摘要/Abstract
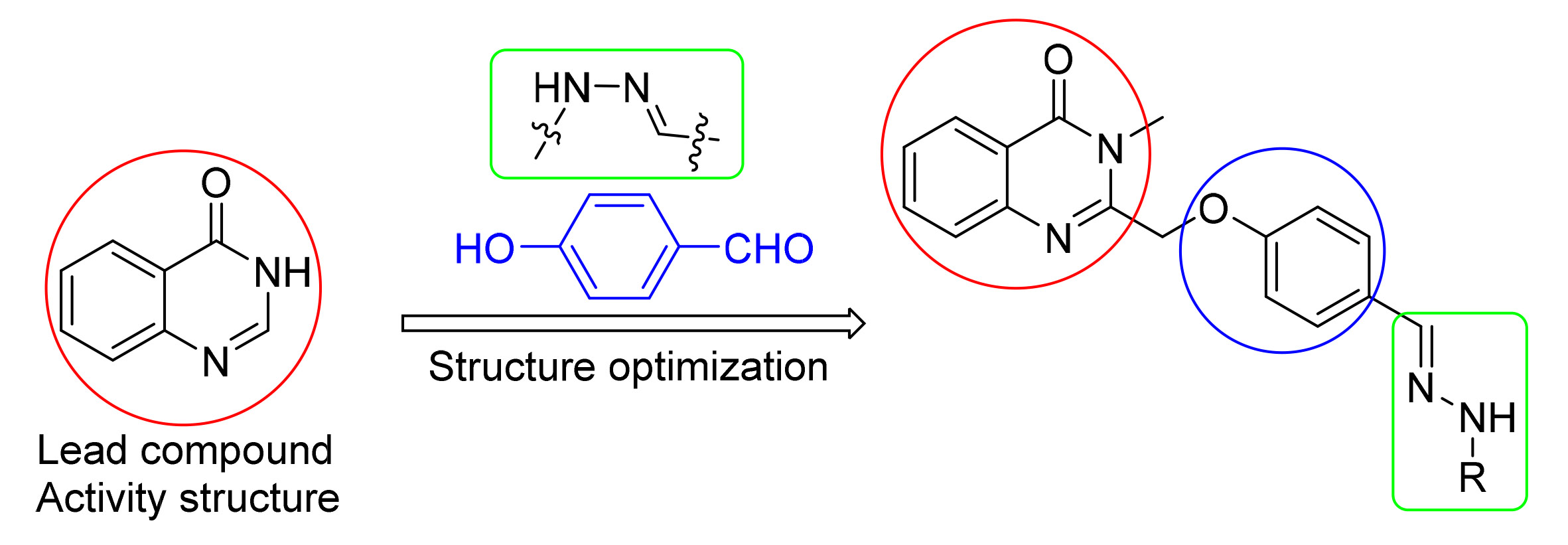
以靛红酸酐为起始原料,设计并合成了一系列新颖的含腙结构单元的喹唑啉酮类衍生物.所有目标化合物经1H NMR、13C NMR和高分辨质谱(HRMS)表征确证其结构.初步抗菌活性结果显示,该类化合物对水稻白叶枯病菌(Xanthomonas oryzae pv. Oryzae,Xoo)、猕猴桃溃疡病菌(Pseudomonassyringae pv. actinidae,Psa)和柑橘溃疡病菌(Xanthomonas axonopodis pv. Citri,Xac)均表现出一定的抑制活性.其中3-甲基-2-(((4-((2-(4-甲基苯磺酰基)肼基)甲基)苯氧基)甲基)喹唑啉-4(3H)-酮(G18)、3-甲基-2-(((4-((2-(2,6-二氯苯基)肼基)甲基)苯氧基)甲基)喹唑啉-4(3H)-酮(G12)和3-甲基-2-(((4-((2-(苯磺酰基)肼基)甲基)苯氧基)甲基)喹唑啉-4(3H)-酮(G16)对Xoo、Psa和Xac三种细菌的抑制活性分别优于对照药叶枯唑和噻菌铜.另外,3-甲基-2-(((4-((2-(3,5-二氯苯基)肼基)甲基)苯氧基)甲基)喹唑啉-4(3H)-酮(G5)对Xoo、Psa和Xac三种细菌均表现出良好的广谱抗菌活性.
关键词: 喹唑啉酮衍生物, 腙, 抑菌活性, 构效关系
A series of novel quinazolinone derivatives containing hydrazone structural units were designed and synthesized with isatoic anhydride as the starting material. All target compounds were characterized by 1H NMR, 13C NMR and HRMS. The preliminary antibacterial activity results showed that the compounds exhibited a certain inhibitory activity against Xanthomonas oryzae pv. oryzae (Xoo), Pseudomonas syringae pv. actinidiae (Psa) and Xanthomonas axonopodis pv. citri (Xac). Among them, (E)-4-methyl-N'-(4-((3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)methoxy)benzylidene)benzenesulfonohydrazi-de (G18), (E)-2-((4-((2-(2,6-dichlorophenyl)hydrazono)methyl)phenoxy)methyl)-3-methylquinazolin-4(3H)-one (G12) and (E)-N'-(4-((3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)methoxy)benzylidene)benzenesulfonohydrazide (G16) displayed better antibacterial activity against Xoo, Xac and Psa than the control drugs of bismerthiazol and thiediazole-copper, respectively. Notably, (E)-2-((4-((2-(3,5-dichlorophenyl)hydrazono)methyl)phenoxy)methyl)-3-methylquinazolin-4(3H)-one (G5) displayed fine broad-spectrum antimicrobial activity against Xoo, Xac and Psa.
Key words: quinazolinone derivative, hydrazone, antibacterial activity, structure-activity relationship
PDF全文下载地址:
点我下载PDF
