摘要/Abstract
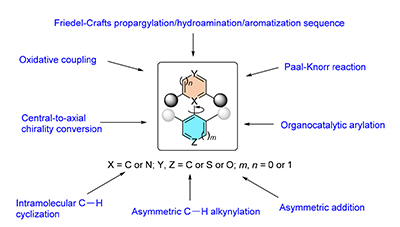
轴手性联芳化合物广泛存在于天然产物和药物分子中,并作为手性配体和催化剂应用于不对称合成.因此,其不对称合成备受关注.然而,目前已知的合成方法大多局限于合成以C-C键为轴的六元联芳结构,而以C-C键或C-N键连接的含有五元杂芳环的联芳轴手性化合物的合成方法却鲜有报道.含有五元杂芳环的轴手性结构其邻位基团与中心轴的距离更远,构象稳定性更差,因而其不对称构建更加困难.综述了五元杂芳轴手性骨架的对映选择性合成的最新进展.
关键词: 五元杂芳阻旋异构体, 对映选择性合成, 轴手性, 联芳
Axially chiral biaryl skeletons are ubiquitous stuctural motifs that are widely represented in pharmaceuticals and natural products, and have been widely used as privileged chiral ligands/catalysts in asymmetric synthesis. Therefore, the asymmetric construction of these compounds has received tremendous attention. However, the established strategies are mainly limited to the construction of biaryls containing hexatomic aromatics, and the approaches towards atropisomers featuring pentatomic heteroaromatics connected through C-C or C-N bond have emerged gradually only until recently. The main hurdle is basically due to the increased distance of substituents ortho to the axis, which is responsible for lower barriers to rotation, thus rendering the asymmetric synthesis more challenging. This review summarizes recent advances on the enantioselective synthesis of atropisomers featuring pentatomic heteroaromatics.
Key words: atropisomers featuring pentatomic heteroaromatic, enantioselective synthesis, axial chirality, biaryl
PDF全文下载地址:
点我下载PDF
