摘要/Abstract
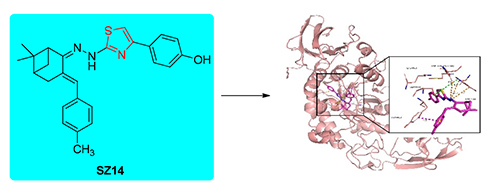
以天然β-蒎烯衍生物诺蒎酮为原料,经缩合和环化等反应,合成了24个诺蒎酮基噻唑腙类化合物,采用1H NMR,13C NMR,HRMS等方法对其结构进行表征,研究了噻唑腙类化合物对α-淀粉酶的抑制活性.结果表明,与阳性对照阿卡波糖相比,有6种化合物对α-淀粉酶表现出优良的的抑制活性,其中4-(2-(2-(6,6-二甲基-3-(4-甲基苄亚基)双环[3.1.1]庚烷-2-亚基)肼基)噻唑-4-基)苯酚(SZ14)的IC50值可达到4.11 μmol/L.从化合物的结构与活性关系看,R2的结构对活性具有显著的影响.抑制动力学结果表明,这6种化合物是针对α-淀粉酶的非竞争性抑制剂.采用分子对接方法评价了噻唑腙类化合物与α-淀粉酶的结合亲和力,并分析探索了化合物SZ14与α-淀粉酶的结合方式.
关键词: 诺蒎酮, 噻唑腙, α-淀粉酶抑制剂, 分子对接
A series of nopinone-based thiazolyhydrazone derivatives were synthesized by using nopinone derivated from natural β-pinene as the starting material in three steps, including aldol reaction with aromatic aldehydes, condensation with aminothiourea, and cyclization with bromoacetophenone. The structures of synthesized compounds were characterized by 1H NMR, 13C NMR and HRMS. α-Amylase inhibitory activities of these compounds were also investigated. The results showed that 6 compounds among them had good inhibitory activities compared with the positive control acarbose. Especially, 4-(2-(2-(6,6-dimethyl-3-(4-methylbenzylidene))bicyclo[3.1.1]heptane-2-ylidene)indolyl-4-thiazol-4-yl)phenol (SZ14) exhibited remarkable α-amylase inhibitory activity with IC50 value of 4.11 μmol/L. From the structure-activity relationship, the structure of R2 gave great influence on the activities of thiazolyhydrazone derivatives. The kinetic inhibition study revealed that those 6 compounds were the noncompetitive inhibitor against α-amylase. It was used for molecular docking study to find out binding affinities for thiazolylhydrazone derivatives, and the binding mode of compound SZ14 with α-amylase was primarily investigated with molecular docking method.
Key words: nopinone, thiazolylhydrazone, α-amylase inhibitor, molecular docking
PDF全文下载地址:
点我下载PDF
