摘要/Abstract
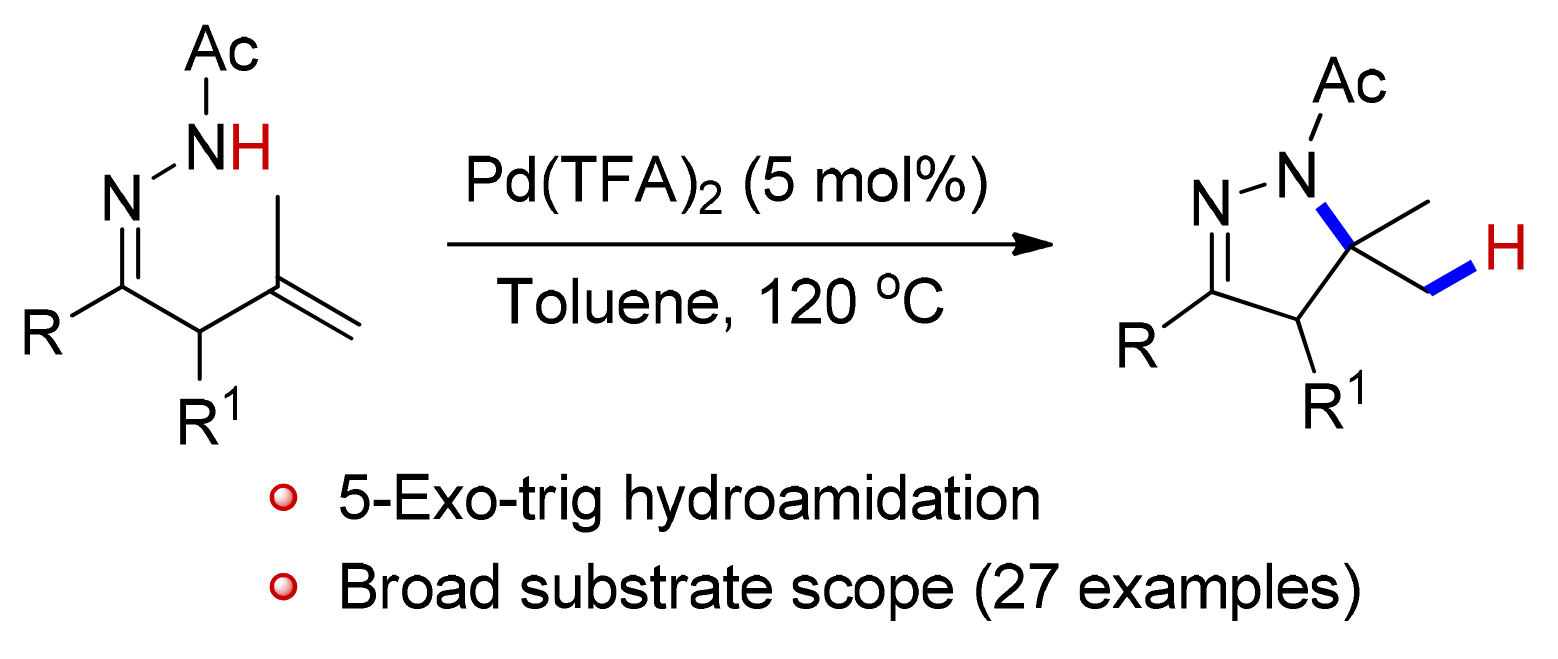
报道了钯催化β,γ-不饱和腙的5-exo-trig型分子内氢酰胺化反应,高效地合成了一系列二氢吡唑类化合物.该反应原料简单易得,官能团兼容性高,底物适用范围宽,产率高,反应不需要配体和添加剂.机理研究表明,与烯烃加成的氢原子来源于酰基腙氮原子上的氢,并且可能是通过三氟乙酸间接转化而来.
关键词: 钯催化, 氢酰胺化, 不饱和腙, 二氢吡唑, 含氮杂环
Hydroamination reaction is the addition of a N-H unit across the unsaturated C-C bond, which provides a convenient route for the formation of C-N bond. Considerable effort has been directed toward the development of multiple catalytic protocols for the hydroamination reaction in the past decades. Despite appreciable progress in this field, the development of general and practical strategy for the hydroamination of amides remains challenging, because the strong electron-with-drawing substituents (acyl, sulfonyl, or phosphinyl) strongly declined the nucleophilicity of the nitrogen. In this paper, a mild and efficient palladium-catalyzed 5-exo-trig hydroamidation of β,γ-unsaturated hydrazones for the synthesis of dihydropyrazoles has been developed. The reaction employs readily available starting materials, tolerates a wide range of functional groups, and produces a series of valuable dihydropyrazoles in good to high yields (63%~88%) under mild reaction conditions. Furthermore, a gram-scale hydroamidation of 1a afforded the dihydropyrazole 2a in 85% yield. In addition, the deuterium labeling experiment demonstrated that N-H is the hydrogen source in this hydroamidation process.
Key words: palladium-catalyzed, hydroamidation, unsaturated hydrazones, dihydropyrazole, N-heterocycles
PDF全文下载地址:
点我下载PDF
