摘要/Abstract
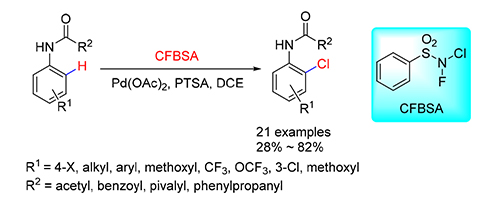
报道了一种以N-氟-N-氯苯磺酰胺(CFBSA)同时作为氯源、氧化剂和促进剂的钯催化乙酰苯胺氯化方法.反应后的副产物N-氟苯磺酰胺在Pd(OAc)2存在下分解,促进了反应进程.表明钯催化下酰胺键导向的碳氢活化可以得到一系列邻位氯化的产物,产率在28%~82%.
关键词: 氯化试剂, 氟原子, 钯催化, 合成方法学
A mild method for palladium-catalyzed halogenation of acetanilide with N-chloro-N-fluorobenzenesulfonylamide (CFBSA) as a chlorinating reagent, oxidant, and novel promoting reagent was achieved. The decomposition of byproduct N-fluoroben-zenesulfonylamine in the presence of Pd(OAc)2 could accelerate the process of chlorination. Preliminary mechanism investigation showed that Pd catalyzed anilide directed C-H activation lead to the ortho chlorination selectivity. A series of ortho-chlorinated anilides were obtained in 28%~82% yields.
Key words: chlorinating reagent, fluorine atom, Pd-catalyzed, synthetic methodology
PDF全文下载地址:
点我下载PDF
