摘要/Abstract
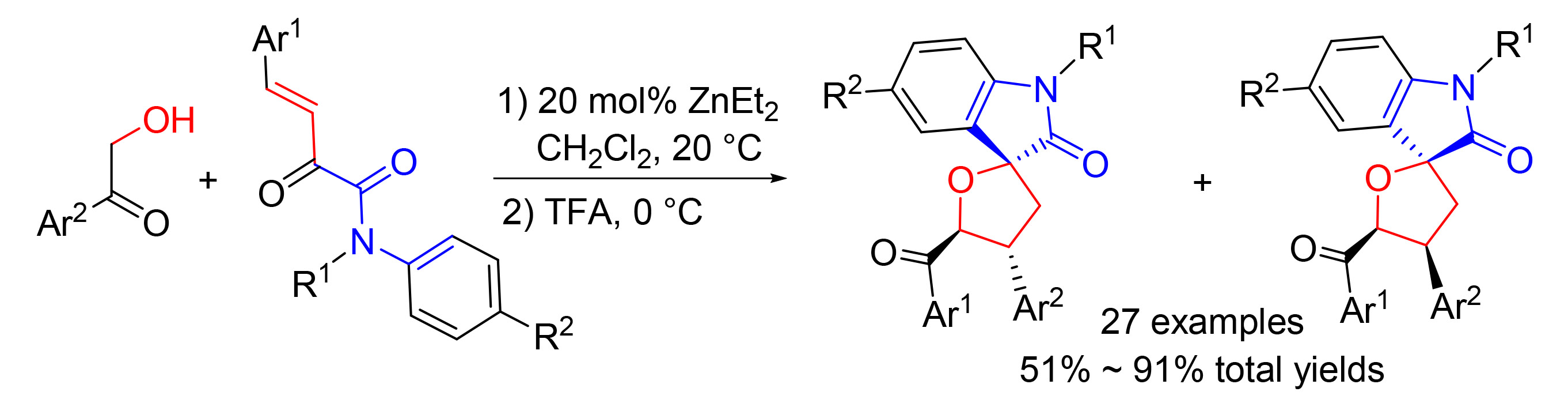
报道了α-羟基芳基酮和β,γ-不饱和-α-酮酰胺发生的Michael/半缩酮化和傅-克(Friedel-Crafts)反应的两步一锅反应.该方法利用不包含氧化吲哚和四氢呋喃结构的链状底物,高效构建出包含螺碳原子、氧化吲哚环和四氢呋喃环的四氢呋喃螺氧化吲哚衍生物.
关键词: 一锅法, 串联反应, 四氢呋喃螺氧化吲哚
A one-pot reaction of Michael/hemiketalization and Fridel-Crafts reaction of α-hydroxy aryl ketones and β,γ-unsaturated α-ketoamides has been developed. The process enables efficient synthesis of tetrahydrofuran spirooxindoles using chain substrates that do not contain oxindole and tetrahydrofuran skeletons. A spiro-carbon center, an oxindole ring and a tetrahydrofuran ring, are constructed in this process.
Key words: one-pot, cascade reaction, tetrahydrofuran spirooxindoles
PDF全文下载地址:
点我下载PDF
