 , 高杰, 杜伟莉, 张仁和
, 高杰, 杜伟莉, 张仁和 , 薛吉全
, 薛吉全*通讯作者(Corresponding author): 张仁和, E-mail:zhangrenhe1975@163.com 第一作者联系方式: E-mail:zhxh4569@163.com
收稿日期:2014-09-30 基金:
摘要
关键词:玉米品种; 干旱胁迫; 光合速率; OJIP荧光
Effects of Drought Stress on Photosynthetic Characteristics of Maize Hybrids at Seedling Stage
ZHANG Xing-Hua
 , GAO Jie, DU Wei-Li, ZHANG Ren-He
, GAO Jie, DU Wei-Li, ZHANG Ren-He , XUE Ji-Quan
, XUE Ji-QuanAbstract
Keyword:Maize; Drought stress; Photosynthetic characteristics; OJIP
Show Figures
Show Figures





玉米是陕西渭北旱区重要的粮食作物之一。该区在玉米生长季节具有足够的光照时间和热量, 显示出高产潜力, 平均产量达12 t hm-1 [1]。该区属典型的旱地农业区, 玉米生产中经常受到季节性不同程度的干旱胁迫, 干旱已成为限制该区玉米生长与产量形成的主要环境因素之一[2]。干旱逆境下玉米的生存率依赖于避旱或耐旱的能力[3]。光合作用是一个十分关键的代谢过程[4], 而光能捕获与能量利用间的平衡是光合作用系统对干旱胁迫响应的核心[5]。因此, 研究干旱胁迫下光系统结构与功能为理解干旱逆境下作物叶片如何控制能量平衡提供有价值的信息[6, 7]。
众多****对干旱胁迫下作物光合作用系统结构和功能大量研究表明, 抗旱作物品种气体交换特征具较小的可塑性, 且在干旱下维持较高的气孔导度[8, 9, 10]。也有****报道中度干旱造成作物光合作用相关酶活性下降[11, 12]。随着光合作用研究的深入, 叶绿素荧光分析技术提供了关于植物光合器官的结构和功能丰富的信息[13], 利用调制荧光技术分析了干旱胁迫引起植物光系统II (PSII)的主要光化学变化[14]。如干旱逆境下光合作用受到抑制, 吸收的光能就会超出光合机构所能利用的范围, 植物叶片可以通过光合碳同化以外的途径如非光化学耗散、热耗散等消耗过剩光能保护光系统过氧化[15, 16]; 同时, 另一种监测光合器官结构的途径JIP-test被发展[17], 这种快速叶绿素荧光诱导动力学曲线(OJIP)已成为研究光合作用特别是原初光化学反应的最有力工具之一[18], 它包含丰富和有价值的信息可以反映光系统II (PSII)反应中心能量捕获和PSII供、受体侧电子传递变化[19]。这些研究大多集中在大麦、百香果和豌豆植物上[20, 21, 22]。而有关玉米在干旱胁迫下光系统电子传递过程是怎样处理能量不平衡来保护光合机构的研究报道较少。这些信息是深入理解玉米适应干旱逆境光合作用调控机制的重要补充[23]。鉴于此, 本研究在前期研究基础上[24], 探索玉米叶片光合电子传递与气体交换参数在不同干旱胁迫下的变化规律, 以期为玉米抗旱光合特性研究提供理论依据。
1 材料与方法1.1 试验设计于2013年5月至9月在西北农林科技大学农作物示范园活动式防雨棚内进行盆栽试验。用规格相同的塑料桶(内径26 cm, 深38 cm), 分别装风干黏壤土18 kg, 土壤田间最大持水量为26.8%, pH 7.31, 含有机质1.64%和全氮0.064%。桶底装鹅卵石, 上铺滤纸与土隔离, 通过插到鹅卵石上的硬质塑料管浇水。供试玉米品种为陕单609和郑单958。设正常供水(CK)、轻度干旱(LS)、中度干旱(MS)和重度干旱(SS) 4个处理, 其土壤相对含水量分别为土壤田间最大持水量的70%~80%、60%~70%、50%~60%和35%~45%。共设6次重复, 三叶期定苗每盆3株并开始控水, 自然干旱至设定土壤含水量标准范围, 每天8:00和18:00采用称重法补水控水并记录, 处理期间除桶内土壤水分明显差异外其他管理一致, 土壤相对含水量达到预期设定的4个不同干旱程度后持续7 d, 开始连续3 d测定。用每个重复样品第3片叶测定各项指标。
1.2 测定项目与方法1.2.1 气体交换参数 利用Li-6400便携式光合作用
测定系统(Li-Cor, USA)测定净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)等参数。采用Li-6400-02B红蓝光光源设定光强, PAR=1600 µ mol m-2 s-1, 大气CO2浓度(Ca)为400 μ mol mol-1, 空气相对湿度为50%~70%。选择晴天上午10:00至12:00。连续测定2 d, 重复测定6次。
1.2.2 快速叶绿素荧光动力学曲线 参考Schansker等[25]的方法, 叶片经暗适应20 min后用M-PEA (Hansatech, UK)同时测定叶片快速叶绿素荧光诱导动力学曲线(OJIP)的光吸收曲线。饱和脉冲光(PFD = 3000 µ mol m-2 s-1)下1 s的红光诱导, 测定时间为2 s, 记录的初始速率为每秒种105个数据。测定时选择健康叶中部, 相同处理分别测定6盆。用JIP-test分析O-J-I-P荧光诱导曲线, 计算如下相关参数[26]:
在K相、J相和I相的相对可变荧光(Vk、Vj和Vi) = (Ft-Fo)/(Fm-Fo), t= 300 µ s、2 ms和30 ms可变荧光Fk占Fj-Fo振幅的比例Wk= (Fk-Fo)/(Fj-Fo)
捕获的激子将电子传递到电子传递链中QA-下游的其他电子受体的概率Ψ 0= ET0/TR0= 1-Vj
单位反应中心吸收ABS/RC=Mo× (1/Vj)× (1/φ Po)
PSII最大光化学效率 Fv/Fm=1-(Fo/Fm)
用于电子传递的量子产额ET0/ABS = (1-Fo/Fm)/Ψ 0
PSI末端受体还原的量子产额RE0/ABS = Fv/Fm× Ψ 0× (1-Vj)/(1-Vj)
以吸收光能为基础的性能指数PIABS= (RC/ABS)× [Fv/Fm/(1-Fv/Fm)]× [Ψ 0/(1-Ψ 0)]
1.3 数据分析用SPSS12.0软件统计分析数据。
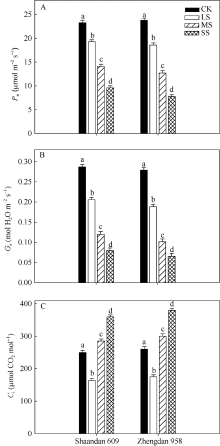
2 结果与分析2.1 干旱胁迫对玉米叶片气体交换特性的影响由图1-A可知, 在LS、MS和SS胁迫处理下陕单609叶片Pn较对照分别下降17.4%、40.0%和58.8%; 郑单958叶片Pn较对照分别下降21.8%、46.5%和67.2%。重度干旱下陕单609叶片Pn高于郑单958。
图1
Fig. 1
| Figure OptionViewDownloadNew Window | |
 | 图1 干旱胁迫对玉米品种陕单609和郑单958叶片净光合速率(Pn, A)、气孔导度(Gs, B)和胞间CO2浓度(Ci, C)的影响不同小写字母表示在0.05水平上差异显著。CK: 正常供水; LS: 轻度干旱; MS: 中度干旱; SS: 重度干旱。Fig. 1 Effects of drought stress on photosynthetic rate (Pn, A), stomatal conductance (Gs, B) and intercellular CO2 concentration (Ci, C) of maize cultivars Shaandan 609 and Zhengdan 958Different letters above the colums indicate significant differences between treatments (P < 0.05) and determined by Tukey’ s test.CK: control, LS: low drought stress, MS: moderate drought stress, SS: severe drought stress. |
图1-B表明, 在LS、MS、SS胁迫处理下陕单609叶片气孔导度Gs较对照分别下降30.3%、58.2%和72.1%; 郑单958叶片Gs较对照分别下降32.6%、63.8%和76.7%。
2个品种叶片胞间CO2浓度(Ci)变化则不同, 随着干旱胁迫进程出现先下降后上升的趋势。LS胁迫处理下陕单609的Ci降低34.4%; 而MS和SS胁迫处理下Ci较对照分别增加28.4%和37.5%; 郑单958在LS胁迫处理下Ci降低32.7%, 而MS和SS胁迫处理下Ci较对照分别增加26.2%和37.8% (图1-C)
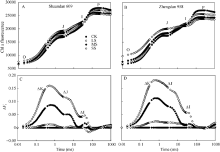
2.2 干旱胁迫对玉米叶片PSII供体侧和受体侧的影响利用JIP-test分析进一步研究干旱胁迫对PSII的影响。将从Fo到Fm间原始荧光曲线标准化, 更清楚了解叶绿素荧光诱导曲线O、J、I、P相(OJIP)的特征。不同干旱胁迫下陕单609和郑单958叶片OJIP曲线均显示典型的多相上升, 包含O-J-I-P四个基本点(图2-A, B)。为进一步分析干旱对玉米叶片光合伤害位点变化, 对OJIP瞬时荧光标准化后的相对可变荧光Δ Vt(图2-C, D)。与对照相比, 在SS胁迫下陕单609和郑单958叶片可变荧光(Δ Vt)均在2 ms和30 ms附近显示正的Δ J-带和Δ I-带, 分别增加13.4%、4.8%和15.3%、5.7%, 郑单958可变荧光(Δ Vt)增加较为明显。表明郑单958叶片PSII的供体侧受到了更严重伤害, 向下游提供电子的能力更弱。
图2
Fig. 2
| Figure OptionViewDownloadNew Window | |
 | 图2 干旱胁迫对玉米品种陕单609和郑单958叶片PSII荧光诱导动力学曲线OJIP和相对可变荧光曲线(Δ Vt)影响OJIP曲线按照VOP = (Ft-Fo)/(Fm-Fo)进行标准化; Δ Vt是干旱处理与对照的曲线的差值Δ Vt = VOP (treatment) - VOP (control)。CK:正常供水; LS: 轻度干旱; MS: 中度干旱; SS: 重度干旱。Fig. 2 Effects of drought stress on the mean OJIP transients of Shaandan 609 (A) and Zhengdan 958 (B) on a logarithmic time scaleThe relative variable fluorescence derived from the mean OJIP transients between F0 and Fm is defined as: VOP= (Ft-F0)/(Fm-F0). The difference kinetics Δ Vt=VOP (treatment) - VOP (control) in different drought treatments are shown. CK: control, LS: low drought stress, MS: moderate drought stress, SS: severe drought stress. |
在O与J点间(20 μ s和2 ms)相对可变荧光的标准化 (图3)表明, 不同干旱下2个品种动力曲线Δ VOJ间在 300 μ s处出现显著的峰值, 即K-band。在MS和SS胁迫处理下, 陕单609的K-band较对照分别增加4.8%和8.5%; 郑单958的K-band较对照分别增加5.4%和9.9%。表明郑单958叶片PSII能量交换较少、光系统II放氧复合体(OEC)的稳定状态较差。
图3
Fig. 3
| Figure OptionViewDownloadNew Window | |
 | 图3 干旱胁迫下玉米品种陕单609和郑单958叶片PSII荧光诱导动力学OJIP曲线K-band的变化A, B: VOj = (Ft-Fo)/(Fj-Fo)进行标准化。CK: 正常供水; LS: 轻度干旱; MS: 中度干旱; SS: 重度干旱Fig. 3 Changes in the shape of the Chl a fluorescence transient curves of Shaandan 609 (A) and Zhengdan 958 (B) under drought stress revealing K-bandA and B: relative variable fluorescence between Fo and Fj [VOj= (Ft-Fo)/(Fj-Fo)]. The difference kinetics Δ VOj = VOj (treatment) - VOj (control) in different drought treatments are shown. CK: control, LS: low drought stress, MS: moderate drought stress, SS: severe drought stress. |
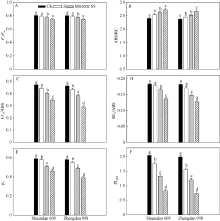
2.3 干旱胁迫对玉米叶片光系统II特性的影响最大光化学效率(Fv/Fm)反映PSII天线吸收的光能被反应中心捕获的概率, 在LS和MS胁迫处理2个品种叶片的Fv/Fm都没有明显变化, 而SS胁迫后陕单609和郑单958的Fv/Fm分别下降5.7%和6.8% (图4-A)。
图4
Fig. 4
| Figure OptionViewDownloadNew Window | |
 | 图4 干旱胁迫对玉米陕单609和郑单958叶片单位PSII反应中心活性的6个参数的影响各个参数为PSII最大光化学效率(Fv/Fm)、单位反应中心吸收的电子流(ABS/RC)、用于电子传递的量子产额(ET0/ABS)、PSI末端受体还原的量子产额(RE0/ABS)、捕获的激子将电子传递到电子传递链中QA-下游的其他电子受体的概率(Ψ o)、以吸收光能为基础的性能指数(PIABS)。图注上不同小写字母表示处理间在0.05水平上差异显著。CK: 正常供水; LS: 轻度干旱; MS: 中度干旱; SS: 重度干旱。Fig. 4 Effects of drought stress on six fluorescence parameters in dark-adapted leaves of Shaandan 609 and Zhengdan 958Fv/Fm: maximum quantum yield of primary photochemistry, ABS/RC: the specific energy fluxes per RC for absorption; ET0/ABS: quantum yield for electron transport, RE0/ABS: quantum yield for the reduction of end acceptors of PSI per photon absorbed; Ψ o: the efficiency with which a trapped exciton can transfer an electron into the down stream electron transport chain further than QA-; PIABS: the performance index on an absorption basis. Different letters above columns indicate significant differences between treatments (P < 0.05) and determined by Tukey’ s test. CK: control, LS: low drought stress, MS: moderate drought stress, SS: severe drought stress. |
电子传递的量子产额(ET0/ABS)反映了被PSII天线吸收的光能最终进入电子传递链的概率。在MS、SS胁迫处理下陕单609和郑单958的ET0/ABS分别下降15.0%、26.6%和17.1%、37.2% (图4-B)。PSI末端受体还原的量子产额(RE0/ABS)下降分别为9.8%、25.1%和18.7%、30.2% (图4-C)。
叶片捕获的激子将电子传递到电子传递链中QA-下游的电子受体的概率(Ψ o)反映被PSII反应中心捕获的能量进入电子传递链的概率。在MS和SS胁迫处理下陕单609和郑单958的Ψ o下降分别为13.6%、22.1%和15.5%、32.8% (图4-D)。而随着干旱胁迫加剧叶片单位反应中心吸收能量(ABS/RC)的增加, 在MS、SS胁迫处理下陕单609和郑单958的ABS/RC分别增加17.3%、26.5%和22.7%、30.3% (图4-E), 以光能吸收为基础的性能指数(PIABS)在MS和SS胁迫处理陕单609和郑单958的PIABS分别下降40.1%、64.2%和49.1%、72.9% (图4-F)。
3 讨论Pn、Gs和Ci间显著相关表明气孔关闭是降低Gs和Ci, 以及反馈下调Pn主要因素[27]; 干旱胁迫初期(或轻度干旱)首先是气孔关闭以减少蒸腾作用, 进而阻碍了CO2进入叶内, 而中度和重度干旱下上升Ci水平或者保持不变, 非气孔因素是Pn下降的主要原因[20, 28]。本研究中轻度干旱时2个玉米品种叶片气孔导度(Gs)对Pn起重要的调控作用, 这时受旱玉米降低Gs使植株节约水分具有保护效应; 而中度和重度干旱下气孔导度(Gs)和Pn显著下降, 胞间二氧化碳浓度(Ci)上调, 此时2个玉米品种主要受到非气孔因素限制。这种反应方式与前人的研究相一致[7, 8]。说明受旱玉米限制光合速率可能是光能捕获效率和光合电子传递系统影响的结果[22, 24]。
JIP-test能够反应植株光合电子传递过程的重要变化, OJIP曲线的O-J-I-P阶段变化对干旱逆境十分敏感[26, 33]。且O-J、J-I和I-P阶段分别与QA的氧化还原状态、质体醌的氧化还原状态和PSI末端电子受体氧化还原状态有关[26]。本研究中干旱胁迫下郑单958叶片VJ和VI增加的幅度均比陕单609叶片大(图2), 这意味着干旱胁迫下郑单958叶片PSII受体侧比陕单609叶片更还原, 而其PSI 受体侧比陕单609更氧化, 此时受旱玉米郑单958叶片PSII受体侧受光抑制伤害程度比陕单609大。类似的研究结果在干旱胁迫下10个不同抗旱性大麦品种上被发现[19]。
OJIP曲线上K-band正值(约在300 μ s处)作为OEC受伤害的一个特殊标记, 表明PSII供体侧和受体侧电子流的不平衡[25, 30]。本试验中干旱下陕单609叶片在300 μ s处的正Δ K-band不如郑单958明显(图3), 意味着干旱胁迫下陕单609比郑单958叶片PSII放氧复合体(OEC)受损的程度轻。进一步说明干旱下陕单609能够较好地维持PSII受体和供体侧电子传递的平衡。
光合电子传递中, 最大光化学效率(Fv/Fm)、电子传递的量子产额(ET0/ABS)、PSI末端受体还原的量子产额(RE0/ABS)、捕获的激子将电子传递到电子传递链中QA-下游的电子受体的概率(Ψ o)光合行为的结构参数能量化从最初的天线复合体光化学吸收到最后的PSI末端受体还原光化学表现[25]。本研究结果显示, 中度和重度干旱胁迫下陕单609叶片最大光化学效率(Fv/Fm)、电子传递产额(ET0/ABS)、PSI末端受体还原的量子产额(RE0/ABS)和捕获的激子将电子传递到电子传递链中QA-下游的电子受体的概率(Ψ o)降低幅度比郑单958小, 意味着陕单609具有较强的从PSII到PSI的电子传递能力。而光合功能参数ABS/RC代表活性PSII反应中心的有效天线大小, 受到活性与失活反应中心比例的影响[29]。本研究中重度干旱下郑单958叶片单位反应中心吸收的光能(ABS/RC)较对照增加30.3%, 而陕单609较对照增加26.5% (图4-E), 说明郑单958光系统II反应中心失活比例较大。Gomes等[21]
也阐述了耐旱百香果叶片通过调控PSII反应中心失活比例控制光合电子流。这也进一步支持了干旱胁迫下陕单609较郑单958在光合结构和功能上具有较强的优势。此外, 以光能吸收为基础的性能指数(PIABS)综合了RC/ ABS、Fv/Fm和Ψ 0单个效应, 是JIP-test的参数中最敏感的参数之一[26]。本文在重度干旱下陕单609叶片的PIABS较对照下降幅度小于郑单958, 说明陕单609叶片光合机构的耐旱性强于郑单958。
The authors have declared that no competing interests exist.
参考文献View Option
原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] |
