摘要/Abstract
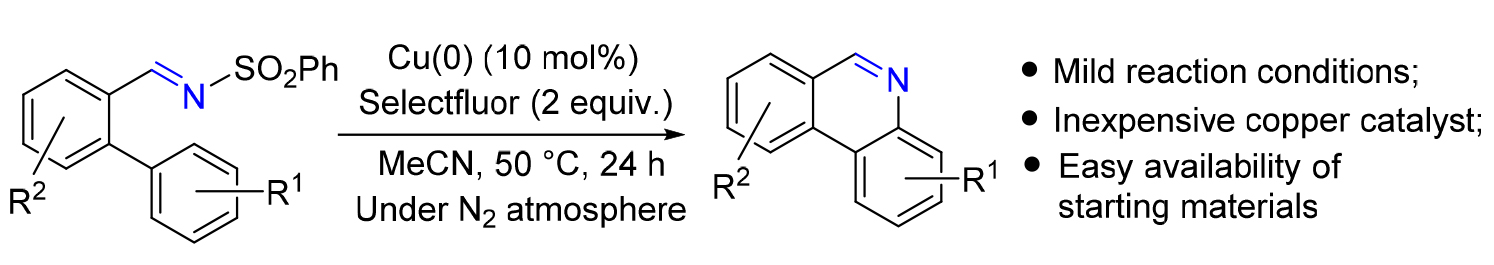
发展了Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应, 于温和的反应条件下以中等到良好的产率简便、高效地构建了一系列6H-菲啶类化合物. 机理研究表明, 反应的关键步骤经历了由Cu(0)/Selectfluor体系现场原位产生XCuOH (X=F, BF4)物种, 进而诱导对C=N键的羟铜化反应和分子内C—H键胺化反应, 从而合成了6H-菲啶类化合物.
关键词: 铜催化, Selectfluor, 6H-菲啶, 环化反应, C—H键胺化
A facile and efficient method for the synthesis of 6H-phenanthridines has been successfully developed involving a copper(0)/Selectfluor system-catalyzed tandem annulation/aromatization of o-aryl benzenesulfonylimides. A variety of substituted 6H-phenanthridines were synthesized in moderate to good yields under mild reaction conditions. Mechanistic experiments revealed that the reaction might involve an oxycupration of C=N bond followed by an intramolecular C—H bond amination as the key steps triggered by an in situ generated copper species XCuOH (X=F or BF4) from the Cu(0)/Selectfluor system.
Key words: copper catalysis, Selectfluor, 6H-phenanthridine, annulation reaction, C—H bond amination
PDF全文下载地址:
点我下载PDF
