their mobility and molecular weights by SDS-PAGE. Therefore, the SDS-CE was unsuitable for MW determination of HMW-GS. A linear response was obtained from SDS-CE of a plot of the concentration of HMW-GS of the 40% acetone precipitate versus corrected areas for absorbance at 214 nm. Quantification of HMW-GS for the two biotypes (subunits 5+10 vs. 2+12) of an Australian wheat cultivar Warigal confirmed the differences between the two biotypes in their quantity of HMW-GS. Therefore, the technique could be used to quantify HMW-GS in con- junction with SDS-PAGE.
DOI:10.1094/CCHEM.2004.81.5.561URL [本文引用: 1]

Abstract High molecular weight glutenin subunits (HMW-GS) from three hexaploid wheat species (AABBDD, 2n=6x=42, Triticum aestivum L., T spelta L., and T compactum L.) were separated and identified by acidic capillary electrophoresis (A-CE) with phosphate-glycine buffer (pH 2.5) in uncoated fused-silica capillaries (50 mum, i.d. x 25.5 cm) at 12.5 kV and 40degreesC. The rapid separations (<15 min) of HMW-GS with good repeatability (RSD < 2%) were obtained using a fast capillary rising protocol. All 17 HMW-GS analyzed could be well separated and their relative migration orders were ranked. In particular, the good quality subunit pair 5+10 could be differentiated from poor quality subunit pair 2+12. In addition, the other three allelic pairs of 13+16, 17+18, and 7+8 subunits that were considered to have positive effects on dough properties, as well as three pairs of novel subunits 13+22*, 13*+19*, and 6.1+22.1 detected from spell and club wheat, can also be readily separated and identified. An additional protein subunit presented in Chinese bread wheat cultivar Jing 411 and club wheat TRI 4445/75, respectively, was detected by both A-CE and 2-D gel electrophoresis (A-PAGE x SDS-PAGE), for which further identification is needed.
DOI:10.1016/j.talanta.2014.04.055URLPMID:25127558 [本文引用: 2]

61A novel method of the identification and charge characterisation of wheat HMW-GS is presented.61From eight to twelve isoforms of wheat HMW-GS with pI points in the range of 4.72–6.98 were separated.61The isoelectric point peak profiles were compared with CZE electrophoregrams of these subunits.
URL [本文引用: 2]

High molecular weight glutenin subunits (HMW-GS) are among the major determinant on wheat bread-making quality. Rapid ultra-performance liquid chromatography (UPLC) methods with high resolution and reproducibility for HMW-GS separation were established in this work. A sample could be completely analyzed in less than 12 min by using the optimized UPLC conditions: gradually increasing elution gradient from 21% to 47% in 30 min at flow rate 0.55 ml/min and separation temperature 55C. The results from 15 consecutive runs showed that UPLC patterns of HMW-GS including peak migration time, height and peak area displayed high reproducibility. In addition, the analysis consumes small amounts of reagent. A total of 34 subunits from 111 wheat cultivars and gene bank accessions were well separated and showed clearly different UPLC patterns. Thus all subunits could be readily identified, especially for 1Dx and 1By subunits that were not separable by traditional HPLC. The results from different growing environments demonstrated that the subunit elution times were highly stable, displaying significant changes in the subunit peak heights and areas, reflecting their expression amounts. In comparison with the state-of-the-art HPLC method, UPLC has obvious advantages in separation time, resolution and reagent consuming, which provides an alternative powerful technique for wheat storage protein studies and quality improvement.
DOI:10.1021/pr040035oURL [本文引用: 1]

review: capillary electrophoresis of proteins and peptides
DOI:10.3321/j.issn:0496-3490.2004.05.014URL [本文引用: 1]

Twelve wheat cultivars with different HMW-GS were used to analyze dynamics of protein components and ratio of GMP to protein of wheat grain. The fast accumulation for albumin and globulin appeared in early stage of grain filling, then descended little by little, but slightly ascended at mature stage
DOI:10.3321/j.issn:0496-3490.2004.05.014URL [本文引用: 1]

Twelve wheat cultivars with different HMW-GS were used to analyze dynamics of protein components and ratio of GMP to protein of wheat grain. The fast accumulation for albumin and globulin appeared in early stage of grain filling, then descended little by little, but slightly ascended at mature stage
DOI:10.3969/j.issn.1005-7129.2010.04.002URL [本文引用: 1]

蛋白质的分离技术在蛋白质研究中起到了举足轻重的作用。毛细管电泳作为一项较新的分离技术,以其高通量、高灵敏度、快捷低耗的优势弥补了传统双向凝胶电泳的不足。本文分别从毛细管电泳技术的不同分离方式出发,对其在植物蛋白质分离中的研究进展进行了综述,重点分析了毛细管区带电泳和涂层毛细管电泳对植物蛋白质进行分离的优缺点。
DOI:10.3969/j.issn.1005-7129.2010.04.002URL [本文引用: 1]

蛋白质的分离技术在蛋白质研究中起到了举足轻重的作用。毛细管电泳作为一项较新的分离技术,以其高通量、高灵敏度、快捷低耗的优势弥补了传统双向凝胶电泳的不足。本文分别从毛细管电泳技术的不同分离方式出发,对其在植物蛋白质分离中的研究进展进行了综述,重点分析了毛细管区带电泳和涂层毛细管电泳对植物蛋白质进行分离的优缺点。
DOI:10.1016/S0378-5173(96)04769-2URL [本文引用: 1]

Film coating of pharmaceuticals involves interfacial interactions based on adhesion and spreading of the polymer over the substrate surface. The surface tension of the polymer solution will have a major influence on these interfacial events. It is known that solutions of macromolecules exhibit surface ageing; however, data are not available concerning the dynamic surface tension (DST) of such systems in the first few seconds of surface formation. In this study, the DST of hydroxypropylmethylcellulose (HPMC) has been measured using a maximum bubble pressure method. It was found that, at all concentrations, the DST was higher than the equilibrium surface tension, but that at concentrations above 6% (w/w) this difference was much greater. This can be related to difficulties in film coating which can occur with more concentrated solutions. It was shown that additions of additives, such as poly(ethylene) glycol or lactose, had a detrimental impact on DST for the high-concentration solutions only. These data provide an improved understanding of the film coating process and give a route by which film-coating formulations may be optimised.
DOI:10.1007/s00216-009-2875-9URLPMID:19543716 [本文引用: 1]

The extraction and separation of dyes present on textile fibers offers the possibility of enhanced discrimination between forensic trace fiber evidence. An automated liquid sample handling workstation was programmed to deliver varying solvent combinations to acid-dyed nylon samples, and the resulting extracts were analyzed by an ultraviolet/visible microplate reader to evaluate extraction efficiencies at different experimental conditions. Combinatorial experiments using three-component mixture designs varied three solvents (water, pyridine, and aqueous ammonia) and were employed at different extraction temperatures for various extraction durations. The extraction efficiency as a function of the three solvents (pyridine/ammonia/water) was modeled and used to define optimum conditions for the extraction of three subclasses of acid dyes (anthraquinone, azo, and metal complex) from nylon fibers. The capillary electrophoresis analysis of acid dye extracts is demonstrated using an electrolyte solution of 15 mM ammonium acetate in acetonitrile/water (40:60, v/v) at pH 9.3. Excellent separations and discriminating diode array spectra are obtained even for dyes of similar color.
DOI:10.1016/S0304-3975(97)00126-6URLPMID:20146422 [本文引用: 1]

High molecular weight glutenin subunits (HMW-GS) from 60 germplasms including 30 common wheat cultivars and 30 related species were separated and characterized by a suite of separation methods including sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), reversed-phase high-performance liquid chromatography (RP-HPLC), high-performance capillary electrophoresis (HPCE), and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Comparative analysis demonstrated that each methodology has its own advantages and disadvantages. The main drawback of SDS-PAGE was its overestimation of molecular mass and incorrect identification of HMW-GS due to its low resolution. However, it had the advantages of technical simplicity and low requirements of equipment; thus, it is suitable for large-scale and high-throughput HMW-GS screening for breeding programs, especially when the glutenin composition is clear in the breeding material. MALDI-TOF-MS clearly expressed many technical advantages among the four methods evaluated, including high throughput, high resolution, and accuracy; it was, however, associated with high equipment cost, thus preventing many breeding companies from accessing the technology. RP-HPLC and HPCE were found to be intermediate between SDS-PAGE and MALDI-TOF-MS. Both RP-HPLC and HPCE demonstrated higher resolution and reproducibility over SDS-PAGE but lower detection power than MALDI-TOF-MS. Results demonstrated that MALDI-TOF-MS is suitable for analyzing HMW-GS for routine breeding line screening and for identifying new genotypes.
