摘要/Abstract
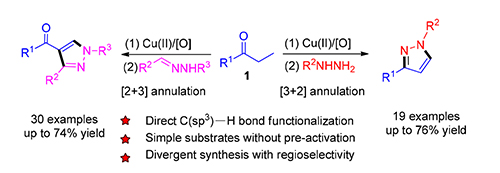
报道了一种经由二价铜催化饱和酮与肼或醛腙的串联反应合成1,3-二取代吡唑或1,3,4-三取代吡唑类化合物的简便和区域选择性新方法.从机理上看,1,3-二取代吡唑的生成经历了烯酮中间体的原位生成及其与肼的[3+2]环化反应,而1,3,4-三取代吡唑的生成则经历了烯酮中间体的原位生成及其与醛腙的[2+3]环化反应.与文献方法相比,该方法具有原料简单易得、底物适用范围广、区域选择性好、效率高、原子经济性优异等优势.
关键词: 吡唑合成, 二价铜催化反应, C(sp3)-H官能团化, [3+2]环化, [2+3]环化
A highly convenient and regioselective synthesis of 1,3-disubstituted pyrazoles or 1,3,4-trisubstituted pyrazoles from Cu(Ⅱ)-catalyzed cascade reactions of saturated ketones with hydrazines or aldehyde hydrazones is presented. Mechanistically, the formation of 1,3-disubstituted pyrazoles involves the in situ generation of an enone intermediate followed by its[3+2] annulations with hydrazine. On the other hand, the formation of 1,3,4-trisubstituted pyrazoles is believed to go through a cascade process including enone formation and its subsequent[2+3] annulation with aldehyde hydrazone. Compared with literature methods, the notable features of the protocol include simple starting materials, general and broad substrate scope, high regioselectivity, good efficiency and excellent atom-economy.
Key words: pyrazole synthesis, Cu(Ⅱ) catalyzed reaction, C(sp3)-H functionalization, [3+2] annulation, [2+3] annulation
PDF全文下载地址:
点我下载PDF
