摘要/Abstract
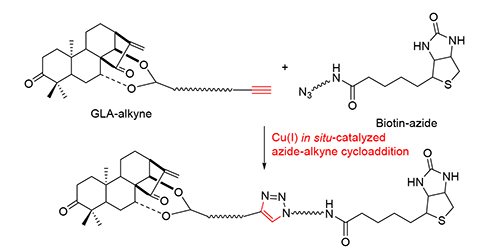
新型生物素标记的蓝萼甲素可以作为潜在的、基于生物活性的靶标蛋白质的探针.利用原位Cu(I)催化的叠氮-炔环加成反应,以高区域选择性和较高收率合成了系列蓝萼甲素的生物素长链轭合分子.研究发现,在优选的反应条件下,原位还原生成的Cu(I)可以很好地被溶剂化,从而展现了高催化活性.本方法较好地解决了针对双长链的叠氮-炔环加成收率普遍较低的问题,简便,条件温和,同样适用于其他以三氮唑链接的长链双功能分子的合成.
关键词: 生物素标记, 蓝萼甲素, 基于活性的探针, 原位Cu(I)催化, 叠氮-炔环加成
The novel biotin conjugated glaucocalyxin A analogs as potential activity-based protein probes were synthesized with good yields via Cu(I) in situ-catalyzed azide-alkyne cycloaddition. Cu(I) in situ was good solvated under the optimized condition and shown high catalytic activity. The approach efficiently constructs the even longer linker containing 1,2,3-triazole nucleus between bifunctional groups with the advantages of high regioselectivity and operational simplicity.
Key words: biotin tag, glaucocalyxin A, activity-based probe, Cu(I) in situ catalysis, azide-alkyne cycloaddition
PDF全文下载地址:
点我下载PDF
